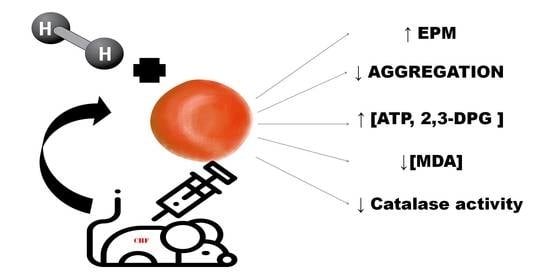
The Effect of Molecular Hydrogen on Functional States of Erythrocytes in Rats with Simulated Chronic Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Care
2.2. Verification of CHF Rats Model
2.3. Experiment Grouping and Process
2.4. Blood Collection
2.5. Materials
2.5.1. The Electrophoretic Mobility of Erythrocytes (EPM)
2.5.2. Erythrocyte Aggregation
2.5.3. Adenosine Triphosphate (ATP)
2.5.4. 2,3-Diphosphoglyceric Acid (2,3-DPG)
2.5.5. Lipid Peroxidation
2.5.6. Antioxidant Capacity
2.5.7. Hematological Parameters
2.6. Statistical Analysis
3. Results
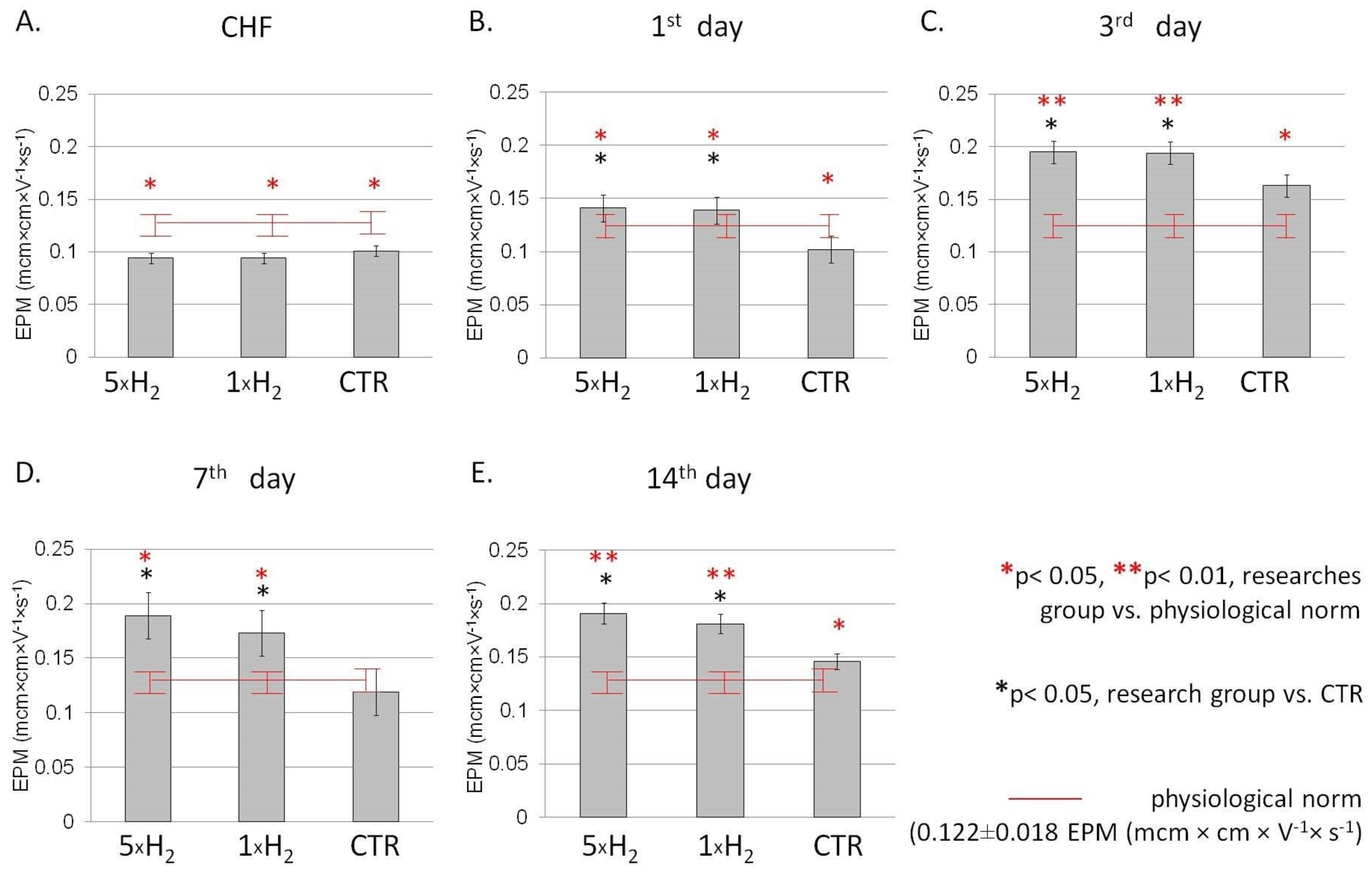
3.1. The Electrophoretic Mobility of the Cell
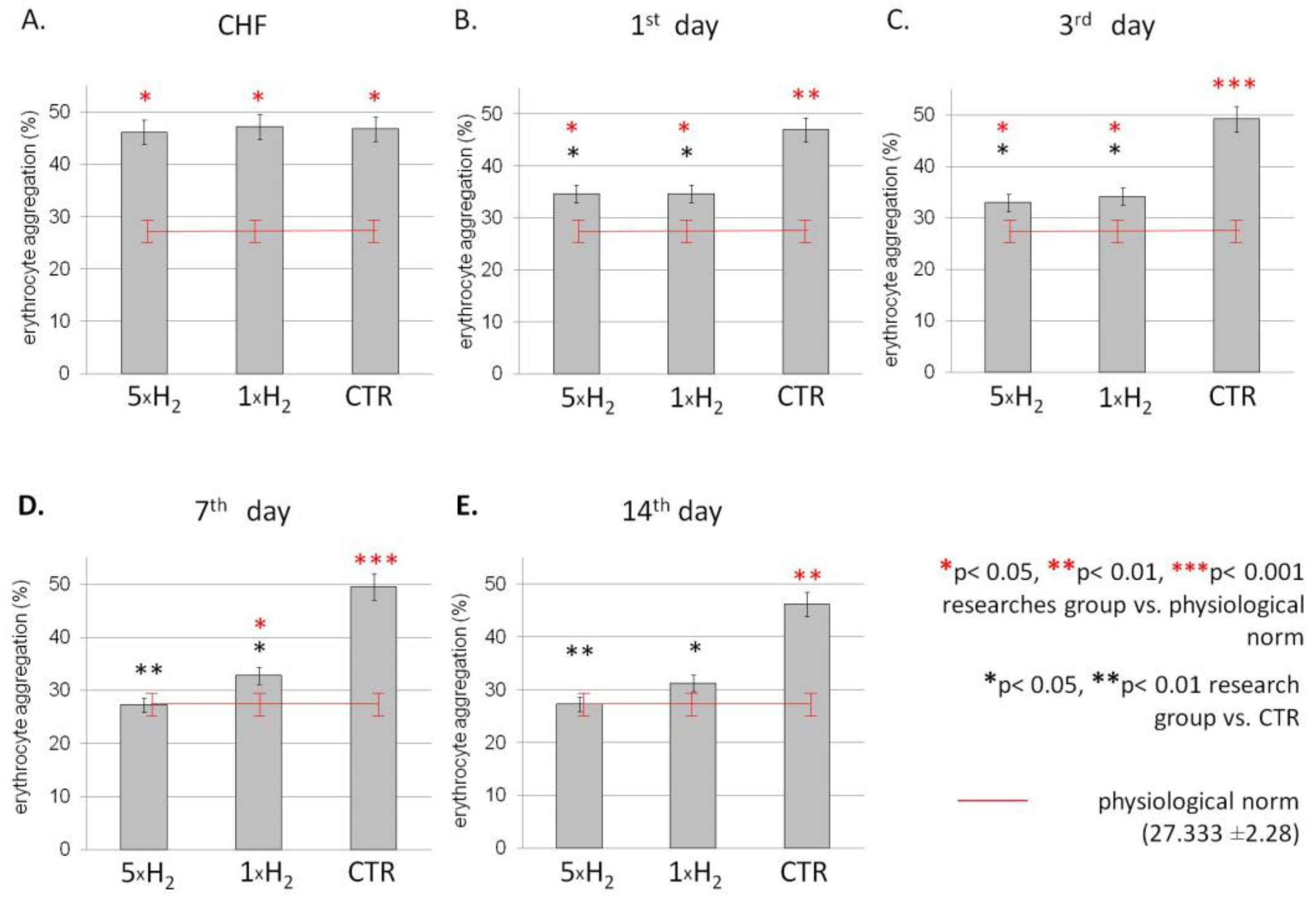
3.2. The Aggregation
3.3. The Energetic Metabolism
3.4. Oxidative Stress Markers
3.5. Hematological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Riet, E.E.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.; Rutten, F.H. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 2016, 18, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Sarhene, M.; Wang, Y.; Wei, J.; Huang, Y.; Li, M.; Li, L.; Acheampong, E.; Zhengcan, Z.; Xiaoyan, Q.; Yunsheng, X.; et al. Biomarkers in heart failure: The past, current and future. Heart Fail. Rev. 2019, 24, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Remme, W.J.; Swedberg, K. Task force for the diagnosis and treatment of chronic heart failure, European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure. Eur. Heart J. 2001, 22, 1527–1560. [Google Scholar] [CrossRef]
- Kim, I.C.; Yoo, B.S. Multidimensional Approach of Heart Failure Diagnosis and Prognostication Utilizing Cardiac Imaging with Biomarkers. Diagnostics 2022, 12, 1366. [Google Scholar] [CrossRef]
- Misiti, F.; Carelli-Alinovi, C.; Rodio, A. ATP release from erythrocytes: A role of adenosine1. Clin. Hemorheol. Microcirc. 2022, 80, 61–71. [Google Scholar] [CrossRef]
- Zhou, Z.; Mahdi, A.; Tratsiakovich, Y.; Zahoran, S.; Kovamees, O.; Nordin, F.; Uribe Gonzalez, A.E.; Alvarsson, M.; Ostenson, C.G.; Andersson, D.C.; et al. Erythrocytes from patients with type 2 diabetes induce endothelial dysfunction via arginase I. J. Am. Coll. Cardiol. 2018, 72, 769–780. [Google Scholar] [CrossRef]
- Wautier, J.L.; Wautier, M.P. Molecular basis of erythrocyte adhesion to endothelial cells in diseases. Clin. Hemorheol. Microcirc. 2013, 53, 11–21. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, S.; Zhang, J.M. Hydrogen as a selective antioxidant: A review of clinical and experimental studies. J. Int. Med. Res. 2010, 38, 1893–1903. [Google Scholar] [CrossRef]
- Ohta, S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. Curr. Pharm. Des. 2011, 17, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Alshami, A.; Einav, S.; Skrifvars, M.B.; Varon, J. Administration of inhaled noble and other gases after cardiopulmonary resuscitation: A systematic review. Am. J. Emerg. Med. 2020, 38, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Yoshida, A.; Asanuma, H.; Sasaki, H.; Sanada, S.; Yamazaki, S.; Asano, Y.; Shinozaki, Y.; Mori, H.; Shimouchi, A.; Sano, M.; et al. H(2) mediates cardioprotection via involvements of K(ATP) channels and permeability transition pores of mitochondria in dogs. Cardiovasc. Drugs Ther. 2012, 26, 217–226. [Google Scholar] [CrossRef]
- Shibata, A.; Sugano, Y.; Shimouchi, A.; Yokokawa, T.; Jinno, N.; Kanzaki, H.; Ohta-Ogo, K.; Ikeda, Y.; Okada, H.; Aiba, T.; et al. Decrease in exhaled hydrogen as marker of congestive heart failure. Open Heart 2018, 5, e000814. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Deryugina, A.V.; Danilova, D.A.; Skokova, A.A.; Brichkin, Y.D.; Pichugin, V.V.; Medvedev, A.P.; Ryazanov, M.V.; Fedorov, S.A. Dynamics of Metabolic and Oxidative Parameters of Erythrocytes during Treatment of Chronic Heart Failure with Molecular Hydrogen. Bull. Exp. Biol. Med. 2022, 173, 602–605. [Google Scholar] [CrossRef]
- Tsai, C.K.; Chen, B.H.; Chen, H.H.; Hsieh, R.J.; Lee, J.C.; Chu, Y.T.; Lu, W.H. Low-Dose Propranolol Prevents Functional Decline in Catecholamine-Induced Acute Heart Failure in Rats. Toxics 2022, 10, 238. [Google Scholar] [CrossRef]
- Muders, F.; Friedrich, E.; Luchner, A.; Pfeifer, M.; Ickenstein, G.; Hamelbeck, B.; Riegger, G.A.; Elsner, D. Hemodynamic changes and neurohumoral regulation during development of congestive heart failure in a model of epinephrine-induced cardiomyopathy in conscious rabbits. J. Card. Fail. 1999, 5, 109–116. [Google Scholar] [CrossRef]
- Deryugina, A.V.; Ivashchenko, M.N.; Ignatiev, P.S.; Balalaeva, I.V.; Samodelkin, A.G. Low-level laser therapy as a modifier of erythrocytes morphokinetic parameters in hyperadrenalinemia. Lasers Med. Sci. 2019, 34, 1603–1612. [Google Scholar] [CrossRef]
- Shumilova, A.V.; Deryugina, A.V.; Gordleeva, S.Y.; Boyarinov, G.A. Cytoflavin action on electro-kinetic and aggregation indices of erythrocytes in the post-traumatic period of cerebrocranial injury in experiment. Russ. J. Exp. Clin. Pharmacol. 2018, 3, 20–23. [Google Scholar] [CrossRef]
- Deryugina, A.V.; Danilova, D.A.; Brichkin, Y.D.; Taranov, E.V.; Nazarov, E.I.; Pichugin, V.V.; Medvedev, A.P.; Riazanov, M.V.; Fedorov, S.A.; Andrej, Y.S.; et al. Molecular hydrogen exposure improves functional state of red blood cells in the early postoperative period: A randomized clinical study. Med. Gas. Res. 2023, 13, 59–66. [Google Scholar] [CrossRef]
- Volchegorskii, I.A.; Nalimov, A.G.; Iarovinskii, B.G.; Lifshits, R.I. Sopostavlenie razlichnykh podkhodov k opredeleniiu produktov perekisnogo okisleniia lipidov v heptan-izopropanol’nykh ékstraktakh krovi [Comparison of various approaches to the determination of the products of lipid peroxidation in heptane-isopropanol extracts of blood]. Vopr. Med. Khim. 1989, 35, 127–131. [Google Scholar] [PubMed]
- Beers, R.F.; Sizer, J.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Popel, A.S.; Johnson, P.C. Microcirculation and hemorheology. Annu. Rev. Fluid Mech. 2005, 37, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Provotorov, V.M.; Avdeeva, S.A. Anemia in patients with chronic cardiac failure. Clin. Med. 2012, 3, 55–59. [Google Scholar]
- Tokumasu, F.; Nardone, G.A.; Ostera, G.R.; Fairhurst, R.M.; Beaudry, S.D.; Hayakawa, E.; Dvorak, J.A. Altered membrane structure and surface potential in homozygous hemoglobin C erythrocytes. PLoS ONE 2009, 4, e5828. [Google Scholar] [CrossRef]
- Cicco, G.; Cicco, S. The influence of oxygen supply, hemorheology and microcirculation in the heart and vascular systems. Adv. Exp. Med. Biol. 2010, 662, 33–39. [Google Scholar] [CrossRef]
- Hayashida, K.; Sano, M.; Ohsawa, I.; Shinmura, K.; Tamaki, K.; Kimura, K.; Endo, J.; Katayama, T.; Kawamura, A.; Kohsaka, S.; et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2008, 373, 30–35. [Google Scholar] [CrossRef]
- Liu, S.; Liu, K.; Sun, Q.; Liu, W.; Xu, W.; Denoble, P.; Tao, H.; Sun, X. Consumption of hydrogen water reduces paraquat-induced acute lung injury in rats. J. Biomed. Biotechnol. 2011, 2011, 305086. [Google Scholar] [CrossRef]
- Li, H.M.; Shen, L.; Ge, J.W.; Zhang, R.F. The transfer of hydrogen from inert gas to therapeutic gas. Med. Gas Res. 2018, 7, 265–272. [Google Scholar] [CrossRef]
- Fistal EYa Nosenco, V.M. Pathogenetic justification of parenteral use of ozone in emergency conditions in kombustiology. Emerg. Med. Care 2007, 3, 86–89. [Google Scholar]
- Musielak, M. Are there two functionally distinguished Neu5Gc pools with respect to rouleau formation on the bovine red blood cell? Clin. Hemorheol. Microcirc. 2004, 30, 435–438. [Google Scholar]
- Nunomura, W.; Takakuwa, Y. Regulation of protein 4.1R interactions with membrane proteins by Ca2+ and calmodulin. Front. Biosci. 2006, 11, 1522–1539. [Google Scholar] [CrossRef]
- Minetti, M.; Agati, L.; Malorni, W. The microenvironment can shift erythrocytes from a friendly to a harmful behavior: Pathogenetic implications for vascular diseases. Cardiovasc. Res. 2007, 75, 21–28. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Fukuzaki, S. ATP effects on response of human erythrocyte membrane to high pressure. Biophys. Physicobiol. 2019, 16, 158–166. [Google Scholar] [CrossRef]
- Glenn, A.; Armstrong, C.E. Physiology of red and white blood cells. Anaesth. Intensive Care Med. 2019, 20, 170–174. [Google Scholar] [CrossRef]
- Ulker, P.; Sati, L.; Celik-Ozenci, C.; Meiselman, H.J.; Baskurt, O.K. Mechanical stimulation of nitric oxide synthesizing mechanisms in erythrocytes. Biorheology 2009, 46, 121–132. [Google Scholar] [CrossRef]
- Price, A.K.; Fischer, D.J.; Martin, R.S.; Spence, D.M. Deformation-induced release of ATP from erythrocytes in a poly(dimethylsiloxane)-based microchip with channels that mimic resistance vessels. Anal. Chem. 2004, 76, 4849–4855. [Google Scholar] [CrossRef]
- Jensen, F.B. The dual roles of red blood cells in tissue oxygen delivery: Oxygen carriers and regulators of local blood flow. J. Exp. Biol. 2009, 212 Pt 21, 3387–3393. [Google Scholar] [CrossRef]
- Hsu, L.L.; Champion, H.C.; Campbell-Lee, S.A.; Bivalacqua, T.J.; Manci, E.A.; Diwan, B.A.; Schimel, D.M.; Cochard, A.E.; Wang, X.; Schechter, A.N.; et al. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood 2007, 109, 3088–3098. [Google Scholar] [CrossRef]
- Kim-Shapiro, D.B.; Schechter, A.N.; Gladwin, M.T. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 697–705. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta 2012, 1820, 586–594. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [Green Version]
- Deryugina, A.V.; Ivashchenko, M.N.; Ignatiev, P.S.; Lodyanoy, M.S.; Samodelkin, A.G. Alterations in the phase portrait and electrophoretic mobility of erythrocytes in various diseases. Mod. Technol. Med. 2019, 11, 63–68. [Google Scholar] [CrossRef]
- Krylov, V.N.; Deriugina, A.V.; Zakharova, O.A.; Antipenko, E.A. Nonspecific adaptive reactions blood in chronic brain ischemia. Klin. Lab. Diagn. 2010, 12, 28–30. [Google Scholar]
- de Lucia, C.; Piedepalumbo, M.; Paolisso, G.; Koch, W.J. Sympathetic nervous system in age-related cardiovascular dysfunction: Pathophysiology and therapeutic perspective. Int. J. Biochem. Cell Biol. 2019, 108, 29–33. [Google Scholar] [CrossRef]
- Wehrwein, E.A.; Orer, H.S.; Barman, S.M. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr. Physiol. 2016, 6, 1239–1278. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Anderson, A.S. The sympathetic nervous system and heart failure. Cardiol. Clin. 2014, 32, 33–45. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef]
| Indicator | Group | Day of the Experiment | |||
|---|---|---|---|---|---|
| 1 | 3 | 7 | 14 | ||
| ATP (μmol Pi/mL) | 5 × H2 | 1.120 ± 0.063 | 2.724 ± 0.357 * | 3.961 ± 0.090 * | 2.166 ± 0.298 * |
| 1 × H2 | 1.944 ± 0.210 * | 1.464 ± 0.119 | 3.814 ± 0.146 | 2.480 ± 0.756 * | |
| CTR | 0.960 ± 0.240 | 1.380 ± 0.162 | 3.500 ± 0.187 | 1.534 ± 0.124 | |
| 2,3-DPG (μmol Pi/mL) | 5 × H2 | 13.403 ± 1.587 | 13.261 ± 1.444 * | 10.404 ± 1.060 | 16.914 ± 1.460 * |
| 1 × H2 | 12.940 ± 0.601 | 5.428 ± 1.489 | 10.090 ± 0.180 | 10.510 ± 1.970 | |
| CTR | 11.595 ± 0.816 | 8.278 ± 2.885 | 11.532 ± 2.815 | 9.343 ± 0.614 | |
| Indicator | Group | Day of the Experiment | |||
|---|---|---|---|---|---|
| 1 | 3 | 7 | 14 | ||
| MDA (nmol/mL) | 5 × H2 | 0.539 ± 0.270 * | 2.378 ± 0.147 | 2.077 ± 0.107 | 0.549 ± 0.131 * |
| 1 × H2 | 0.404 ± 0.110 * | 2.692 ± 0.530 | 2.570 ± 0.189 | 1.263 ± 0.153 | |
| CTR | 2.563 ± 0.270 | 2.863 ± 0.354 | 2.269 ± 0.172 | 1.539 ± 0.485 | |
| Catalase activity (units/gHb×min) | 5 × H2 | 0.492 ± 0.105 | 0.954 ± 0.166 * | 0.691 ± 0.183 | 1.542 ± 0.210 * |
| 1 × H2 | 0.803 ± 0.194 | 0.786 ± 0.071 | 1.040 ± 0.295 | 1.269 ± 0.227 | |
| CTR | 0.547 ± 0.128 | 0.618 ± 0.140 | 0.617 ± 0.271 | 0.729 ± 0.327 | |
| Indicator | Group | Day of the Experiment | |||
|---|---|---|---|---|---|
| 1 | 3 | 7 | 14 | ||
| DC (relative unit) | 5 × H2 | 0.195 ± 0.008 | 0.206 ± 0.006 | 0.156 ± 0.009 | 0.210 ± 0.021 |
| 1 × H2 | 0.224 ± 0.014 * | 0.181 ± 0.006 | 0.157 ± 0.007 | 0.177 ± 0.015 | |
| CTR | 0.183 ± 0.012 | 0.167 ± 0.026 | 0.155 ± 0.010 | 0.186 ± 0.013 | |
| TC (relative unit) | 5 × H2 | 0.068 ± 0.017 | 0.117 ± 0.014 | 0.079 ± 0.010 | 0.081 ± 0.012 |
| 1 × H2 | 0.068 ± 0.017 | 0.117 ± 0.014 | 0.079 ± 0.010 | 0.081 ± 0.012 | |
| CTR | 0.069 ± 0.011 | 0.090 ± 0.015 | 0.065 ± 0.010 | 0.071 ± 0.006 | |
| SB (relative unit) | 5 × H2 | 5.545 ± 0.920 | 2.382 ± 0.246 * | 2.571 ± 0.747 * | 2.137 ± 0.644 * |
| 1 × H2 | 6.468 ± 1.731 | 4.096 ± 0.929 * | 2.521 ± 0.897 * | 3.745 ± 1.124 * | |
| CTR | 5.054 ± 1.431 | 6.557 ± 1.746 | 4.037 ± 0.837 | 6.555 ± 0.725 | |
| Indicator | Group | Day of the Experiment | |||
|---|---|---|---|---|---|
| 1 | 3 | 7 | 14 | ||
| RBC, (×1012/L) | 5 × H2 | 3.41 ± 0.67 * | 3.83 ± 0.55 * | 4.12 ± 0.34 | 4.49 ± 0.63 * |
| 1 × H2 | 4.13 ± 0.74 * | 4.33 ± 0.51 | 4.45 ± 0.36 | 5.51 ± 0.32 | |
| CTR | 5.46 ± 0.57 | 4.56 ± 0.13 | 4.410 ± 0.28 | 5.52 ± 0.31 | |
| Hb (g/L) | 5 × H2 | 63.83 ± 9.11 * | 76.63 ± 5.17 * | 90.21 ± 5.77 | 82.85 ± 11.80 * |
| 1 × H2 | 85.47 ± 4.01 * | 81.81 ± 8.32 | 92.61 ± 2.92 | 111.22 ± 3.56 | |
| CTR | 94.54 ± 5.18 | 87.08 ± 5.01 | 94.13 ± 7.01 | 111.16 ± 2.91 | |
| MCV, (fL) | 5 × H2 | 57.42 ± 2.46 * | 58.67 ± 4.65 | 58.41 ± 1.60 | 56.62 ± 0.51 * |
| 1 × H2 | 57.61 ± 1.21 * | 59.43 ± 1.77 | 62.61 ± 3.08 | 62.01 ± 3.13 | |
| CTR | 63.03 ± 1.47 | 61.33 ± 1.33 | 61.67 ± 2.18 | 60.67 ± 2.18 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deryugina, A.V.; Danilova, D.A.; Pichugin, V.V.; Brichkin, Y.D. The Effect of Molecular Hydrogen on Functional States of Erythrocytes in Rats with Simulated Chronic Heart Failure. Life 2023, 13, 418. https://doi.org/10.3390/life13020418
Deryugina AV, Danilova DA, Pichugin VV, Brichkin YD. The Effect of Molecular Hydrogen on Functional States of Erythrocytes in Rats with Simulated Chronic Heart Failure. Life. 2023; 13(2):418. https://doi.org/10.3390/life13020418
Chicago/Turabian StyleDeryugina, Anna Vyacheslavovna, Darya Andreevna Danilova, Vladimir Viktorovich Pichugin, and Yurii Dmitrievich Brichkin. 2023. "The Effect of Molecular Hydrogen on Functional States of Erythrocytes in Rats with Simulated Chronic Heart Failure" Life 13, no. 2: 418. https://doi.org/10.3390/life13020418
APA StyleDeryugina, A. V., Danilova, D. A., Pichugin, V. V., & Brichkin, Y. D. (2023). The Effect of Molecular Hydrogen on Functional States of Erythrocytes in Rats with Simulated Chronic Heart Failure. Life, 13(2), 418. https://doi.org/10.3390/life13020418