Effects of Microencapsulated Essential Oils on Equine Health: Nutrition, Metabolism and Methane Emission
Abstract
1. Introduction
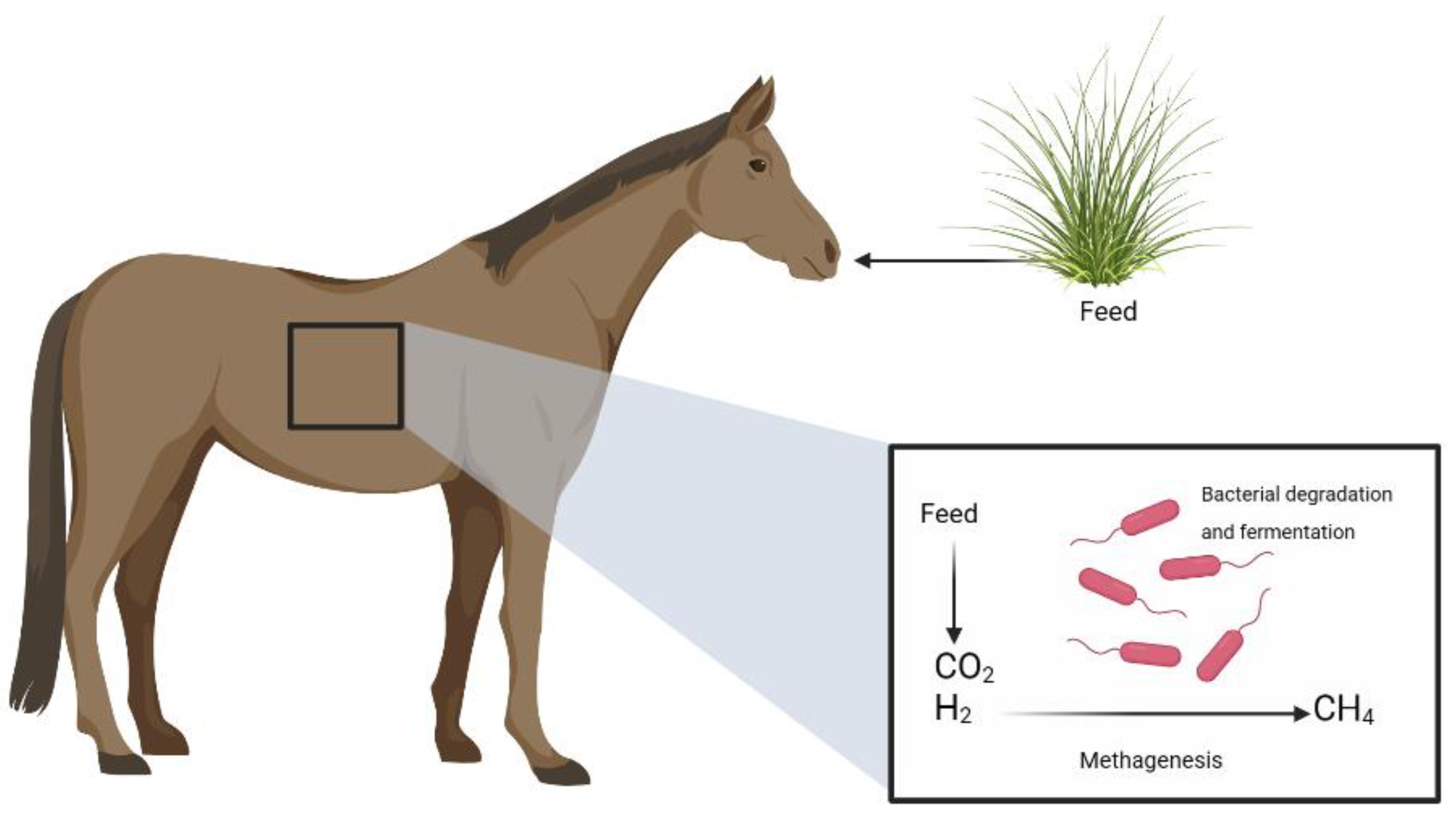
2. Enteric Fermentation in Horses
3. Essential Oils (EOs)
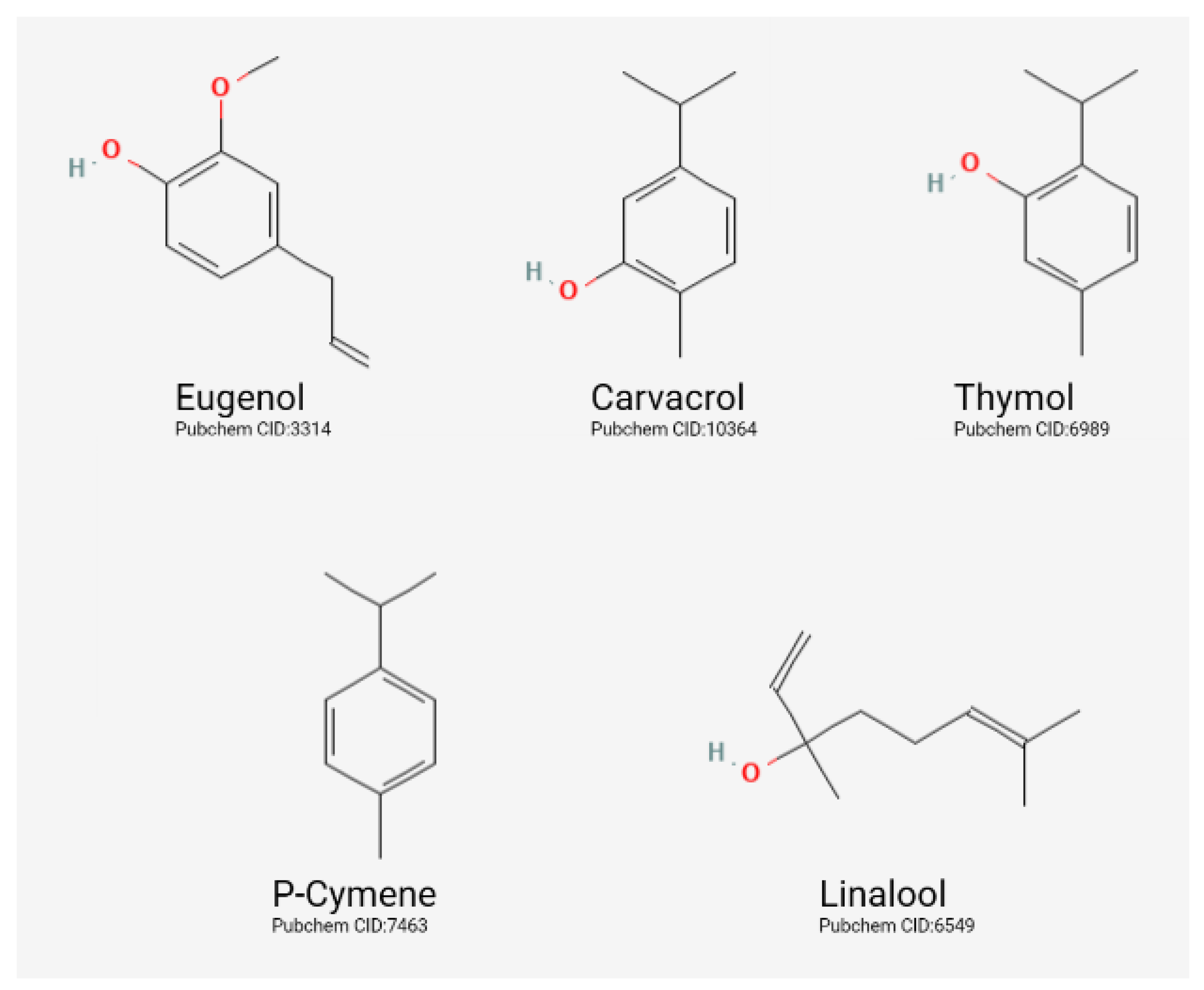
3.1. Classification of EOs
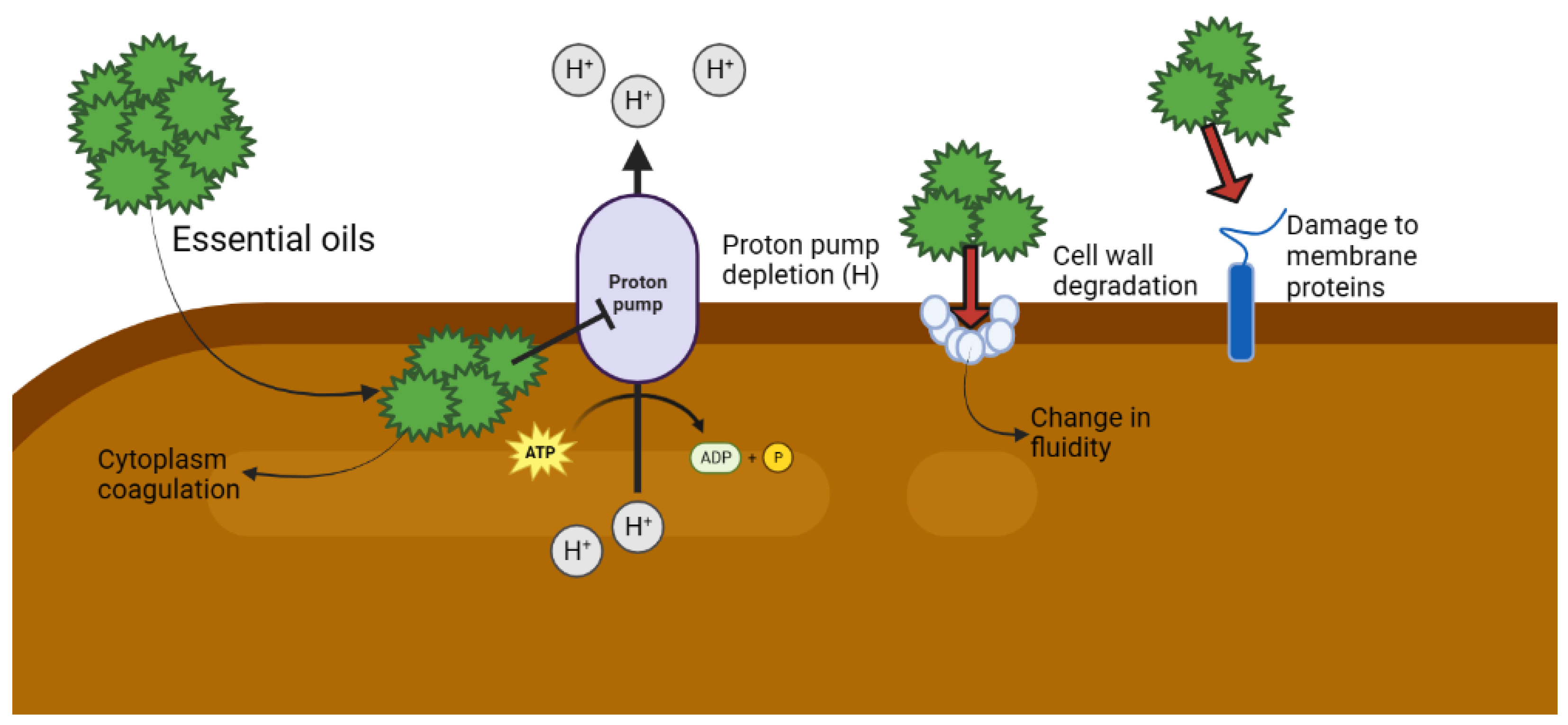
3.2. Mechanisms of Antimicrobial Action
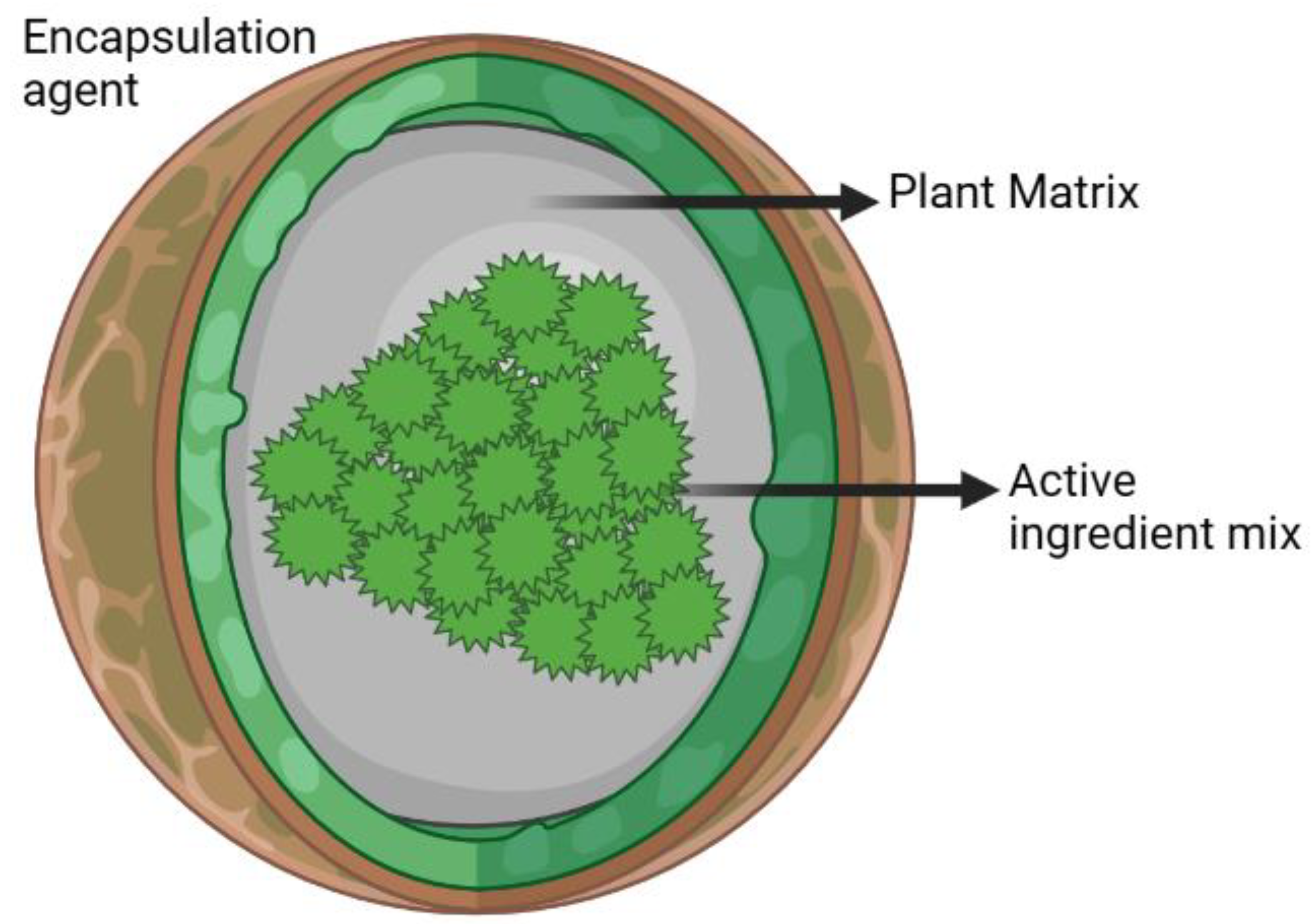
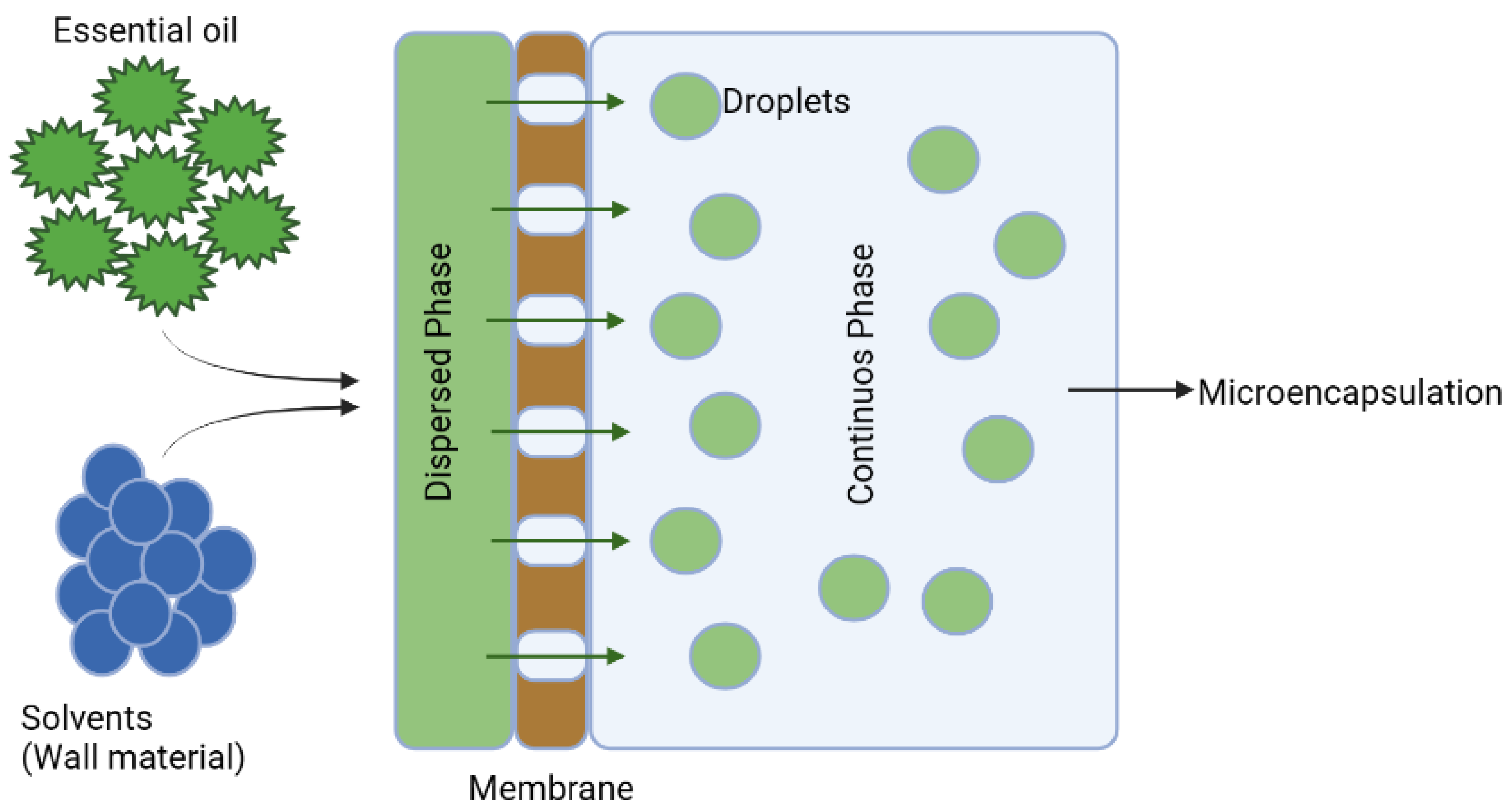
3.3. Microencapsulation
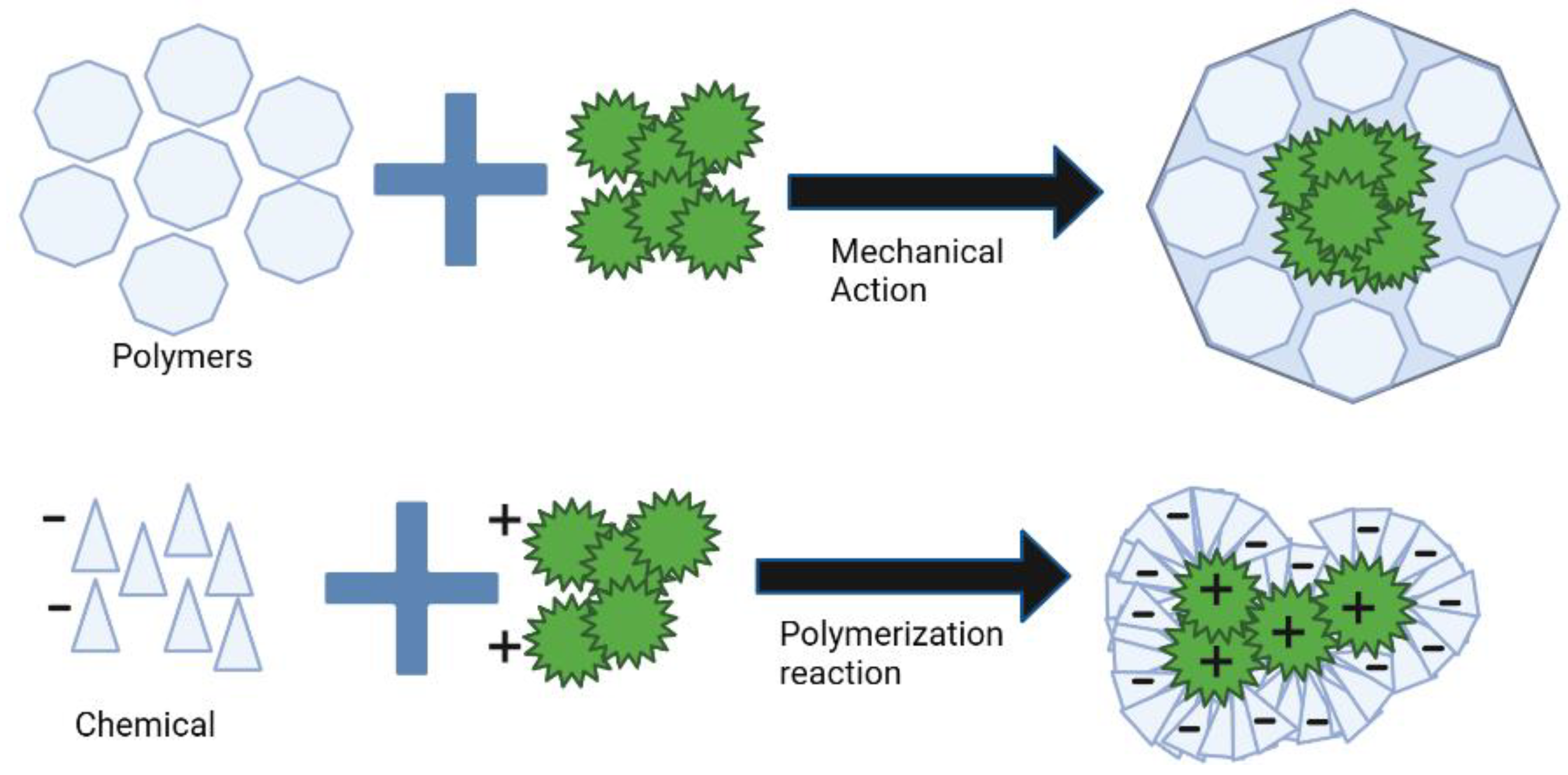
3.4. Encapsulation Techniques
| Technique | Type | Process | Reference |
|---|---|---|---|
| Lyophilization | Physical | Lyophilization consists of two basic steps: freezing and drying. During the drying process, the water is removed from the sample. Loss of essential oils may be experienced in the drying process, due to temperature and the volatility of the essential oils. | [54] |
| Extrusion | Physical | The process requires the essential oil to flow under different conditions (depending on the technique) through a certain orifice. Extrusion techniques can be divided into the following:
| [52,55] |
| Fluidization | Physical | Fluidization is a method that keeps solid particles floating in a flow of gas. So, in this process, the particles are encapsulated using hot air in a coating chamber. | [56] |
| Spray dryer | Physical | It consists of atomizing essential oils with hot air, creating small particles, and evaporating the water. In this process, these small particles can also be covered with a “wall material”, such as polysaccharides. | [52] |
| Solvent removal | Physical | It consists of 4 steps:
| [52,57] |
| Coacervation | Chemical | This process is a coacervation that occurs between oppositely charged molecules. After polymerization, there are 2 separate liquid phases, a polymer-rich phase and polymer-depleted phase. Then, the polymer-rich phase is extracted. The process is strongly recommended for encapsulating essential oils. | [52,58] |
| Mini-emulsion polymerization | Chemical | In this process, monomer droplets are formed. These droplets act to promote the polymerization reaction, which results in the formation of polymeric particles. | [52,59] |
| Ionic gelation | Chemical | This technique is based on the ionic crosslinking of a polymer. When the essential oil is added to the reaction, it can be trapped inside the polymer. | [60] |
| Emulsification | Chemical | This process occurs by mixing two immiscible liquids. To form the encapsulation, some of the 2 liquids must be dispersed as droplets in the other. | [61] |
| Co-extrusion | Chemical | This process begins with the formation of droplets through vibration. These droplets then fall onto a solution with the gelling agent, resulting in the encapsulation of the active ingredient. Co-extrusion/gelation is widely used in the encapsulation of volatile substances. | [61,62] |
4. Influence on Horses’ Cecal Fermentation
5. Influence on Cecal Microbial Population
6. Influence on Methane Production
7. Influence on Horse Nutrition
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Purba, R.A.P.; Yuangklang, C.; Paengkoum, S.; Paengkoum, P. Piper oil decreases in vitro methane production with shifting ruminal fermentation in a variety of diets. Int. J. Agric. Biol. 2021, 25, 231–240. [Google Scholar] [CrossRef]
- Faccia, M.; Maggiolino, A.; Natrella, G.; Zizzadoro, C.; Mazzone, A.; Poulopoulou, I.; Bragaglio, A.; De Palo, P. Ingested versus inhaled limonene in sheep: A pilot study to explore potential different transfer to the mammary gland and effects on milk and Caciotta cheese aroma. J. Dairy Sci. 2022, 105, 8143–8157. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Maggiolino, A.; Faccia, M.; Holman, B.W.; Hopkins, D.L.; Bragaglio, A.; Natrella, G.; Mazzone, A.; De Palo, P. The effect of oral or respiratory exposure to limonene on goat kid performance and meat quality. Meat Sci. 2022, 191, 108865. [Google Scholar] [CrossRef]
- Dinardo, F.; Maggiolino, A.; Casalino, E.; Deflorio, M.; Centoducati, G. A Multi-Biomarker Approach in European Sea Bass Exposed to Dynamic Temperature Changes under Dietary Supplementation with Origanum vulgare Essential Oil. Animals 2021, 11, 982. [Google Scholar] [CrossRef]
- Peterfalvi, A.; Miko, E.; Nagy, T.; Reger, B.; Simon, D.; Miseta, A.; Czéh, B.; Szereday, L. Much More Than a Pleasant Scent: A Review on Essential Oils Supporting the Immune System. Molecules 2019, 24, 4530. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Park, J.; Kim, M.S.; Seol, G.H.; Min, S.S. Analgesic effects of eucalyptus essential oil in mice. Korean J. Pain 2019, 32, 79–86. [Google Scholar] [CrossRef]
- Purba, R.A.P.; Yuangklang, C.; Paengkoum, P. Enhanced conjugated linoleic acid and biogas production after ruminal fermentation with Piper betle L. supplementation. Ciênc. Rural 2020, 50, e20191001. [Google Scholar] [CrossRef]
- Simitzis, P.E. Enrichment of Animal Diets with Essential Oils—A Great Perspective on Improving Animal Performance and Quality Characteristics of the Derived Products. Medicines 2017, 4, 35. [Google Scholar] [CrossRef]
- Besharati, M.; Giannenas, I.; Palangi, V.; Ayasan, T.; Noorian, F.; Maggiolino, A.; Lorenzo, J.M. Chitosan/Calcium–Alginate Encapsulated Flaxseed Oil on Dairy Cattle Diet: In Vitro Fermentation and Fatty Acid Biohydrogenation. Animals 2022, 12, 1400. [Google Scholar] [CrossRef] [PubMed]
- Castillejos, L.; Calsamiglia, S.; Martín-Tereso, J.; Ter Wijlen, H. In vitro evaluation of effects of ten essential oils at three doses on ruminal fermentation of high concentrate feedlot-type diets. Anim. Feed. Sci. Technol. 2008, 145, 259–270. [Google Scholar] [CrossRef]
- Rivera, R.A.; Camino, M.D.C.P.; Silva, N.C. Evalución de la vida útil de los aceites de Sacha Inchi (Plukenetia huayllabambana y Plukenetia volubilis) microencapsulados. Rev. Soc. Quím. Perú 2019, 85, 327–337. [Google Scholar]
- Wang, X.; Gao, S.; Yun, S.; Zhang, M.; Peng, L.; Li, Y.; Zhou, Y. Microencapsulating Alginate-Based Polymers for Probiotics Delivery Systems and Their Application. Pharmaceuticals 2022, 15, 644. [Google Scholar] [CrossRef]
- Dougal, K.; de la Fuente, G.; Harris, P.A.; Girdwood, S.E.; Pinloche, E.; Newbold, C.J. Identification of a Core Bacterial Community within the Large Intestine of the Horse. PLoS ONE 2013, 8, e77660. [Google Scholar] [CrossRef] [PubMed]
- Wartell, B.A.; Krumins, V.; Alt, J.; Kang, K.; Schwab, B.J.; Fennell, D. Methane production from horse manure and stall waste with softwood bedding. Bioresour. Technol. 2012, 112, 42–50. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of Essential Oils on Methane Production and Fermentation by, and Abundance and Diversity of, Rumen Microbial Populations. Appl. Environ. Microbiol. 2012, 78, 4271–4280. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, N.; Torres, S.H.; De Sanctis, J.B.; Pulido, M.M.; Sucre, L.E. A comparative study of metabolic characteristics of M-gluteus medius in equines and bovines. Rev. Cient. Fac. Cienc. Vet. 2004, 14, 153–161. [Google Scholar]
- Bailac, P.N.; Dellacasa, A.D.; Bernasconi, H.O.; Firpo, N.H.; Ponzi, M.I. Composicion del aceite esencial y actividad antimicrobiana de Eupatorium patens. Bol. Soc. Chil. Quím. 2000, 45, 207–211. [Google Scholar] [CrossRef]
- Pathania, A.S.; Guru, S.K.; Verma, M.; Sharma, C.; Abdullah, S.T.; Malik, F.; Chandra, S.; Katoch, M.; Bhushan, S. Disruption of the PI3K/AKT/mTOR signaling cascade and induction of apoptosis in HL-60 cells by an essential oil from Monarda citriodora. Food Chem. Toxicol. 2013, 62, 246–254. [Google Scholar] [CrossRef]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef]
- Ruiz, C.; Díaz, C.; Rojas, R. Composición química de aceites esenciales de 10 plantas aromáticas peruanas. Rev. Soc. Quím. Perú. 2015, 81, 81–94. [Google Scholar] [CrossRef]
- Martínez, J.; Sulbarán de Ferrer, B.; Ojeda de Rodríguez, G.; Ferrer, A.; Nava, R. Actividad antibacteriana del aceite esencial de mandarina. Rev. Fac. Agron. 2003, 20, 502–512. [Google Scholar]
- Vega, F.E.A.; Montenegro, Z.J.S.; Delgado, M.E.T.; Alvarez, J.A.P.; Benavidez, A.M.H.; Ospina, J.D. Evaluación de la capacidad inhibitoria de aceites esenciales en Staphylococcus aureus y Escherichia coli. Biotecnol. Sect. Agropecu. Agroind. 2017, 15, 52–60. [Google Scholar] [CrossRef]
- Plaus, E.A.; Flores, G.S.; Ataucusi, S.G. Composición química y actividad antibacteriana del aceite esencial del Origanum vulgare (orégano). Rev. Med. Hered. 2013, 12, 16. [Google Scholar] [CrossRef]
- Sanchez Perez, Y.; Correa Vidal, T.; Abreu Machado, Y.; Cotilla Pelier, L.; Berroa Navarro, G.; Pino Pérez, O. Chemical composition of the essential oil of Piper hispidum Sw. and antibacterial activity against Xanthomonas albilineans (Ashby) Dowson and Xanthomonas campestris pv. campestris (Pammel) Dowson. Rev. Prot. Veg. 2014, 29, 185–191. [Google Scholar]
- Pino, O.; Sánchez, Y.; Rojas, M.M.; Abreu, Y.; Correa, T.M. Composición química y actividad antibacteriana del aceite esencial de Pimpinella anisum L. Rev. Prot. Veg. 2012, 27, 181–187. [Google Scholar]
- Coy Barrera, C.C.A.; Eunice Acosta, G. Actividad antibacteriana y determinación de la composición química de los aceites esenciales de romero (Rosmarinus officinalis), tomillo (Thymus vulgaris) y cúrcuma (Curcuma longa) de Colombia. Rev. Cuba Plantas Med. 2013, 18, 237–246. [Google Scholar]
- Rueda, Y.; Mogollón, C.; Fernando, O. Composición química y actividad antibacteriana del aceite esencial de las especies Eucalyptus globulus y E. camaldulensis de tres zonas de Pamplona (Colombia). Bioagro 2012, 17, 137–141. [Google Scholar]
- Granados, C.; Yáñez, X.; Acevedo, D. Evaluación de la Actividad Antioxidante del Aceite Esencial Foliar de Myrcianthes leucoxyla de Norte de Santander (Colombia). Inf. Tecnol. 2014, 25, 11–16. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R.; Durán, D.C.; Córdoba, Y.; Caballero, D. Estudio comparativo de la composición química y la actividad antioxidante de los aceites esenciales de algunas plantas del género Lippia (Verbenaceae) cultivadas en Colombia. Rev. Acad. Colomb. Cienc. Exactas Fís Nat. 2014, 38, 89–105. [Google Scholar] [CrossRef]
- Montero-Recalde, M.; Revelo, I.J.; Avilés-Esquivel, D.; Valle, E.V.; Guevara-Freire, D. Efecto Antimicrobiano del Aceite Esencial de Canela (Cinnamomum zeylanicum) sobre Cepas de Salmonella. Rev. Investig. Vet. Perú 2017, 28, 987–993. [Google Scholar] [CrossRef]
- Gómez-Castellanos, J.R. Epazote (Chenopodium ambrosioides). Revisión a sus características morfológicas, actividad farmacológica, y biogénesis de su principal principio activo, ascaridol. Bol. Latinoam. Caribe Plantas Med. Aromat. 2008, 7, 2–7. [Google Scholar]
- Granados Conde, C.; Yáñez Rueda, X.; Santafé Pariño, G.G. Evaluaciòn de la actividad antioxidante del aceite esencial foliar de Calycolpus moritzianus y Minthostachys mollis de Norte de Santander. Bistua Rev. Fac. Cienc. Básicas 2012, 10, 12–23. [Google Scholar]
- Santana, P.M.; Miranda, M.; Gutiérrez, Y.; García, G.; Orellana, T.; Orellana, A. Antinflammatory effect and chemical composition of bursera graveolens Triana & Planch. branch oil (palo santo) from Ecuador. Rev. Cuba Plantas Med. 2009, 14, 45–53. [Google Scholar]
- Purba, R.A.P.; Paengkoum, S.; Yuangklang, C.; Paengkoum, P.; Salem, A.Z.M.; Boo, L.J. Mammary gene expressions and oxidative indicators in ruminal fluid, blood, milk, and mammary tissue of dairy goats fed a total mixed ration containing piper meal (Piper betle L.). Ital. J. Anim. Sci. 2022, 21, 129–141. [Google Scholar] [CrossRef]
- Hu, F.; Tu, X.-F.; Thakur, K.; Hu, F.; Li, X.-L.; Zhang, Y.-S.; Zhang, J.-G.; Wei, Z.-J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Cho, T.J.; Park, S.M.; Yu, H.; Seo, G.H.; Kim, H.W.; Kim, S.A.; Rhee, M.S. Recent Advances in the Application of Antibacterial Complexes Using Essential Oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef]
- Bouyahya, A.; Lagrouh, F.; El Omari, N.; Bourais, I.; El Jemli, M.; Marmouzi, I.; Salhi, N.; Faouzi, M.E.A.; Belmehdi, O.; Dakka, N.; et al. Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatal. Agric. Biotechnol. 2020, 23, 101471. [Google Scholar] [CrossRef]
- Venable, E.B.; Fenton, K.A.; Braner, V.M.; Reddington, C.E.; Halpin, M.J.; Heitz, S.A.; Francis, J.M.; Gulson, N.A.; Goyer, C.L.; Bland, S.D.; et al. Effects of Feeding Management on the Equine Cecal Microbiota. J. Equine Vet. Sci. 2017, 49, 113–121. [Google Scholar] [CrossRef]
- Cuevas-Bernardino, J.C.; Pérez-Alonso, C.; Nieto-Ángel, R.; Aguirre-Mandujano, E. Microencapsulation of grape seed oil by spray drying using whey protein and hawthorn pectin. Ing. Agríc. Biosist. 2019, 11, 127–145. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Leal, A.L.A.B.; Coutinho, H.D.M.; Vitalini, S.; Kręgiel, D.; Antolak, H.; Sharifi-Rad, M.; et al. Tagetes spp. Essential Oils and Other Extracts: Chemical Characterization and Biological Activity. Molecules 2018, 23, 2847. [Google Scholar] [CrossRef] [PubMed]
- Rasekh, M.; Karami, H.; Wilson, A.D.; Gancarz, M. Classification and Identification of Essential Oils from Herbs and Fruits Based on a MOS Electronic-Nose Technology. Chemosensors 2021, 9, 142. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Klevenhusen, F.; Muro-Reyes, A.; Khiaosa-Ard, R.; Metzler-Zebeli, B.; Zebeli, Q. A meta-analysis of effects of chemical composition of incubated diet and bioactive compounds on in vitro ruminal fermentation. Anim. Feed. Sci. Technol. 2012, 176, 61–69. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A. Essential oils and phytogenic feed additives in ruminant diet: Chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Purba, R.A.P.; Paengkoum, P. Bioanalytical HPLC method of Piper betle L. for quantifying phenolic compound, water-soluble vitamin, and essential oil in five different solvent extracts. J. Appl. Pharm. Sci. 2019, 9, 33–39. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Veiga, R.D.S.D.; Aparecida Da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Hernández, O.D.L. Microencapsulación de sustancias oleosas mediante secado por aspersión. Rev. Cubana Farm. 2010, 44, 381–389. [Google Scholar]
- Hussain, S.A.; Hameed, A.; Nazir, Y.; Naz, T.; Wu, Y.; Suleria, H.A.R.; Song, Y. Microencapsulation and the Characterization of Polyherbal Formulation (PHF) Rich in Natural Polyphenolic Compounds. Nutrients 2018, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.R.; Ambrosi, A.; Di Luccio, M. Encapsulated essential oils: A perspective in food preservation. Futur. Foods 2022, 5, 100126. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T.; Ennahar, S.; Marchioni, E. Microencapsulation of Lactobacillus plantarum spp. in an alginate matrix coated with whey proteins. Int. J. Food Microbiol. 2009, 129, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Dima, Ş.; Dima, C.; Iordăchescu, G. Encapsulation of Functional Lipophilic Food and Drug Biocomponents. Food Eng. Rev. 2015, 7, 417–438. [Google Scholar] [CrossRef]
- Caballero, B.; Trugo, L.C.; Finglas, P.M. Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Li, M.; Rouaud, O.; Poncelet, D. Microencapsulation by solvent evaporation: State of the art for process engineering approaches. Int. J. Pharm. 2008, 363, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Chadha, S. Recent advances in nano-encapsulation technologies for controlled release of biostimulants and antimicrobial agents. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 29–55. [Google Scholar] [CrossRef]
- Asua, J.M. Miniemulsion polymerization. Prog. Polym. Sci. 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Lu, W.; Kelly, A.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Lucía, C.; Marcela, F.; Ainhoa, L. Encapsulation of Almond Essential Oil by Co-Extrusion/Gelling Using Chitosan as Wall Material. J. Encapsul. Adsorpt. Sci. 2017, 7, 67–74. [Google Scholar] [CrossRef]
- Cichorska, B.; Komosa, M.; Nogowsk, L.; Maćkowiak, P.; Józefia, D. Significance of Nutrient Digestibility in Horse Nutrition—A Review. Ann. Anim. Sci. 2014, 14, 779–797. [Google Scholar] [CrossRef]
- Oliver-Espinosa, O. Diagnostics and Treatments in Chronic Diarrhea and Weight Loss in Horses. Vet. Clin. N. Am. Equine Pr. 2018, 34, 69–80. [Google Scholar] [CrossRef]
- Castillo-González, A.; Burrola-Barraza, M.; Domínguez-Viveros, J.; Chávez-Martínez, A. Rumen microorganisms and fermentation. Arch. Med. Vet. 2014, 46, 349–361. [Google Scholar] [CrossRef]
- Martínez, R.M.; Cerrilla, M.E.O.; Haro, J.G.H.; Garza, J.R.K.; Ramos, J.Z.; Soriano, R.R. Uso de aceites esenciales en animales de granja. Interciencia 2015, 40, 744–750. [Google Scholar]
- Van Weyenberg, S.; Sales, J.; Janssens, G. Passage rate of digesta through the equine gastrointestinal tract: A review. Livest. Sci. 2006, 99, 3–12. [Google Scholar] [CrossRef]
- Arroyo, L.G.; Rossi, L.; Santos, B.P.; Gomez, D.E.; Surette, M.G.; Costa, M.C. Luminal and Mucosal Microbiota of the Cecum and Large Colon of Healthy and Diarrheic Horses. Animals 2020, 10, 1403. [Google Scholar] [CrossRef]
- Maggiolino, A.; Lorenzo, J.; Quiñones, J.; Latorre, M.; Blando, F.; Centoducati, G.; Dahl, G.; De Palo, P. Effects of dietary supplementation with Pinus taeda hydrolyzed lignin on in vivo performances, in vitro nutrient apparent digestibility, and gas emission in beef steers. Anim. Feed. Sci. Technol. 2019, 255, 114217. [Google Scholar] [CrossRef]
- De Bellis, P.; Maggiolino, A.; Albano, C.; De Palo, P.; Blando, F. Ensiling Grape Pomace With and Without Addition of a Lactiplantibacillus plantarum Strain: Effect on Polyphenols and Microbiological Characteristics, in vitro Nutrient Apparent Digestibility, and Gas Emission. Front. Vet. Sci. 2022, 9, 808293. [Google Scholar] [CrossRef] [PubMed]
- Misiukiewicz, A.; Gao, M.; Filipiak, W.; Cieslak, A.; Patra, A.; Szumacher-Strabel, M. Review: Methanogens and methane production in the digestive systems of nonruminant farm animals. Animal 2021, 15, 100060. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, T.D.; Elghandour, M.M.; Ekanem, N.J.; Alcala-Canto, Y.; Velázquez, A.E.; Pacheco, E.B.F.; Purba, R.A.P.; Salem, A.Z. Influence of Azadirachta indica and Cnidoscolus angustidens Dietary Extracts on Equine Fecal Greenhouse Gas Emissions. J. Equine Vet. Sci. 2022, 116, 104049. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Yan, J.; Xia, J.; Huang, L.; Li, M.; Ding, H.; Xu, L. Effect of different combinations of emulsifier and wall materials on physical properties of spray-dried microencapsulated swida wilsoniana oil. J. Bioresour. Bioprod. 2020, 5, 44–50. [Google Scholar] [CrossRef]
- Kauter, A.; Epping, L.; Semmler, T.; Antao, E.-M.; Kannapin, D.; Stoeckle, S.D.; Gehlen, H.; Lübke-Becker, A.; Günther, S.; Wieler, L.H.; et al. The gut microbiome of horses: Current research on equine enteral microbiota and future perspectives. Anim. Microbiome 2019, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, G.; Trabalza-Marinucci, M.; Yu, Z. Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Sci. Total Environ. 2016, 545–546, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.; Castillejos, L.; Ferret, A. Invited Review: Essential Oils as Modifiers of Rumen Microbial Fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [PubMed]
- Bandoni, A.L.; Retta, D.; Lira, P.M.D.L.; Baren, C.M.V. ¿Son realmente útiles los aceites esenciales? Bol. Latinoam. Caribe Plantas Med. Aromat. 2009, 8, 317–322. [Google Scholar]
| EOs | Effect | Main Composition % | Reference |
|---|---|---|---|
| Jungia paniculata | High MICs, inhibition of parasite growth (L. amazonensis) | β-Caryophyllene (35.91%), Caryophyllene oxide (36.49%), α-curcumene (3.85%) | [22] |
| Tangerine (Dancy variety and commercial Gerch) | Bactericidal of Staphylococcus aureus, Listeria monocytogenes and Bacillus subtilis | EOs pure Tangerine (100%)/linalool (dimethyl-2,7-octadien-6-ol.) and thymol (2-isopropyl-5-methylphenol) chemotypes a | [23,24] |
| Tangerine (Dancy variety and commercial Gerch) | MICs for Bacillus subtilis 9% and 19%, respectively; for Staphylococcus aureus and Listeria monocytogenes 7% | EOs pure Tangerine (100%)/linalool (dimethyl-2,7-octadien-6-ol.) and thymol (2-isopropyl-5-methylphenol) chemotypes a | [23,24] |
| Eucalyptus spp. (Eucalyptus) | Staphylococcus aureus CMI 6.8 µL/ML MBC (6.8 µL/mL), Escherichia coli CMI (13.2 µL/mL), MBC (13.2 µL/mL) | Eucalyptol (57.85%), α-pinene (22.81%), α-terpinyl acetate (3.72%), β-Myrcene (1.85%), Viridiflorol (1.6%), β-pinene (1.53), Aromadendrene (1.49%), α-Terpineol (1.27%) | [24] |
| Citrus lemon (L.) Osbeck (Lemon) | Staphylococcus aureus MICs 7.6 µL/Ml MBC (7.6 µL/mL) Escherichia coli MICs (14 µL/mL), MBC (13.2 µL/mL) | Limonene (58.17%), β-pinene (13.22%), γ-Terpinene (11.72%), β-Myrcene (1.75%), Octanal (1.67%), Citronellal (1.5%), α-Terpineol (1.19%) | [24] |
| Origanum vulgare (Labiate) (oregano) | Antimicrobial effect on Gram-positive bacteria Staphylococcus aureus and Bacillus cereus and on Gram-negative bacteria | 9,12-octadecadienoic acid (8.29%),9, 12, 15. octadecatrienal (8.29%), Cis sabinene hydrate (18.66%), 4-terpineol (9.43%), Carvacrol (7.72%) | [25] |
| Piper hispidum (matico hoja lisa) | Antibacterial activity in X. albilineans | a-Phellandrene (22.30%) a-Pinene (14.82%) Eucalyptol (15.49%) NI (CHO) (12.90%) | [22,26] |
| Pimpinella anisum L. | Antibacterial activity on Bacillus subtilis, Pseudomona aeruginosa, Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, and Klebsiella pneumoniae | canfeno (0.11%), linalol (0.11%), 4-ciclopropil-2-metoxifenol (0.19%) metil chavicol (97.76%), B-cariofileno (0.12%), germacreno-D (0.44%) | [27] |
| Thymus vulgaris (thyme) | 70% inhibition against S. aureus strain and 20% E. faecalis strain | β-pineno (29.0%), 1,8-cineol (21.5%), and o-cimeno (17.9%) | [28] |
| Curcuma longa (cúrcuma) | 70% inhibition against S. aureus strain and 50% E. faecalis strain | turmerona (36.9%), α-turmerona (18.9%), and β-turmerona (13.6%); | [28] |
| Eucalyptus globulus | Inhibition of salmonella, Bacillus subtilis, Enterococcus faecalis, Escherichia coli | 1,8-Cineol or Eucalyptol (82.27%), Limonene (3.70%), α-Pinene (3.16%) | [29] |
| E. camaldulensis | Higher concentrations of the oil inhibit bacterial strains of Bacillus subtilis, Enterococcus faecalis, Escherichia coli | 1,8-Cineol (77.41%), Terpinen-4-ol (3.68%), α-Pinene (3.64%), Limonene (3.21%) | [29] |
| Myrcianthes leucoxyla | Antioxidant activity from 500 ppm when inhibition percentages higher than 60% are reached | Pineno (28.40%), 1,8-Cineol (15.70%), Z-Cariofileno (3.79%), Cariofileno Guaiol (3.13%) | [30] |
| Lippia graveolens | Antimicrobial activity, antifungal, antibacterial, antioxidant, antiprotozoal | 1,8-Cineol, phellandrenes, p-cymene, terpinenes, carvacrol, thymol and their ethers and esters, b-caryophyllene | [31] |
| Cinnamomum zeylanicum (Cinnamon) | Inhibition to salmonella strains, mainly sensitive to concentrations of cinnamon essential oil at concentrations of 50% or higher | eugenol, present in 70–95% | [32] |
| Chenopodium ambrosioides (Epazote) | Analgesic effects and against arthritis | 1-methyl-4-isopropyl-2,3-dioxa bicyclo[2.2.2]hept-5-ene) occurs in 60–80% of the essential oil of C. ambrosioides, and in 1% by fresh weight | [33] |
| Calycolpus moritzianus | Increased antioxidant activity | monoterpenes α-Pinene, Eucalyptol and α-Terpineol | [34] |
| Minthostachys mollis | Presents antioxidant activity, but in concentrations higher than 200 ppm | 1,8-Cineol or Eucalyptol (6.39%) | [34] |
| Bursera graveolens Triana | Acetylcholinesterase inhibitory activity | Major compound (viridiflorol) | [35] |
| Type of Essential Oil | Dose | Effect | Reference |
|---|---|---|---|
| Roman chamomile Marjoram | Apply 20–30 drops of pure oil (dilute with V-6 vehicular oil if phenol-rich oils are used). | Can be placed in a capsule and mixed with food. It helps to combat nerve problems and anxiety in horses. | [65] |
| Mint Cypress | Essential oil drops on the coat approximately 10 cm in size. | Therapeutic application for horses. | [66] |
| Valeriana | 20–30 pure drops per application. | Relieves anxiety in animals; the calming effect lasts from days to weeks. | [77] |
| Marjoram | Application of essential oil drops on the coat, approximately 10 cm in size. | Therapeutic application for horses. | [65] |
| Gauteria | Apply diluted in a vegetable oil such as almond oil or even arnica, which is also an anti-inflammatory. | It is a powerful anti-inflammatory and analgesic, but contraindicated during pregnancy and lactation. | [66] |
| Rosemary chemotype camphor | Apply diluted in a vegetable oil such as almond oil or even arnica, which is also anti-inflammatory. | Muscle relaxant and analgesic, ideal for muscle contractures, torticollis, and other types of pain. | [66] |
| BIOBRON is a food supplement based on a synergistic blend of essential oils. | 20 mL/day per 100 kg of live weight for 5 days (there are also more concentrated versions). | It helps animals recover due to essential oils’ expectorant and fluidizing properties. Stimulates food consumption and general health thanks to plant extracts and vitamin C. | [65] |
| Soybean or sunflower oil | 20 percent or 450 mL daily of the total diet. | Contributes to the proper growth of the foal. Enhances absorption of vitamins A, D, E, K, and linoleic acid. Prevents colic. | [19] |
| Corn oil | 90 mL in a single meal; after 3 days, 90 mL in each meal and continue to increase gradually until reaching the desired level. | Maintains reserves of muscle glycogen and glycemia levels for a longer time. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elghandour, M.M.M.Y.; Maggiolino, A.; García, E.I.C.; Sánchez-Aparicio, P.; De Palo, P.; Ponce-Covarrubias, J.L.; Pliego, A.B.; Salem, A.Z.M. Effects of Microencapsulated Essential Oils on Equine Health: Nutrition, Metabolism and Methane Emission. Life 2023, 13, 455. https://doi.org/10.3390/life13020455
Elghandour MMMY, Maggiolino A, García EIC, Sánchez-Aparicio P, De Palo P, Ponce-Covarrubias JL, Pliego AB, Salem AZM. Effects of Microencapsulated Essential Oils on Equine Health: Nutrition, Metabolism and Methane Emission. Life. 2023; 13(2):455. https://doi.org/10.3390/life13020455
Chicago/Turabian StyleElghandour, Mona M. M. Y., Aristide Maggiolino, Erendira Itzel Ceja García, Pedro Sánchez-Aparicio, Pasquale De Palo, José Luis Ponce-Covarrubias, Alberto Barbabosa Pliego, and Abdelfattah Z. M. Salem. 2023. "Effects of Microencapsulated Essential Oils on Equine Health: Nutrition, Metabolism and Methane Emission" Life 13, no. 2: 455. https://doi.org/10.3390/life13020455
APA StyleElghandour, M. M. M. Y., Maggiolino, A., García, E. I. C., Sánchez-Aparicio, P., De Palo, P., Ponce-Covarrubias, J. L., Pliego, A. B., & Salem, A. Z. M. (2023). Effects of Microencapsulated Essential Oils on Equine Health: Nutrition, Metabolism and Methane Emission. Life, 13(2), 455. https://doi.org/10.3390/life13020455









