Laboratory- and Pilot-Scale Cultivation of Tetraselmis striata to Produce Valuable Metabolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae and Maintenance Conditions
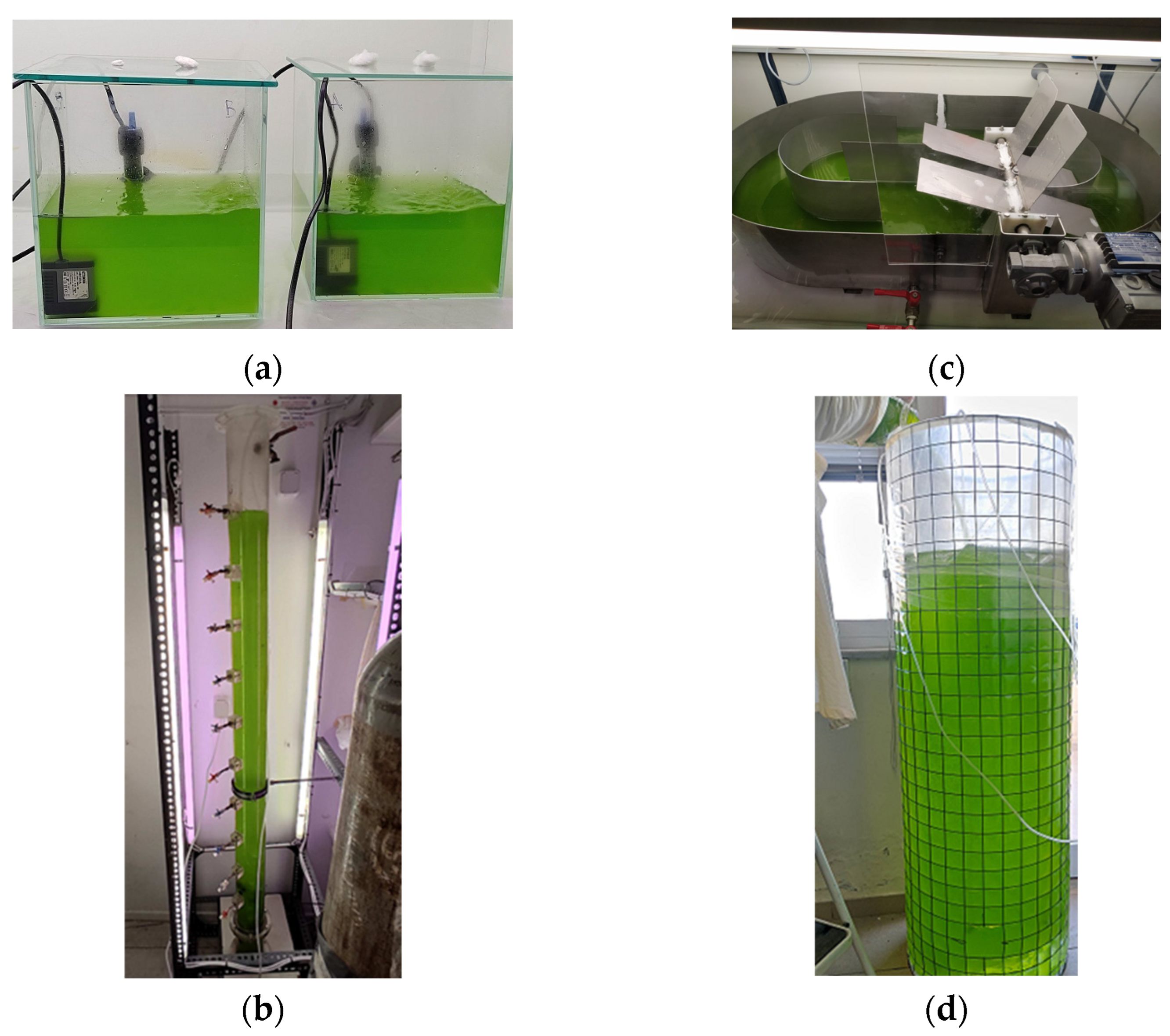
2.2. Photobioreactors
2.2.1. Laboratory-Scale Photobioreactors
2.2.2. Pilot-Scale PBRs
2.3. Tested Growth Substrates and Culture Conditions
2.4. Scale-Up of Tetraselmis Cultures
2.5. Analytical Methods and Calculations
2.5.1. Medium Analyses
2.5.2. Biomass Analyses
Estimation of Dry Biomass and Biomass Yields
Lipid and Carbohydrate Estimation
Fatty Acid Analysis
Protein Estimation
Pigment Estimation
2.6. Statistical Treatment of the Data
3. Results and Discussion
3.1. Biomass Growth and Nutrient Removal in the Different PBRs
3.2. Effect of the Different PBRs on Biomass Composition
3.2.1. Effect of the Different PBRs on Fatty Acid Composition
4. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds. Biology 2022, 11, 1146. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Ulloa, R.G.; Sineiro, J.; Feijoo, G.; Moreira, M.T. Life cycle assessment of the production of bioactive compounds from Tetraselmis suecica at pilot scale. J. Clean. Prod. 2014, 64, 323–331. [Google Scholar] [CrossRef]
- Chen, F.; Leng, Y.; Lu, Q.; Zhou, W. The application of microalgae biomass and bio-products as aquafeed for aquaculture. Algal Res. 2021, 60, 102541. [Google Scholar] [CrossRef]
- Trovão, M.; Pereira, H.; Silva, J.; Páramo, J.; Quelhas, P.; Santos, T.; Silva, J.T.; Machado, A.; Gouveia, L.; Varela, J. Growth performance, biochemical composition and sedimentation velocity of Tetraselmis sp. CTP4 under different salinities using low-cost lab- and pilot-scale systems. Heliyon 2019, 5, E01553. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.I.; Hakim, M.A.Q.M.A.; Al-Jabri, H.M.S.J.; Alghasal, G.S.H.S. A comparative study of the growth of Tetraselmis sp. in large scale fixed depth and decreasing depth raceway ponds. Bioresour. Technol. 2016, 216, 114–120. [Google Scholar] [CrossRef]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of microalgae cultivation in photobioreactor, open raceway pond, and a two-stage hybrid system. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
- Raes, E.J.; Isdepsky, A.; Muylaert, K.; Borowitzka, M.A.; Moheimani, N.R. Comparison of growth of Tetraselmis in a tubular photobioreactor (Biocoil) and a raceway pond. J. Appl. Phycol. 2013, 25, 247–255. [Google Scholar] [CrossRef]
- Isdepsky, A.; Borowitzka, M.A. In-pond strain selection of euryhaline Tetraselmis sp. strains for reliable long-term outdoor culture as potential sources of biofuel and other products. J. Appl. Phycol. 2019, 31, 3359–3370. [Google Scholar] [CrossRef]
- Fon Sing, S.; Isdepsky, A.; Borowitzka, M.A.; Lewis, D.M. Pilot-scale continuous recycling of growth medium for the mass culture of a halotolerant Tetraselmis sp. in raceway ponds under increasing salinity: A novel protocol for commercial microalgal biomass production. Bioresour. Technol. 2014, 161, 47–54. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Thaher, M.; Khan, S.; AbdulQuadir, M. Potential utilization of waste nitrogen fertilizer from a fertilizer industry using marine microalgae. Sci. Total Environ. 2021, 755, 142532. [Google Scholar] [CrossRef]
- Lee, W.K.; Ryu, Y.K.; Choi, W.Y.; Kim, T.; Park, A.; Lee, Y.J.; Jeong, Y.; Lee, C.G.; Kang, D.H. Year-Round Cultivation of Tetraselmis sp. for Essential Lipid Production in a Semi-Open Raceway System. Mar. Drugs 2021, 19, 314. [Google Scholar] [CrossRef]
- Gorbunova, S.Y.; Avsiyan, A.L. Diurnal dynamics of green microalga Tetraselmis viridis culture density in open pond monitored by optical density sensor. Bioresour. Technol. Rep. 2022, 20, 101251. [Google Scholar] [CrossRef]
- Zittelli, G.C.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Productivity and photosynthetic efficiency of outdoor cultures of Tetraselmis suecica in annular columns. Aquaculture 2006, 261, 932–943. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Guidi, F.; Bustamante, B.; Venuleo, M.; de Assunçao, P.A.C.J.; Portillo, E. Scaling-Up and Semi-Continuous Cultivation of Locally Isolated Marine Microalgae Tetraselmis striata in the Subtropical Island of Gran Canaria (Canary Islands, Span). Processes 2021, 9, 1326. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of Photobioreactors for Mass Cultivation of Photosynthetic Organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Chanquia, S.N.; Vernet, G.; Kara, S. Photobioreactors for cultivation and synthesis: Specifications, challenges, and perspectives. Eng. Life Sci. 2021, 22, 712–724. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, and fermenters. J. Biotechnol. 1999, 35, 313–321. [Google Scholar] [CrossRef]
- Kim, Z.H.; Park, Y.S.; Ryu, Y.J.; Lee, C.G. Enhancing biomass and fatty acid productivity of Tetraselmis sp. in bubble column photobioreactors by modifying light quality using light filters. Biotechnol. Bioprocess Eng. 2017, 22, 397–404. [Google Scholar] [CrossRef]
- Pereira, H.; Páramo, J.; Silva, J.; Marques, A.; Barros, A.; Maurício, D.; Santos, T.; Schulze, P.; Barros, R.; Gouveia, L.; et al. Scale-up and large-scale production of Tetraselmis sp. CTP4 (Chlorophyta) for CO2 mitigation: From an agar plate to 100-m3 industrial photobioreactors. Sci. Rep. 2018, 8, 5112. [Google Scholar] [CrossRef]
- Makri, A.; Bellou, S.; Birkou, M.; Papatrehas, K.; Dolapsakis, N.P.; Bokas, D.; Papanikolaou, S.; Aggelis, G. Lipid synthesized by micro-algae grown in laboratory- and industrial-scale bioreactors. Eng. Life Sci. 2011, 11, 52–58. [Google Scholar] [CrossRef]
- Erkelens, M.; Ward, A.J.; Ball, A.S.; Lewis, D.M. Microalgae digestate effluent as a growth medium for Tetraselmis sp. in the production of biofuels. Bioresour. Technol. 2014, 167, 81–86. [Google Scholar] [CrossRef]
- Moheimani, N.R. Long-term outdoor growth and lipid productivity of Tetraselmis suecica, Dunaliella tertiolecta and Chlorella sp. (Chlorophyta) in bag photobioreactors. J. Appl. Phycol. 2013, 25, 167–176. [Google Scholar] [CrossRef]
- Danquah, M.K.; Harun, R.; Halim, R.; Forde, G.M. Cultivation medium design via elemental balancing for Tetraselmis suecica. Chem. Biochem. Eng. Q. 2010, 24, 361–369. [Google Scholar]
- Michels, M.H.A.; Slegers, P.M.; Vermuë, M.H.; Wijffels, R.H. Effect of biomass concentration on the productivity of Tetraselmis suecica in a pilot-scale tubular photobioreactor using natural sunlight. Algal Res. 2014, 4, 12–18. [Google Scholar] [CrossRef]
- Cuello, M.C.; Cosgrove, J.J.; Randhir, A.; Vadiveloo, A.; Moheimani, N.R. Comparison of continuous and day time only mixing on Tetraselmis suecica (Chlorophyta) in outdoor raceway ponds. J. Appl. Phycol. 2015, 27, 1783–1791. [Google Scholar] [CrossRef]
- Holdt, S.L.; Christensen, L.; Iversen, J.J.L. A novel closed system bubble column photobioreactor for detailed characterisation of micro- and macroalgal growth. J. Appl. Phycol. 2014, 26, 825–835. [Google Scholar] [CrossRef]
- Boopathy, A.B.; Jayakumar, T.; Chinnasamy, S.; Rajaram, M.G.; Mohan, N.; Nagaraj, S.; Rengasamy, R.; Manubolu, M.; Sheu, J.R.; Chang, C.C. Biomass and Lipid Production Potential of an Indian Marine Algal Isolate Tetraselmis striata BBRR1. Energies 2020, 13, 341. [Google Scholar] [CrossRef]
- Patrinou, V.; Daskalaki, A.; Kampantais, D.; Kanakis, D.C.; Economou, C.N.; Bokas, D.; Kotzamanis, Y.; Aggelis, G.; Vayenas, D.V.; Tekerlekopoulou, A.G. Optimization of Cultivation Conditions for Tetraselmis striata and Biomass Quality Evaluation for Fish Feed Production. Water 2022, 14, 3162. [Google Scholar] [CrossRef]
- Economou, C.N.; Marinakis, N.; Moustaka-Gouni, M.; Kehayias, G.; Aggelis, G.; Vayenas, D.V. Lipid production by the filamentous cyanobacterium Limnothrix sp. growing in synthetic wastewater in suspended and attached-growth photobioreactor systems. Ann. Microb. 2015, 65, 1941–1948. [Google Scholar] [CrossRef]
- Patrinou, V.; Tsolcha, O.N.; Tatoulis, T.I.; Stefanidou, N.; Dourou, M.; Moustaka-Gouni, M.; Aggelis, G.; Tekerlekopoulou, A.G. Biotreatment of poultry waste coupled with biodiesel production using suspended and attached growth microalgal-based systems. Sustainability 2020, 12, 5024. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Stefanidou, N.; Moustaka-gouni, M.; Genitsaris, S.; Aggelis, G.; Tekerlekopoulou, A.G.; Vayenas, D.V. A semi-continuous algal-bacterial wastewater treatment process coupled with bioethanol production. J. Environ. Manage. 2023, 326, 116717. [Google Scholar] [CrossRef]
- Windy: Wind Map & Weather Forecast. Available online: https://www.windy.com (accessed on 17 January 2022).
- Pai, S.C.; Tsau, Y.J.; Yang, T.I. pH and buffering capacity problems involved in the determination of ammonia in saline water using the indophenol blue spectrophotometric method. Anal. Chim. Acta. 2001, 434, 209–216. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Tsonis, S.P. A modified method for the examination of chemical oxygen demand in sea water. WIT Trans. Ecol. Environ. 1993, 2, 1743–3541. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.A. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1951, 226, 497–509. [Google Scholar] [CrossRef]
- Association Francaise de Normalisation. Collection of French Standards for Fats, Oleaginous Grains and Derived Products, 3rd ed.; French Association for Standardization, Paris (AFNOR): Paris, France, 1984; p. 95. [Google Scholar]
- Papadopoulos, K.P.; Economou, C.N.; Tekerlekopoulou, A.G.; Vayenas, D.V. A cyanobacteria-based biofilm system for advanced brewery wastewater treatment. Appl. Sci. 2021, 11, 174. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. In Handbook of Food Analytical Chemistry; Wiley Publishes: Hoboken, NJ, USA, 2005; Volume 2, pp. 171–178. [Google Scholar]
- Bácsi, I.; Tóthfalusi, F.; Márton, K.; B-Béres, V.; Gonda, S. The Effects of Photobioreactor Type on Biomass and Lipid Production of the Green Microalga Monoraphidium pusillum in Laboratory Scale. Appl. Sci. 2022, 12, 2196. [Google Scholar] [CrossRef]
- Lisondro, I.; Gómez Serrano, C.; Sepúlveda, C.; Batista Ceballos, A.I.; Acién Fernández, F.G. Influence of irradiance on the growth and biochemical composition of Nitzschia aff. pellucida. J. Appl. Phycol. 2021, 34, 19–30. [Google Scholar] [CrossRef]
- Benner, P.; Meier, L.; Pfeffer, A.; Krüger, K.; Oropeza Vargas, J.E.; Weuster-Botz, D. Lab-scale photobioreactor systems: Principles, applications, and scalability. Bioprocess Biosyst. Eng. 2022, 45, 791–813. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Microalgal reactors: A review of enclosed system designs and performances. Biotechnol. Prog. 2006, 22, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lan, C.Q. Effects of shear stress on microalgae—A review. Biotechnol. Ad. 2018, 36, 986–1002. [Google Scholar] [CrossRef]
- Rodríguez, J.J.G.; Mirón, A.S.; Camacho, F.G.; García, M.C.C.; Belarbi, E.H.; Chisti, Y.; Grima, E.M. Carboxymethyl cellulose and Pluronic F68 protect the dinoflagellate Protoceratium reticulatum against shear-associated damage. Bioprocess Biosyst. Eng. 2011, 34, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, O.; Bashan, Y. Microalgal heterotrophic and mixotrophic culturing for bio-refining: From Metabolic Routes to Techno-economics. In Algal Biorefineries; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer: Cham, Switzerland, 2015; Volume 2, pp. 61–131. [Google Scholar]
- Yang, Z.; Pei, H.; Han, F.; Wang, Y.; Hou, Q.; Chen, Y. Effects of air bubble size on algal growth rate and lipid accumulation using fine-pore diffuser photobioreactors. Algal Res. 2018, 32, 293–299. [Google Scholar] [CrossRef]
- Laroche, C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef]
- Imamoglu, E.; Demirel, Z.; Conk Dalay, M. Process optimization and modeling for the cultivation of Nannochloropsis sp. and Tetraselmis striata via response surface methodology. J. Phycol. 2015, 51, 442–453. [Google Scholar] [CrossRef]
- Patidar, S.K.; Kim, S.H.; Kim, J.H.; Park, J.; Park, B.S.; Han, M.S. Pelagibaca bermudensis promotes biofuel competence of Tetraselmis striata in a broad range of abiotic stressors: Dynamics of quorum-sensing precursors and strategic improvement in lipid productivity. Biotechnol. Biofuels. 2018, 11, 102. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Montero, Z.; Cuaresma, M.; Ruiz-Domínguez, M.C.; Mogedas, B.; Nores, I.G.; González del Valle, M.; Vílchez, C. Outdoor large-scale cultivation of the acidophilic microalga Coccomyxa onubensis in a vertical close photobioreactor for lutein production. Processes 2020, 8, 324. [Google Scholar] [CrossRef]
- Ma, R.; Wang, B.; Chua, E.T.; Zhao, X.; Lu, K.; Ho, S.H.; Shi, X.; Liu, L.; Xie, Y.; Lu, Y.; et al. Comprehensive utilization of marine microalgae for enhanced co-production of multiple compounds. Mar. Drugs 2020, 18, 467. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Carvalho, C.F.; Pereira, H.; Gangadhar, K.N.; Schüler, L.M.; Santos, T.F.; Varela, J.C.; Barreira, L. Urban wastewater treatment by Tetraselmis sp. CTP4 (Chlorophyta). Bioresour. Technol. 2017, 223, 175–183. [Google Scholar] [CrossRef]
- He, Q.N.; Yang, H.J.; Wu, L.; Hu, C.X. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Chauton, M.S.; Winge, P.; Brembu, T.; Vadstein, O.; Bones, A.M. Gene regulation of carbon fixation, storage, and utilization in the diatom Phaeodactylum tricornutum acclimated to light/dark cycles. Plant Physiol. 2013, 161, 1034–1048. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, M.; Sanudi; Arifin, N.B.; Ekawati, A.W.; Yuniarti, A.; Hariati, A.M. Effect of photoperiod regimes on growth, biomass and pigment content of Nannochloropsis sp. BJ17. Asian J. Microbiol. Biotechnol. Environ. Sci. 2017, 19, 263–267. [Google Scholar]
- Schüler, L.M.; Bombo, G.; Duarte, P.; Santos, T.F.; Maia, I.B.; Pinheiro, F.; Marques, J.; Jacinto, R.; Schulze, P.S.C.; Pereira, H.; et al. Carotenoid biosynthetic gene expression, pigment and n-3 fatty acid contents in carotenoid-rich Tetraselmis striata CTP4 strains under heat stress combined with high light. Bioresour. Technol. 2021, 337, 125385. [Google Scholar] [CrossRef]
- Schüler, L.M.; Walter, J.M.; Kato, H.; Suzuki, C.; Hulatt, C.J.; Rautenberger, R.; Navalho, S.; Schmid, B.; Varela, J.; Kiron, V.; et al. High-value compound induction by flashing light in Diacronema lutheri and Tetraselmis striata CTP4. Bioresour. Technol. Rep. 2022, 19, 101158. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y.; Ho, S.; Xing, D.; Ren, N.; Liu, B. Cell growth and lipid accumulation of a microalgal mutant Scenedesmus sp. Z-4 by combining light/dark cycle with temperature variation. Biotechnol. Biofuels 2017, 10, 260–272. [Google Scholar] [CrossRef]
- Vendruscolo, R.G.; Fagundes, M.B.; Maroneze, M.M.; do Nascimento, T.C.; de Menezes, C.R.; Barin, J.S.; Zepka, L.Q.; Jacob-Lopes, E.; Wagner, R. Scenedesmus obliquus metabolomics: Effect of photoperiods and cell growth phases. Bioprocess Biosyst. Eng. 2019, 42, 727–739. [Google Scholar] [CrossRef]
- Lim, K.C.; Zaleha, K. Effect of photoperiod on the cellular fatty acid composition of three tropical marine microalgae. Malaysian J. Appl. Biol. 2013, 42, 41–49. [Google Scholar]
- Bellou, S.; Triantaphyllidou, I.E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef]
- Kumar, K.; Mishra, S.K.; Shrivastav, A.; Park, M.S.; Yang, J.W. Recent trends in the mass cultivation of algae in raceway ponds. Renew. Sust. Energ. Rev. 2015, 51, 875–885. [Google Scholar] [CrossRef]



| Initial Concentrations (mg L−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| PBRs 1 | NH4+-N | NO3−-N | TN | PO43−-P | Total Carbohydrates | d-COD | C:N | N:P |
| Aq-F/2 2 | 70.0 ± 2.3 | 0.7 ± 0.1 | 74.2 ± 2.7 | 6.7 ± 0.4 | 4.7 ± 0.5 | 125.0 ± 7.1 | 1.7 | 11.1 |
| Pilot column 3 F/2 | 69.9 ± 1.2 | 4.5 ± 0.1 | 85.3 ± 2.9 | 7.6 ± 0.1 | 2.3 ± 0.2 | 152.7 ± 17.9 | 1.8 | 11.2 |
| Pilot column 4 | 18.3 ± 0.5 | 9.7 ± 0.1 | 71.7 ± 0.1 | 9.8 ± 0.4 | 4.1 ± 0.8 | 147.5 ± 22.5 | 2.1 | 7.3 |
| Pilot pond 5 | 9.5 ± 0.2 | 9.7 ± 0.1 | 96.2 ± 1.7 | 12.5 ± 0.4 | 2.9 ± 0.6 | 100.0 ± 4.1 | 1.0 | 7.7 |
| Pilot p-bag 6 | 14.0 ± 1.2 | 10.5 ± 0.7 | 89.0 ± 1.7 | 11.6 ± 0.3 | 2.1 ± 0.2 | 166.2 ± 3.5 | 1.9 | 7.7 |
| % Removal Rate | Maximum Biomass Productivity (mg L−1 d−1) | Specific Growth Rate (d−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| PBR 1 | NH4+-N | NO3−-N | TN | PO43−-P | Total Carbohydrates | d-COD | ||
| Aq-F/2 2 | 92.6 ± 0.2 | 43.1 ± 0.7 | 90.0 ± 1.5 | 91.7 ± 0.1 | 0.0 | 50.4 ± 3.2 | 55.2 ± 2.7 | 0.230 ± 0.06 |
| Pilot column 3 F/2 | 84.9 ± 0.4 | 22.8 ± 0.4 | 96.4 ± 0.1 | 96.6 ± 0.1 | 0.0 | 74.8 ± 1.8 | 78.5 ± 17.7 | 0.272 ± 0.05 |
| Pilot column 4 | 100 ± 0.1 | 69.0 ± 0.2 | 96.8 ± 0.2 | 100 ± 0.7 | 0.0 | 75.8 ± 2.5 | 83.2 ± 12.8 | 0.303 ± 0.04 |
| Pilot pond 5 | 80.0 ± 0.4 | 69.0 ± 1.3 | 93.0 ± 0.8 | 97.0 ± 0.1 | 0.0 | 70.2 ± 2.3 | 80.0 ± 14.1 | 0.278 ± 0.04 |
| Pilot p-bag 6 | 90.4 ± 0.4 | 45.1 ± 0.4 | 70.2 ± 0.2 | 83.6 ± 0.3 | 0.0 | 47.3 ± 2.5 | 55.0 ± 6.0 | 0.226 ± 0.05 |
| Species | PBR Types, Working Volumes | Operating Conditions | Biomass Productivity (mg L−1 d−1), Specific Growth Rate (d−1) | Reference |
|---|---|---|---|---|
| Tetraselmis sp. | Helical tubular PBR, 40 L | Outdoors, F/2 medium, Semi-continuous mode tubular, without CO2 | (56.0–67.0), (0.10–0.33) | [7] |
| tubular + CO2 | (63.0–85.0), (0.31–0.6) | |||
| paddle wheel raceway pond 1 m−2 | pond, without CO2 | (36.0–39.0), (0.11–0.35) | ||
| pond + CO2 | 15.0, 0.11 | |||
| Tetraselmis sp. | Tubular PBRs, 2.5 m−3 | Outdoors, F/2 medium, Optimization of operations (pH, culture velocity), Semi-continuous mode | 140–430, - | [19] |
| 35 m−3 | 80.0, - | |||
| 100 m−3 | 50.0, - | |||
| Tetraselmis striata | Flasks, 150 mL | Shake flasks, F/2 medium, Agitation rate: 150 rpm, Temperature: 25.5 °C, Light intensity: 56 μmol photons m−2 s−1 | -, 0.250 | [52] |
| Tetraselmis striata | Flasks, 150 mL | O3 medium, Temperature: 20 °C, Continuous high light exposure: 145 μΜ cm−2 s−1 | 56.0, - | [53] |
| Tetraselmis striata | Tubular bubble column, 33 L | F/2 medium, Temperature: 20 °C, Photoperiod: 8:16 (L/D), Light intensity: 30 μmol photons m−2 s−1, Periodic addition of pure CO2, Batch mode | -, (0.064–0.13) h−1 | [26] |
| Tetraselmis striata | Tubular bubble column, 9 L | Indoors, Nutri Leaf 30-10-10 medium + NaHCO3, Continuous illumination of 56 μmol photons m−2 s−1, pH 8, Temperature: 25 °C, batch mode | 83.2, 0.303 | Present study |
| Tetraselmis striata | Raceway pond, 40 L | Indoors, Nutri Leaf 30-10-10 medium + NaHCO3, Continuous illumination of 56 μmol photons m−2 s−1, pH 8, Temperature: 25 °C, batch mode | 80.0, 0.278 | Present study |
| Tetraselmis striata | Polyethylene bag reactor, 280 L | Indoors, Nutri Leaf 30-10-10 medium + NaHCO3, Continuous illumination, during sunshine: 140–180 μmol photons m−2 s−1, cloud cover: 20–30 μmol photons m−2 s−1, night: 4 μmol photons m−2 s−1, pH 8, Temperature: 25 °C, batch mode | 55.0, 0.226 | Present study |
| Tetraselmis suecica | Tubular plexiglass PBR, 40 L | Outdoor greenhouse, Walne medium, Effect of initial biomass concentration | 350, 0.680 | [24] |
| Tetraselmis suecica | Annular columns, 120 L | Outdoors, F medium+ NaHCO3, Periodic addition of pure CO2 | (420–460), - | [13] |
| % d.w. Content | |||||
|---|---|---|---|---|---|
| PBRs 1 | Proteins | Lipids | Carbohydrates | Total Chlorophylls | Total Carotenoids |
| Aq-F/2 2 | 43.7 ± 2.8 | 25.6 ± 2.7 | 15.1 ± 1.6 | 3.6 ± 0.3 | 0.58 ± 0.03 |
| Pilot column 3 F/2 | 40.5 ± 0.7 | 23.3 ± 2.6 | 17.5 ± 1.5 | 4.5 ± 0.1 | 0.76 ± 0.1 |
| Pilot column 4 | 41.8 ± 1.9 | 25.7 ± 1.3 | 18.7 ± 0.4 | 4.2 ± 0.2 | 0.90 ± 0.1 |
| Pilot pond 5 | 45.3 ± 0.9 | 27.6 ± 3.1 | 15.5 ± 1.8 | 4.2 ± 0.1 | 0.91 ± 0.1 |
| Pilot P-bag 6 | 44.2 ± 2.0 | 22.4 ± 0.5 | 13.7 ± 0.5 | 3.7 ± 0.2 | 0.67 ± 0.11 |
| PBRs 1 | Fatty Acid Composition (%, w/w) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C16:1 | C16:2 | C18:0 | C18:1 n-9 | C18:2 | C18:3 Alpha | C18:4 | C20:1 n-9 | C20:5 n-3 | Others | ΣPUFAs | ΣMUFAs | ΣSFAs | |
| Aq-F/2 2 | 3.1 ± 0.7 | 31.5 ± 4.9 | 13.0 ± 1.8 | 3.7 ± 0.6 | 1.2 ± 0.1 | 11.0 ± 0.3 | 11.0 ± 0.7 | 4.7 ± 1.4 | ND * | 2.6 ± 0.6 | 14.1 ± 0.5 | 4.1 ± 1.3 | 33.5 ± 0.8 | 26.6 ± 0.9 | 35.8 ± 3.9 |
| Pilot column 3 F/2 | 3.3 ± 0.6 | 22.1 ± 1.1 | 16.9 ± 0.4 | 5.1 ± 1.2 | 0.2 ± 0.3 | 8.8 ± 0.1 | 11.0 ± 0.2 | 6.8 ± 0.2 | ND * | 2.5 ± 0.5 | 12.5 ± 0.1 | 10.8 ± 0.5 | 35.4± 0.4 | 28.2 ± 0.3 | 25.6± 0.7 |
| Pilot column 4 | 1.6 ± 0.2 | 24.1 ± 1.5 | 10.4 ± 0.4 | 5.1 ± 0.4 | 1.1 ± 0.0 | 13.4 ± 2.3 | 13.2 ± 1.0 | 12.6 ± 1.9 | ND * | 1.1 ± 0.1 | 13.7 ± 3.8 | 3.7 ± 1.2 | 44.6 ± 1.8 | 24.9 ± 0.9 | 26.8 ± 0.9 |
| Pilot pond 5 | 4.0 ± 1.3 | 16.3 ± 1.9 | 17.3 ± 2.7 | 0.3 ± 0.3 | 1.2 ± 0.2 | 13.7 ± 2.9 | 4.4 ± 0.6 | 4.9 ± 0.1 | 2.8 ± 0.6 | 3.9 ± 1.0 | 22.4 ± 3.2 | 8.8 ± 1.4 | 34.8 ± 3.2 | 34.9 ± 1.9 | 21.5 ± 1.3 |
| Pilot P-bag 6 | 3.8 ± 0.2 | 19.4 ± 2.5 | 17.3 ± 2.8 | 0.5 ± 0.1 | 1.0 ± 0.1 | 8.6 ± 0.3 | 7.2 ± 0.7 | 4.8 ± 1.4 | 1.8 ± 0.1 | 3.0 ± 0.6 | 26.3 ± 0.5 | 6.3 ± 0.9 | 40.6 ± 0.9 | 28.9 ± 1.8 | 24.2 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrinou, V.; Patsialou, S.; Daskalaki, A.; Economou, C.N.; Aggelis, G.; Vayenas, D.V.; Tekerlekopoulou, A.G. Laboratory- and Pilot-Scale Cultivation of Tetraselmis striata to Produce Valuable Metabolic Compounds. Life 2023, 13, 480. https://doi.org/10.3390/life13020480
Patrinou V, Patsialou S, Daskalaki A, Economou CN, Aggelis G, Vayenas DV, Tekerlekopoulou AG. Laboratory- and Pilot-Scale Cultivation of Tetraselmis striata to Produce Valuable Metabolic Compounds. Life. 2023; 13(2):480. https://doi.org/10.3390/life13020480
Chicago/Turabian StylePatrinou, Vasiliki, Stefania Patsialou, Alexandra Daskalaki, Christina N. Economou, George Aggelis, Dimitris V. Vayenas, and Athanasia G. Tekerlekopoulou. 2023. "Laboratory- and Pilot-Scale Cultivation of Tetraselmis striata to Produce Valuable Metabolic Compounds" Life 13, no. 2: 480. https://doi.org/10.3390/life13020480
APA StylePatrinou, V., Patsialou, S., Daskalaki, A., Economou, C. N., Aggelis, G., Vayenas, D. V., & Tekerlekopoulou, A. G. (2023). Laboratory- and Pilot-Scale Cultivation of Tetraselmis striata to Produce Valuable Metabolic Compounds. Life, 13(2), 480. https://doi.org/10.3390/life13020480











