Characterization and Quantification of Arsenic Species in Foodstuffs of Plant Origin by HPLC/ICP-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Working Standard Solutions
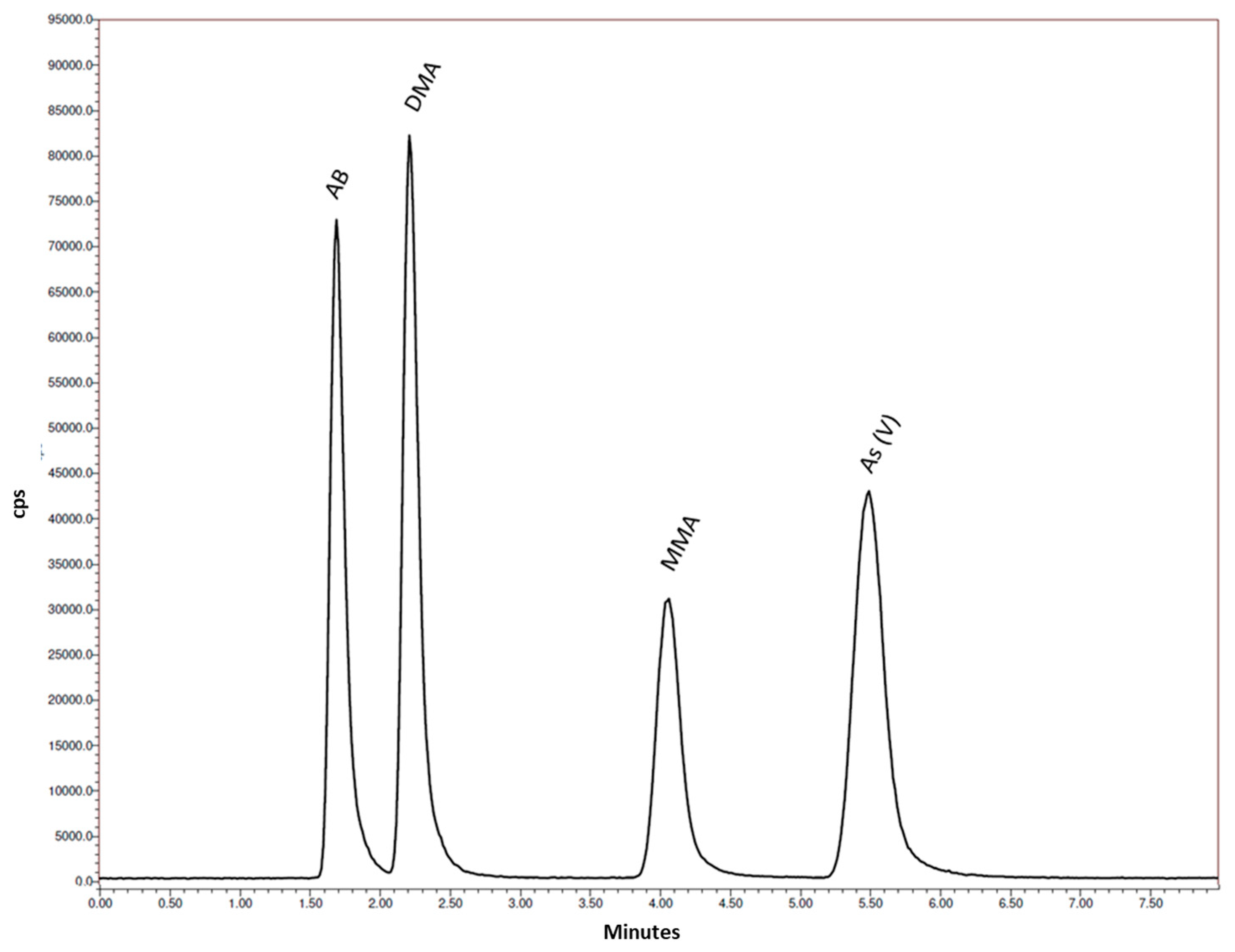
2.2. HPLC/ICP-MS Analysis
2.3. Sample Preparation
2.4. Validation Study
| Performance Characteristics | Evaluation/Measurement Approach |
|---|---|
| Linearity Working Range | Injection of five iAs and oAsCs standard solutions in extractant 0.05, 0.1, 0.5, 2.0, 10.0 μg L−1 Three replicates at each concentration level, injected in three different analytical sessions, with the same instrument, performed on different days and operators); n = 3 Regression of calibration curve with the least square method Mandel’s fitting test to check linearity Calculation of determination coefficient value, acceptable if R2 ≥ 0.99 |
| Selectivity | Analysis of 15 pseudo-blank samples, in two replicates under repeatability conditions |
| Limit of detection Limit of quantification | Estimation of LoD via calibration approach: injection of iAs and oAsCs standard solutions in extractant 0.05, 0.1, 0.5, 2.0, 10.0 μg L−1 (two replicates at each concentration level) Construction of mean calibration curve and usage of calibration function to estimate the standard deviation of intercept and the slope σi is the standard deviation of intercept b is the slope of the calibration function |
| Precision and trueness | Analysis of a blank rice sample fortified at two levels: 15.0 and 30.0 μg kg−1 with a mix of iAs and oAsCs standard solution (6 replicates in 2 different working sessions with the same instrument, different days, operators and instrumental calibrations) Evaluation of relative standard deviation for each analyte and recovery values Usage of a SRM NIST-1568b for the assessment of trueness: recovery values obtained on samples spiked at 15.0 and 30.0 μg kg−1 were used to correct the results of 6 independent tests; n = 18 |
| Measurement uncertainty | Maximum standard uncertainty approach:
Uf is the maximum standard uncertainty (μg kg−1) α = numeric factor depending on the value of C |
| Matrix effect | Calibration graph method: the ratio between the slope of the curve obtained for the matrix-matched extracts and the slope of the curve for the standard calibration curve minus 1, expressed in percentage; n = 3
|
| Matrix Ruggedness | Change of matrix to analyse: conditions of major changes; 10 pseudo-blanks and 6 additional experiments for 3 different pools of samples of legume, cereal and vegetable powders at 30.0 μg kg−1 in matrix. Comparison of precision and recovery data with the results obtained for validation matrix |
2.5. Interlaboratory Comparison: Proficiency Test Round
2.6. Software and Statistical Analysis
3. Results and Discussion
3.1. Procedure Optimization
3.2. Method Validation
3.3. Interlaboratory Comparison: Proficiency Test Round
3.4. Comparison with Other Methods
3.5. Application to Commercial Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- IARC—International Agency for Research on Cancer (Ed.) Arsenic, Metals, Fibres, and Dusts. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization WHO-Press: Lyon, France, 2012; Volume 100C. [Google Scholar]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A Review of Arsenic in Crops, Vegetables, Animals and Food Products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef]
- Sadee, B.; Foulkes, M.E.; Hill, S.J. Coupled Techniques for Arsenic Speciation in Food and Drinking Water: A Review. J. Anal. At. Spectrom. 2014, 30, 102–118. [Google Scholar] [CrossRef]
- Cubadda, F.; Jackson, B.P.; Cottingham, K.L.; Van Horne, Y.O.; Kurzius-Spencer, M. Human Exposure to Dietary Inorganic Arsenic and Other Arsenic Species: State of Knowledge, Gaps and Uncertainties. Sci. Total Environ. 2017, 579, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority—EFSA. Dietary Exposure to Inorganic Arsenic in the European Population. EFSA J. 2014, 12, 3597. [Google Scholar] [CrossRef]
- Kurzius-Spencer, M.; Burgess, J.L.; Harris, R.B.; Hartz, V.; Roberge, J.; Huang, S.; Hsu, C.-H.; O’Rourke, M.K. Contribution of Diet to Aggregate Arsenic Exposures—An Analysis across Populations. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 156–162. [Google Scholar] [CrossRef]
- Hussain, S.; Rengel, Z.; Qaswar, M.; Amir, M.; Zafar-ul-Hye, M. Arsenic and Heavy Metal (Cadmium, Lead, Mercury and Nickel) Contamination in Plant-Based Foods. In Plant and Human Health; Ozturk, M., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 2, pp. 447–490. [Google Scholar]
- Xue, X.-M.; Ye, J.; Raber, G.; Rosen, B.P.; Francesconi, K.; Xiong, C.; Zhu, Z.; Rensing, C.; Zhu, Y.-G. Identification of Steps in the Pathway of Arsenosugar Biosynthesis. Environ. Sci. Technol. 2019, 53, 634–641. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives; Food and Agriculture Organization of the United Nations; World Health Organization (Eds.) Safety Evaluation of Certain Contaminants in Food; WHO Food Additives Series; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Commission Recommendation (EU) No 2015/1381 of 10 August 2015 on the Monitoring of Arsenic in Food; European Commission: Brussels, Belgium, 2015.
- Commission Recommendation (EU) No 2018/464 of 19 March 2018 on the Monitoring of Metals and Iodine in Seaweed, Halophytes and Products Based on Seaweed (Text with EEA Relevance); European Commission: Brussels, Belgium, 2018; Volume 78.
- Commission Recommendation No 2022/C 206/01 of 20 May 2022 on Monitoring the Presence of Inorganic Arsenic in Feed; European Commission: Brussels, Belgium, 2022.
- Arcella, D.; Cascio, C.; Ruiz, J.Á.G. Chronic Dietary Exposure to Inorganic Arsenic. EFSA J. 2021, 19, e06380. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; European Commission: Brussels, Belgium, 2022.
- Commission Regulation (EU) No 2015/1006 of 25 June 2015 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Inorganic Arsenic in Foodstuffs; European Commission: Brussels, Belgium, 2015.
- Nam, S.-H.; Oh, H.-J.; Min, H.-S.; Lee, J.-H. A Study on the Extraction and Quantitation of Total Arsenic and Arsenic Species in Seafood by HPLC–ICP-MS. Microchem. J. 2010, 95, 20–24. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, H.; Kim, Y.-T.; Yoon, H.-O. Development of a Simultaneous Analytical Method to Determine Arsenic Speciation Using HPLC-ICP-MS: Arsenate, Arsenite, Monomethylarsonic Acid, Dimethylarsinic Acid, Dimethyldithioarsinic Acid, and Dimethylmonothioarsinic Acid. Microchem. J. 2017, 134, 295–300. [Google Scholar] [CrossRef]
- Narukawa, T.; Inagaki, K.; Kuroiwa, T.; Chiba, K. The Extraction and Speciation of Arsenic in Rice Flour by HPLC–ICP-MS. Talanta 2008, 77, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S.; Hoy, K.S.; Schofield, J.R.M.; Uppal, J.S.; Lin, Y.; Lu, X.; Peng, H.; Le, X.C. Arsenic Speciation Analysis: A Review with an Emphasis on Chromatographic Separations. TrAC Trends Anal. Chem. 2020, 123, 115770. [Google Scholar] [CrossRef]
- Chen, M.-L.; Ma, L.-Y.; Chen, X.-W. New Procedures for Arsenic Speciation: A Review. Talanta 2014, 125, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, Z.; Kong, Q.; Wang, L. Extraction and Determination of Arsenic Species in Leafy Vegetables: Method Development and Application. Food Chem. 2017, 217, 524–530. [Google Scholar] [CrossRef]
- Bou Khouzam, R.; Szpunar, J.; Holeman, M.; Lobinski, R. Trace Element Speciation in Food: State of the Art of Analytical Techniques and Methods. Pure Appl. Chem. 2012, 84, 169–179. [Google Scholar] [CrossRef]
- ISO/IEC 17025; 2017 General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization—ISO: Geneva, Switzerland, 2017.
- Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on Official Controls and Other Official Activities Performed to Ensure the Application of Food and Feed Law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products, Amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and Repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation); European Commission: Brussels, Belgium, 2017.
- Commission Decision of 14 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results; European Commission: Brussels, Belgium, 2002.
- Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to Be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC; European Commission: Brussels, Belgium, 2021.
- ISO 5725-2; 2019 Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 2: Basic Method for the Determination of Repeatability and Reproducibility of a Standard Measurement Method. International Organization for Standardization—ISO: Geneva, Switzerland, 2019.
- ISO/IEC 17043; 2010 Conformity Assessment—General Requirements for Proficiency Testing. International Organization for Standardization—ISO: Geneva, Switzerland, 2010.
- Pompa, C.; D’Amore, T.; Miedico, O.; Preite, C.; Chiaravalle, A.E. Evaluation and Dietary Exposure Assessment of Selected Toxic Trace Elements in Durum Wheat (Triticum Durum) Imported into the Italian Market: Six Years of Official Controls. Foods 2021, 10, 775. [Google Scholar] [CrossRef]
- Management of Left-Censored Data in Dietary Exposure Assessment of Chemical Substances. EFSA J. 2010, 8, 1557. [CrossRef]
- EN 16802; 2016—Foodstuffs—Determination of Elements and Their Chemical Species—Determination of Inorganic Arsenic in Foodstuffs of Marine and Plant Origin by Anion-Exchange HPLC-ICP-MS. European Committee for Standardization—CEN: Brussels, Belgium, 2016.
- Reis, V.A.T.; Duarte, A.C. Analytical Methodologies for Arsenic Speciation in Macroalgae: A Critical Review. TrAC Trends Anal. Chem. 2018, 102, 170–184. [Google Scholar] [CrossRef]
- Ariga, T.; Zhu, Y.; Inagaki, K. Study on Carbon-Induced Signal Enhancement in Inductively Coupled Plasma Mass Spectrometry: An Approach from the Spatial Distribution of Analyte Signal Intensities. J. Anal. At. Spectrom. 2019, 34, 1865–1874. [Google Scholar] [CrossRef]
- Grindlay, G.; Mora, J.; de Loos-Vollebregt, M.; Vanhaecke, F. A Systematic Study on the Influence of Carbon on the Behavior of Hard-to-Ionize Elements in Inductively Coupled Plasma–Mass Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2013, 86, 42–49. [Google Scholar] [CrossRef]
- Vu, H.A.; Nguyen, M.H.; Vu-Thi, H.-A.; Do-Hong, Q.; Dang, X.H.; Nguyen, T.N.B.; Trinh, H.Q.; Ly Bich, T.; Nguyen, T.-T.; Le-Van, D.; et al. Speciation Analysis of Arsenic Compounds by High-Performance Liquid Chromatography in Combination with Inductively Coupled Plasma Dynamic Reaction Cell Quadrupole Mass Spectrometry: Application for Vietnamese Rice Samples. J. Anal. Methods Chem. 2019, 2019, e5924942. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 333/2007 of 28 March 2007 Laying down the Methods of Sampling and Analysis for the Control of the Levels of Trace Elements and Processing Contaminants in Foodstuffs; European Commission: Brussels, Belgium, 2022.
- Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food; Joint Research Centre—JRC: Brussels Belgium, 2016.
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Yang, G.; Xu, J.; Zheng, J.; Xu, X.; Wang, W.; Xu, L.; Chen, G.; Fu, F. Speciation Analysis of Arsenic in Mya Arenaria Linnaeus and Shrimp with Capillary Electrophoresis-Inductively Coupled Plasma Mass Spectrometry. Talanta 2009, 78, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y. Inorganic Arsenic Contents in Infant Rice Powders and Infant Rice Snacks Marketed in Korea Determined by a Highly Sensitive Gas Chromatography-Tandem Mass Spectrometry Following Derivatization with British Anti-Lewisite. Food Sci. Biotechnol. 2017, 27, 617–622. [Google Scholar] [CrossRef]
- Jung, M.Y.; Kang, J.H.; Jung, H.J.; Ma, S.Y. Inorganic Arsenic Contents in Ready-to-Eat Rice Products and Various Korean Rice Determined by a Highly Sensitive Gas Chromatography-Tandem Mass Spectrometry. Food Chem. 2018, 240, 1179–1183. [Google Scholar] [CrossRef]
- Guillod-Magnin, R.; Brüschweiler, B.J.; Aubert, R.; Haldimann, M. Arsenic Species in Rice and Rice-Based Products Consumed by Toddlers in Switzerland. Food Addit. Contam. Part A 2018, 35, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, Y.; Wang, X.; Chen, S.; Wu, Y.; Fu, F. A Universal Method for the Speciation Analysis of Arsenic in Various Seafood Based on Microwave-Assisted Extraction and Ion Chromatography-Inductively Coupled Plasma Mass Spectrometry. Microchem. J. 2020, 159, 105592. [Google Scholar] [CrossRef]
- Musil, S.; Pétursdóttir, Á.H.; Raab, A.; Gunnlaugsdóttir, H.; Krupp, E.; Feldmann, J. Speciation without Chromatography Using Selective Hydride Generation: Inorganic Arsenic in Rice and Samples of Marine Origin. Anal. Chem. 2014, 86, 993–999. [Google Scholar] [CrossRef]
- Kisomi, A.S.; Alizadeh, T.; Shakeri, A.; Nouri, A.; Farsadrooh, M.; Najafi AsliPashaki, S. Application of μ-TLC for Speciation of Inorganic Arsenic by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Microchem. J. 2020, 159, 105443. [Google Scholar] [CrossRef]
- Commission Directive No 2006/125/EC of 5 December 2006 on Processed Cereal-Based Foods and Baby Foods for Infants and Young Children (Text with EEA Relevance); European Commission: Brussels, Belgium, 2006; Volume 339.
- Signes-Pastor, A.J.; Cottingham, K.L.; Carey, M.; Sayarath, V.; Palys, T.; Meharg, A.A.; Folt, C.L.; Karagas, M.R. Infants’ Dietary Arsenic Exposure during Transition to Solid Food. Sci. Rep. 2018, 8, 7114. [Google Scholar] [CrossRef]
- Bianucci, E.; Peralta, J.M.; Furlan, A.; Hernández, L.E.; Castro, S. Arsenic in Wheat, Maize, and Other Crops. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer: Singapore, 2020; pp. 279–306. [Google Scholar]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 13 December 2022).
- Food and Agriculture Organization of the United Nations—FAO. OECD-FAO Agricultural Outlook 2022—2031; Organisation for Economic Cooperation and Development—OECD: Paris, France, 2022. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations—FAO. OECD-FAO Agricultural Outlook 2020–2029; Organisation for Economic Cooperation and Development—OECD: Paris, France, 2020. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Schneider, R.G. The Role of Amaranth, Quinoa, and Millets for the Development of Healthy, Sustainable Food Products—A Concise Review. Foods 2022, 11, 2442. [Google Scholar] [CrossRef]
- Coelho, J.P. Arsenic Speciation in Algae: Case Studies in Europe. In Comprehensive Analytical Chemistry; Duarte, A.C., Reis, V., Eds.; Arsenic Speciation in Algae; Elsevier: Amsterdam, The Netherlands, 2019; Volume 85, pp. 179–198. [Google Scholar]
- Dìaz, O.; Pastene, R.; Encina-Montoya, F.; Vega, R.; Oberti-Grassau, C. Arsenic Speciation in Algae: Case Studies in American Continent. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 85, pp. 247–265. [Google Scholar]
- World Health Organization—WHO. Report of the Expert Meeting on Food Safety for Seaweed—Current Status and Future Perspectives: Rome, 28–29 October 2021; FAO, WHO, Eds.; Food Safety and Quality Series; Food and Agriculture Organization of the United Nations—FAO: Rome, Italy, 2022. [Google Scholar]


| Parameter | HPLC Conditions |
|---|---|
| Column | UPLC PRP-X100 Anion Exchange HPLC Column—i.d. 2.1 mm, l. 250 mm, p.s. 5 µm |
| Mobile phase | Isocratic elution A/B (85:15) A: 50 mM NH4HCO3 in CH3OH 3% v/v B: ultrapure water |
| pH | 10.3 |
| Flow rate | 0.35 mL min−1 |
| Run time | 7 min + 1 min washing |
| Column T | 25 °C |
| Autosampler T | 20 °C |
| Diverter valve | 0–7 min from HPLC to ICP; 7–8 min form HPLC to waste |
| Injection volume | 30 µL |
| ICP-MS Conditions | |
| RF power | 1600 W |
| Sample introduction system | Meinhard concentric PTFE nebulizer High Purity Quartz Cyclonic Spray Chamber |
| Plasma gas flow | 15.0 L min−1 |
| Aux gas flow | 1.0 L min−1 |
| Peristaltic pump control | sample flush 60 s; sample flush speeding −35 rpm |
| Isotopes monitored | As-75; Cl-35 |
| Dwell time | As: 450 ms; Cl: 50 ms |
| Mode | Standard |
| Quadrupole Ion Deflector | Off |
| Parameter | iAs | AB | DMA | MMA |
|---|---|---|---|---|
| Linearity R2 | ≥0.99 | ≥0.99 | ≥0.99 | ≥0.99 |
| Range µg kg−1 | 0.025–400 | 0.106–400 | 0.079–400 | 0.077–400 |
| LoQ µg kg−1 | 0.075 | 0.321 | 0.241 | 0.235 |
| LoD µg kg−1 | 0.025 | 0.106 | 0.079 | 0.077 |
| Precision (mean) RSD% * | 4.96 | 7.35 | 3.15 | 4.92 |
| Recovery (mean) R% * | 81.3 | 100.4 | 85.9 | 117.8 |
| Uncertainty U% | 18.2–22.0 | 18.2–22.0 | 18.2–22.0 | 18.2–22.0 |
| Selectivity | Verified for plant-based processed and unprocessed foods (cereals, fruits, vegetables, tubers, legumes, seaweeds, nuts and seeds) | |||
| Matrix Effect ME% | <9% | <16% | <12% | <19% |
| Matrix Ruggedness | Verified for plant-based processed and unprocessed foods (cereals, fruits, vegetables, tubers, legumes, seaweeds, nuts and seeds) | |||
| Matrix | Analyte | Result µg kg−1 | Assigned Value µg kg−1 | z Score | σP |
|---|---|---|---|---|---|
| Powdered Brown Rice (1) | Arsenic (total) | 687.0 | 643.2 | 0.4 | 110 |
| Arsenic (inorganic) | 119.0 | 117.5 | 0.1 | 25.9 | |
| Cadmium | 27.8 | 25.3 | 0.4 | 5.58 | |
| Iron | 10700 | 10100 | 0.5 | 1.14 | |
| Lead | 51.2 | 48.3 | 0.3 | 10.6 | |
| Nickel | 586.0 | 479.5 | 1.2 | 85.7 | |
| Zinc | 13600 | 14600 | -0.4 | 1.53 | |
| Infant Cereal (2) | Arsenic (total) | 128.0 | 113.0 | 0.6 | 24.9 |
| Arsenic (inorganic) | 94.0 | 88.5 | 0.3 | 19.5 | |
| Cadmium | 35.3 | 32.8 | 0.3 | 7.23 | |
| Chromium | 149.0 | 126.7 | 0.8 | 27.7 | |
| Lead | 49.1 | 44.9 | 0.4 | 9.89 | |
| Mercury (total) | 29.6 | 33.1 | 0.5 | 6.51 | |
| Selenium | 67.5 | 78.3 | -0.6 | 17.2 |
| References | Extraction | Detection | Analytes | Matrices | Recovery (%) | LoD | Validation Parameters | Notes |
|---|---|---|---|---|---|---|---|---|
| Vu et al. (2019) [35] | MAD | HPLC-ICP-DRC-QMS | AB, DMA, MMA, AsIII, AsV | rice | 70.0–135.5 | 0.5–2.9 ng g−1 | linearity, recovery, LoD, LoQ | species monitored 75As16O+ |
| Ma et al. (2017) [21] | Shaking UAE MAE | HPLC-ICP-MS | AB, AC, DMA, MMA, AsIII, AsV | leafy vegetables | - | - | extraction efficiency (%), LoD, LoQ | different extraction protocols |
| Jeong et al. (2017) [17] | - | HPLC-ICP-MS | DMA, MMA, AsIII, AsV, DMDTA, DMMTA | water | 85.1 | 0.04–0.26 µg L−1 | linearity, recovery, LoD, LoQ | reversed phase C18 column; confirmation of DMDTA and DMMTA by ESI-MS |
| Guillod-Magnin et al. (2017) [42] | oven-heated SLE | IC-ICP-MS | DMA, MMA, AsIII, AsV | rice and rice products | 100–117 | 0.29–2.45 µg kg−1 | linearity, recovery, LoD, LoQ, trueness, precision | |
| Lin et al. (2020) [43] | MAE | IC-ICP-MS | AB, DMA, MMA, AsIII, AsV | seafoods, seaweeds | 92–103 | 0.08–0.12 ng g−1 | linearity, LoD, LoQ trueness, precision | cation exchange column |
| Kisomi et al. (2020) [45] | - | µTLC-LA-ICP-MS | AsIII and AsV | water | 71–101 | 0.037–0.27 µg kg−1 | linearity, trueness, precision, matrix effect | |
| Jung et al. (2018)/Jung (2017) [40,41] | water bath | GC-MS-MS | AsIII and AsV | ready-to-eat rice products, rice based infant foods | 90–117 | 0.0159 ng g−1 | linearity, selectivity LoD, LoQ trueness, precision | derivatization reagent: BAL |
| Yang et al. (2009) [39] | MAE (CH3OH–H2O 1:1 v/v) | CE-ICP-MS | DMA, MMA, AsIII, AsV | Mya arenaria Linnaeus; shrimps | 96–105 | 1.0–1.9 µg kg−1 | linearity, precision, recovery | sheath–flow interface to couple CE with ICP-MS |
| Musil et al. (2014) [44] | MAD | HG-ICP-QQQ | DMA, AsIII, AsV | rice, seafoods, seaweeds | 95.8–100.6 | 0.9–1.1 µg kg−1 | linearity, precision, recovery | derivatization reagent: NaBH4 and HCl |
| This method | shaking water bath UAE | HPLC-ICP-MS | AB, DMA, MMA, sum of AsIII and AsV | cereals, fruits, vegetables, tubers, legumes, seaweeds, nuts, seeds, supplements, infant foods | 81.3–117.8 | 0.025–0.106 ng g−1 | selectivity, linearity, LoD, LoQ, trueness, precision, matrix effect, measurement uncertainty, ruggedness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amore, T.; Miedico, O.; Pompa, C.; Preite, C.; Iammarino, M.; Nardelli, V. Characterization and Quantification of Arsenic Species in Foodstuffs of Plant Origin by HPLC/ICP-MS. Life 2023, 13, 511. https://doi.org/10.3390/life13020511
D’Amore T, Miedico O, Pompa C, Preite C, Iammarino M, Nardelli V. Characterization and Quantification of Arsenic Species in Foodstuffs of Plant Origin by HPLC/ICP-MS. Life. 2023; 13(2):511. https://doi.org/10.3390/life13020511
Chicago/Turabian StyleD’Amore, Teresa, Oto Miedico, Ciro Pompa, Chiara Preite, Marco Iammarino, and Valeria Nardelli. 2023. "Characterization and Quantification of Arsenic Species in Foodstuffs of Plant Origin by HPLC/ICP-MS" Life 13, no. 2: 511. https://doi.org/10.3390/life13020511
APA StyleD’Amore, T., Miedico, O., Pompa, C., Preite, C., Iammarino, M., & Nardelli, V. (2023). Characterization and Quantification of Arsenic Species in Foodstuffs of Plant Origin by HPLC/ICP-MS. Life, 13(2), 511. https://doi.org/10.3390/life13020511









