Pharmacological Activities of Mogrol: Potential Phytochemical against Different Diseases
Abstract
:1. Introduction
2. Literature Search
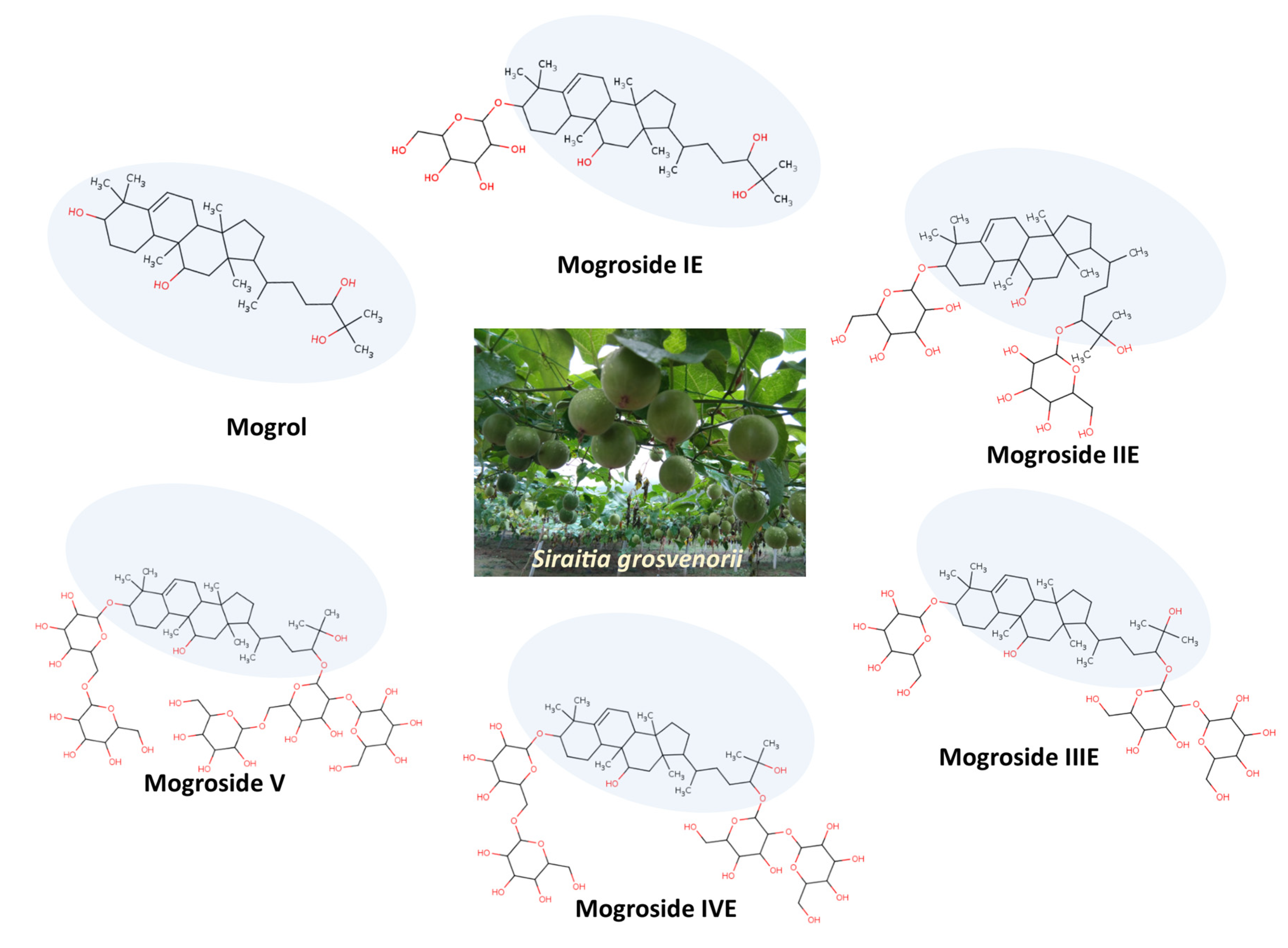
3. Mogrol Structure and Properties
4. Pharmacological Activities of Mogrol
4.1. In Vitro Antiobesity and Antidiabetes Activity of Mogrol
4.2. In Vitro Anti-Inflammatory Activity of Mogrol
4.3. Anticancer Activity of Mogrol
4.3.1. In Vitro Anticancer Activity of Mogrol
4.3.2. In Vivo Anticancer Activity of Mogrol
4.4. Mogrol Activity against Ulcerative Colitis
4.4.1. In Vivo Anti-UC Activity of Mogrol
4.4.2. In Vitro Anti-Colitis Activity of Mogrol
4.5. Activity of Mogrol against the Pulmonary Fibrosis
4.5.1. In Vitro Anti-PF Activity of Mogrol
4.5.2. In Vivo Anti-PF Activity of Mogrol
4.6. Anti-Osteoporosis Activity of Mogrol
4.6.1. In Vitro Antiosteoporosis Activity of Mogrol
4.6.2. In Vivo Antiosteoporosis Activity of Mogrol
4.7. In Vivo Neuroprotective Activity of Mogrol
5. Discussion and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Choi, S.; Yamabe, N.; Kim, K.H.; Kang, K.S. Recent findings on the mechanism of cisplatin-induced renal cytotoxicity and therapeutic potential of natural compounds. Nat. Prod. Sci. 2020, 26, 28–49. [Google Scholar]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Ishihara, M.; Horiuchi, H.; Ito, Y.; Tabata, H.; Suzuki, Y.A.; Nakano, Y.; Yamaji, R.; Inui, H. Mogrol derived from Siraitia grosvenorii mogrosides suppresses 3T3-L1 adipocyte differentiation by reducing cAMP-response element-binding protein phosphorylation and increasing AMP-activated protein kinase phosphorylation. PLoS ONE 2016, 11, e0162252. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; He, R.; Xiong, R.; Fu, T.; Li, N.; Geng, Q. Effect and mechanism of mogrol on lipopolysaccharide-induced acute lung injury. Chin. J. Emerg. Med. 2022, 31, 777–782. [Google Scholar] [CrossRef]
- Chen, X.-b.; Zhuang, J.-j.; Liu, J.-h.; Lei, M.; Ma, L.; Chen, J.; Shen, X.; Hu, L.-h. Potential AMPK activators of cucurbitane triterpenoids from Siraitia grosvenorii Swingle. Bioorganic Med. Chem. 2011, 19, 5776–5781. [Google Scholar] [CrossRef]
- Song, J.-R.; Li, N.; Wei, Y.-L.; Lu, F.-L.; Li, D.-P. Design and synthesis of mogrol derivatives modified on a ring with anti-inflammatory and anti-proliferative activities. Bioorg. Med. Chem. Lett. 2022, 74, 128924. [Google Scholar] [CrossRef]
- Li, H.; Li, R.; Jiang, W.; Zhou, L. Research progress of pharmacological effects of Siraitia grosvenorii extract. J. Pharm. Pharmacol. 2021, 74, 953–960. [Google Scholar] [CrossRef]
- Wu, J.; Jian, Y.; Wang, H.; Huang, H.; Gong, L.; Liu, G.; Yang, Y.; Wang, W. A Review of the Phytochemistry and Pharmacology of the Fruit of Siraitia grosvenorii (Swingle): A Traditional Chinese Medicinal Food. Molecules 2022, 27, 6618. [Google Scholar] [CrossRef]
- Bhusari, S.; Rodriguez, C.; Tarka Jr, S.M.; Kwok, D.; Pugh, G.; Gujral, J.; Tonucci, D. Comparative In vitro metabolism of purified mogrosides derived from monk fruit extracts. Regul. Toxicol. Pharmacol. 2021, 120, 104856. [Google Scholar] [CrossRef]
- Murata, Y.; Ogawa, T.; Suzuki, Y.A.; Yoshikawa, S.; Inui, H.; Sugiura, M.; Nakano, Y. Digestion and absorption of Siraitia grosvenori triterpenoids in the rat. Biosci. Biotechnol. Biochem. 2010, 74, 673–676. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.; Liao, W.; Luo, G.; Qin, Z.; Han, S.; Lin, Y. Modulation of Gut Microbiota Composition and Short-Chain Fatty Acid Synthesis by Mogroside V in an In Vitro Incubation System. ACS Omega 2021, 6, 25486–25496. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, X.; Lv, X.; Liu, Y.; Li, J.; Du, G.; Liu, L. Construction and Optimization of the de novo Biosynthesis Pathway of Mogrol in Saccharomyces cerevisiae. Front. Bioeng. Biotechnol. 2022, 10, 919526. [Google Scholar] [CrossRef]
- Liao, J.; Liu, T.; Xie, L.; Mo, C.; Huang, X.; Cui, S.; Jia, X.; Lan, F.; Luo, Z.; Ma, X. Plant Metabolic Engineering by Multigene Stacking: Synthesis of Diverse Mogrosides. Int. J. Mol. Sci. 2022, 23, 10422. [Google Scholar] [CrossRef]
- Preusche, M.; Ulbrich, A.; Schulz, M. Culturing Important Plants for Sweet Secondary Products under Consideration of Environmentally Friendly Aspects. Processes 2022, 10, 703. [Google Scholar] [CrossRef]
- Li, J.; Mu, S.; Yang, J.; Liu, C.; Zhang, Y.; Chen, P.; Zeng, Y.; Zhu, Y.; Sun, Y. Glycosyltransferase engineering and multi-glycosylation routes development facilitating synthesis of high-intensity sweetener mogrosides. Iscience 2022, 25, 105222. [Google Scholar] [CrossRef]
- Luo, Z.; Qiu, F.; Zhang, K.; Qin, X.; Guo, Y.; Shi, H.; Zhang, L.; Zhang, Z.; Ma, X. In vitro AMPK activating effect and in vivo pharmacokinetics of mogroside V, a cucurbitane-type triterpenoid from Siraitia grosvenorii fruits. RSC Adv. 2016, 6, 7034–7041. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, K.; Shi, H.; Guo, Y.; Ma, X.; Qiu, F. Development and Validation of a Sensitive LC–MS-MS Method for Quantification of Mogrol in Rat Plasma and Application to Pharmacokinetic Study. J. Chromatogr. Sci. 2017, 55, 284–290. [Google Scholar] [CrossRef] [Green Version]
- LIU, S.-g.; DAI, X.-j.; ZHONG, D.-f.; ZHANG, C.-f.; CHEN, X.-y. Identification of metabolites and pharmacokinetics of mogrol in rat plasma. Acta Pharm. Sin. 2017, 12, 1452–1457. [Google Scholar]
- Subedi, L.; Lee, S.E.; Madiha, S.; Gaire, B.P.; Jin, M.; Yumnam, S.; Kim, S.Y. Phytochemicals against TNFα-Mediated Neuroinflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.-G.; Jeong, S.H.; Kim, H.J.; Son, S.W. STAT3 maintains skin barrier integrity by modulating SPINK5 and KLK5 expression in keratinocytes. Exp. Dermatol. 2022, 31, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Xie, Z.; Li, J.; Li, J.; Hu, L. Design, synthesis and biological evaluation of mogrol derivatives as a novel class of AMPKα2β1γ1 activators. Bioorg. Med. Chem. Lett. 2020, 30, 126790. [Google Scholar] [CrossRef]
- Additives, E.P.o.F.; Flavourings; Younes, M.; Aquilina, G.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; et al. Safety of use of Monk fruit extract as a food additive in different food categories. EFSA J. 2019, 17, e05921. [Google Scholar]
- Chen, Y.; Zhang, L.; Li, Z.; Wu, Z.; Lin, X.; Li, N.; Shen, R.; Wei, G.; Yu, N.; Gong, F. Mogrol Attenuates Osteoclast Formation and Bone Resorption by Inhibiting the TRAF6/MAPK/NF-κB Signaling Pathway In vitro and Protects Against Osteoporosis in Postmenopausal Mice. Front. Pharmacol. 2022, 13, 803880. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, J.; Hao, J.; Xie, H.; Shimizu, K.; Li, R.; Zhang, C. Natural product mogrol attenuates bleomycin-induced pulmonary fibrosis development through promoting AMPK activation. J. Funct. Foods 2021, 77, 104280. [Google Scholar] [CrossRef]
- Qin, X.; Xiaojian, S.; Ronggan, L.; Yuxian, W.; Zhunian, T.; Shouji, G.; Heimbach, J. Subchronic 90-day oral (Gavage) toxicity study of a Luo Han Guo mogroside extract in dogs. Food Chem. Toxicol. 2006, 44, 2106–2109. [Google Scholar] [CrossRef]
- Liu, C.; Dai, L.; Liu, Y.; Dou, D.; Sun, Y.; Ma, L. Pharmacological activities of mogrosides. Future Med. Chem. 2018, 10, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.A.; Tomoda, M.; Murata, Y.; Inui, H.; Sugiura, M.; Nakano, Y. Antidiabetic effect of long-term supplementation with Siraitia grosvenori on the spontaneously diabetic Goto–Kakizaki rat. Br. J. Nutr. 2007, 97, 770–775. [Google Scholar] [CrossRef] [Green Version]
- Xiangyang, Q.; Weijun, C.; Liegang, L.; Ping, Y.; Bijun, X. Effect of a Siraitia grosvenori extract containing mogrosides on the cellular immune system of type 1 diabetes mellitus mice. Mol. Nutr. Food Res. 2006, 50, 732–738. [Google Scholar] [CrossRef]
- Desjardins, E.M.; Steinberg, G.R. Emerging role of AMPK in brown and beige adipose tissue (BAT): Implications for obesity, insulin resistance, and type 2 diabetes. Curr. Diabetes Rep. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zeng, Y.; Dai, L.-H.; Cai, T.-Y.; Zhu, Y.-M.; Dou, D.-Q.; Ma, L.-Q.; Sun, Y.-X. Mogrol represents a novel leukemia therapeutic, via ERK and STAT3 inhibition. Am. J. Cancer Res. 2015, 5, 1308. [Google Scholar] [PubMed]
- Wang, L.; Li, L.; Fu, Y.; Li, B.; Chen, B.; Xu, F.; Li, D. Separation, synthesis, and cytotoxicity of a series of mogrol derivatives. J. Asian Nat. Prod. Res. 2019, 22, 663–677. [Google Scholar] [CrossRef]
- Song, J.-R.; Li, N.; Li, D.-P. Synthesis and anti-proliferation activity of mogrol derivatives bearing quinoline and triazole moieties. Bioorg. Med. Chem. Lett. 2021, 42, 128090. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Song, J.; Li, D. Synthesis and Antiproliferative Activity of Ester Derivatives of Mogrol through JAK2/STAT3 Pathway. Chem. Biodivers. 2022, 19, e202100742. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Chen, H.-y.; Yan, X.; Li, R.-l.; Lan, J.; Xue, K.-y.; Li, X.; Zhuo, C.-l.; Lin, L. Mogrol suppresses lung cancer cell growth by activating AMPK-dependent autophagic death and inducing p53-dependent cell cycle arrest and apoptosis. Toxicol. Appl. Pharmacol. 2022, 444, 116037. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, J.; Xie, H.; Li, R.; Shimizu, K.; Zhang, C. Mogrol, an aglycone of mogrosides, attenuates ulcerative colitis by promoting AMPK activation. Phytomedicine 2021, 81, 153427. [Google Scholar]
- Jin, W. Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial–mesenchymal transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef] [Green Version]
- Xiao, B.-D.; Zhao, Y.-J.; Jia, X.-Y.; Wu, J.; Wang, Y.-G.; Huang, F. Multifaceted p21 in carcinogenesis, stemness of tumor and tumor therapy. World J. Stem Cells 2020, 12, 481. [Google Scholar] [CrossRef]
- Spindler, A.; Stefan, K.; Wiese, M. Synthesis and investigation of tetrahydro-β-carboline derivatives as inhibitors of the breast cancer resistance protein (ABCG2). J. Med. Chem. 2016, 59, 6121–6135. [Google Scholar] [CrossRef]
- Chen, G.; Liu, C.; Meng, G.; Zhang, C.; Chen, F.; Tang, S.; Hong, H.; Zhang, C. Neuroprotective effect of mogrol against Aβ1–42-induced memory impairment neuroinflammation and apoptosis in mice. J. Pharm. Pharmacol. 2019, 71, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Meng, G.-L.; Zhang, C.-T.; Wang, H.; Hu, M.; Long, Y.; Hong, H.; Tang, S.-S. Mogrol attenuates lipopolysaccharide (LPS)-induced memory impairment and neuroinflammatory responses in mice. J. Asian Nat. Prod. Res. 2020, 22, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, V.; Chaudhuri, N.; Torrisi, S.E.; Kahn, N.; Müller, V.; Kreuter, M. The therapy of idiopathic pulmonary fibrosis: What is next? Eur. Respir. Rev. 2019, 28, 190021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jegal, Y.; Park, J.S.; Kim, S.Y.; Yoo, H.; Jeong, S.H.; Song, J.W.; Lee, J.H.; Lee, H.L.; Choi, S.M.; Kim, Y.W.; et al. Clinical Features, Diagnosis, Management, and Outcomes of Idiopathic Pulmonary Fibrosis in Korea: Analysis of the Korea IPF Cohort (KICO) Registry. Tuberc. Respir. Dis. 2022, 85, 185–194. [Google Scholar] [CrossRef]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Wang, L.; Liu, B.; Ren, G.; Okubo, R.; Yu, J.; Zhang, C. Bryodulcosigenin attenuates bleomycin-induced pulmonary fibrosis via inhibiting AMPK-mediated mesenchymal epithelial transition and oxidative stress. Phytother. Res. 2022, 36, 3911–3923. [Google Scholar] [CrossRef]
- Lee, S.-K.; Jun, D.-S.; Lee, D.-K.; Baik, J.-M. Clinical Characteristics of Elderly People with Osteoporotic Vertebral Compression Fracture Based on a 12-Year Single-Center Experience in Korea. Geriatrics 2022, 7, 123. [Google Scholar] [CrossRef]
- Jaiswal, V.; Park, M.; Lee, H.-J. Comparative Transcriptome Analysis of the Expression of Antioxidant and Immunity Genes in the Spleen of a Cyanidin 3-O-Glucoside-Treated Alzheimer’s Mouse Model. Antioxidants 2021, 10, 1435. [Google Scholar] [CrossRef]
- Park, M.; Kim, K.H.; Jaiswal, V.; Choi, J.; Chun, J.L.; Seo, K.M.; Lee, M.-J.; Lee, H.-J. Effect of black ginseng and silkworm supplementation on obesity, the transcriptome, and the gut microbiome of diet-induced overweight dogs. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Chun, K.-H.; Jin, H.C.; Kang, K.S.; Chang, T.-S.; Hwang, G.S. Poncirin inhibits osteoclast differentiation and bone loss through down-regulation of NFATc1 in vitro and in vivo. Biomol. Ther. 2020, 28, 337. [Google Scholar] [CrossRef]
- Dang, T.K.; Hong, S.-M.; Dao, V.T.; Tran, P.T.T.; Tran, H.T.; Do, G.H.; Hai, T.N.; Nguyet Pham, H.T.; Kim, S.Y. Anti-neuroinflammatory effects of alkaloid-enriched extract from Huperzia serrata on lipopolysaccharide-stimulated BV-2 microglial cells. Pharm. Biol. 2023, 61, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sanjay; Kim, J.Y. Anti-inflammatory effects of 9-cis-retinoic acid on β-amyloid treated human microglial cells. Eur. J. Inflamm. 2022, 20, 1721727×221143651. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114. [Google Scholar] [PubMed]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshehri, A.; Ahmad, A.; Tiwari, R.K.; Ahmad, I.; Alkhathami, A.G.; Alshahrani, M.Y.; Asiri, M.A.; Almeleebia, T.M.; Saeed, M.; Yadav, D.K.; et al. In Vitro Evaluation of Antioxidant, Anticancer, and Anti-Inflammatory Activities of Ethanolic Leaf Extract of Adenium obesum. Front. Pharmacol. 2022, 13, 847534. [Google Scholar] [CrossRef]
- Kim, S.Y.; An, S.; Park, D.K.; Kwon, K.A.; Kim, K.O.; Chung, J.-W.; Kim, J.H.; Kim, Y.J. Efficacy of iron supplementation in patients with inflammatory bowel disease treated with anti-tumor necrosis factor-alpha agents. Ther. Adv. Gastroenterol. 2020, 13, 1756284820961302. [Google Scholar] [CrossRef]
- Ji, M.; Ryu, H.J.; Baek, H.-M.; Shin, D.M.; Hong, J.H. Dynamic synovial fibroblasts are modulated by NBCn1 as a potential target in rheumatoid arthritis. Exp. Mol. Med. 2022, 54, 503–517. [Google Scholar] [CrossRef]
- Subedi, L.; Gaire, B.P.; Kim, S.-Y.; Parveen, A. Nitric Oxide as a Target for Phytochemicals in Anti-Neuroinflammatory Prevention Therapy. Int. J. Mol. Sci. 2021, 22, 4771. [Google Scholar] [CrossRef]
- Zhang, W.; Huai, Y.; Miao, Z.; Qian, A.; Wang, Y. Systems pharmacology for investigation of the mechanisms of action of traditional Chinese medicine in drug discovery. Front. Pharmacol. 2019, 10, 743. [Google Scholar] [CrossRef] [Green Version]

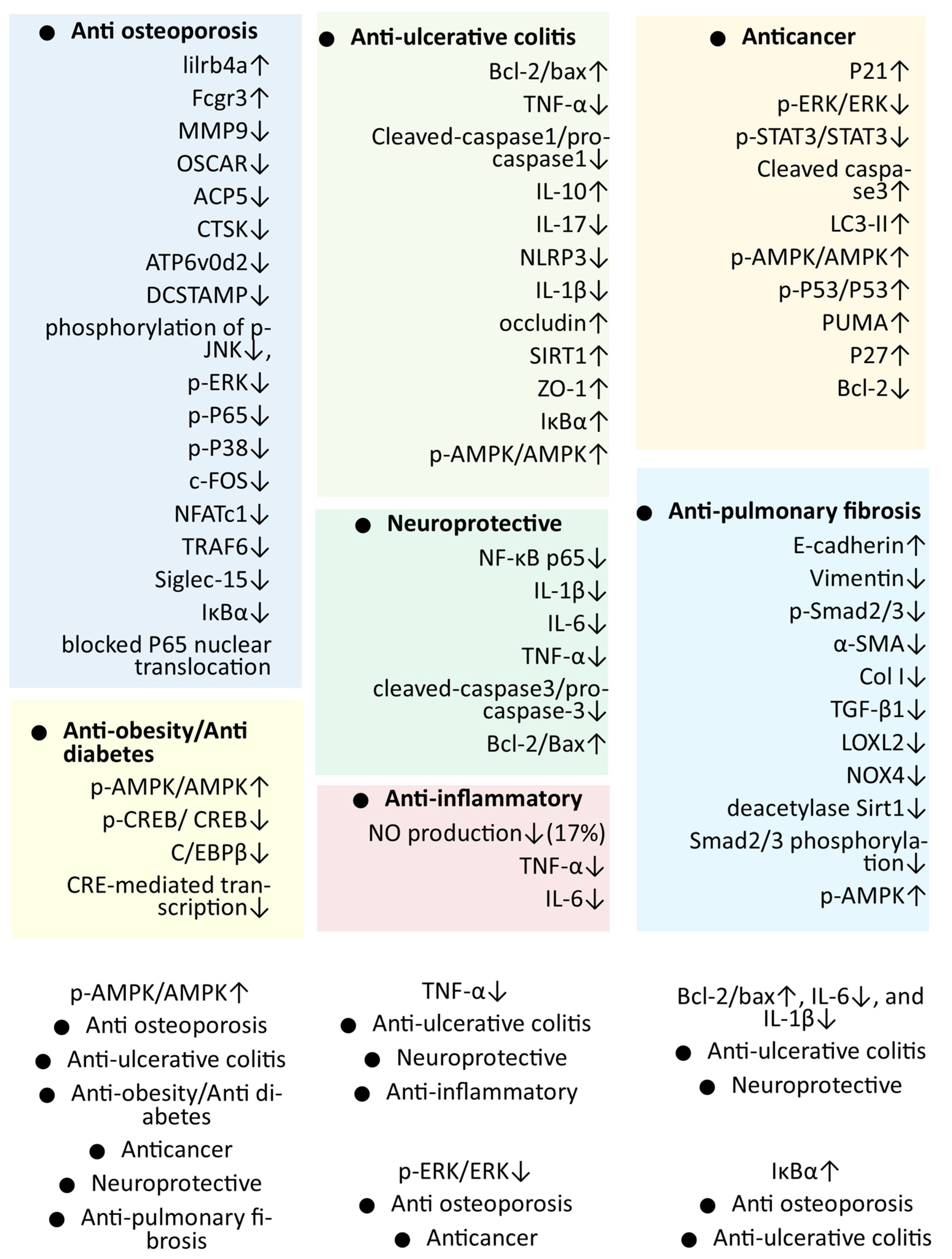
| Activity (and Probable Mechanism) | Dose | Method | Result | Ref. |
|---|---|---|---|---|
| Antiobesity/antidiabetes (through reducing CREB activation and promoting AMPK activation) | 1, 10, and 20 μM | HepG2 cell line AMPK activator (phosphorylation of AMPK) | p-AMPK/AMPK ↑ more efficiently than berberine | [6] |
| 1, 5, 10, and 20 μM | 3T3-L1 cells Oil red O staining and classical Folch method | At 20 μM lipid accumulation ↓ and cellular TG levels ↓ | [4] | |
| 20 μM | WB | p-AMPK/AMPK ↑ and p-CREB/CREB ↓ | ||
| 20 μM | Real-time PCR | C/EBPβ ↓ | ||
| 20 μM | Reporter assay | CRE-mediated transcription ↓ | ||
| 0.625–40 μM | AMPK activations through HTRF assay | A769662 (EC50 of 24.9 nM) and AMP (EC50 of 1.4 nM) EC50 of 4.2 μM. | [17] | |
| mogrol | HepG2 cells AMPK allosteric activator screening based on SPA assay | EC50 = 3.0 ± 0.20 for mogrol and 1.4 ± 0.10 μM for AMP | [23] | |
| Anti-inflammatory (inhibition of TNF-α mediated inflammation) | 10 μM of mogrol | RAW 264.7 cells NO production | NO production ↓ (17%) | [7] |
| 10 μM of mogrol | ELISA | The levels of TNF-α ↓ and IL-6 ↓ | ||
| Anticancer (autophagy and autophagic cell death via activating AMPK signaling pathway, ERK, and STAT3 inhibition) | 0.1, 1, 10, 100, and 250 μM | Cell proliferation (K562 cells) study through MTT assay | Growth inhibition 88% at 250 μM. | [32] |
| WB | P21 ↑, Bcl2 ↓, p-ERK/ERK ↓, and p-STAT3/STAT3 ↓ | |||
| 50 and 100 μM | The A549 and CNE1 cell lines MTT assay | IC50 (μM) 89.51 ± 3.95 (A549) 81.48 ± 4.73 (CNE1) | [33] | |
| 30 μM | CCK8 against lung cancer cell lines (A549 and NCI-H460) | IC50 (μM) 27.78 ± 0.98 (A549) >100 (NCI-H460) | [34] | |
| A549, NCI-H460, H1299 and H1975 | IC50 (μM) 28.56 ± 1.98 (A549) >100 (NCI-H460) >100 (H1299) 87.14 ± 2.56 (H1975) | [7] | ||
| A549, NCI-H460, and CNE1 CCK8 assay | IC50 (μM) 27.78 ± 0.98 (A549) >100 (NCI-H460) >100 (CNE1) | [35] | ||
| 50 μM | A549, H1299, H1975, and SK-MES-1 analyzed through CCK-8 kit and celigo cell counter | IC50 (μM) <25 μM | [36] | |
| 50 μM | Inverted confocal microscopy and surface area were measured by ImageJ | Surface area ↓ and the number of lung cancer ↓ in A549, H1299, and SK-MES-1 cells | ||
| 50 μM | Scratch-wound migration assay | Migration of all studied cells ↓ | ||
| 50 μM | WB | LC3-II ↑ | ||
| 50 μM | Adenovirus expressing fluorescent-mRFP-GFP-LC3 | Autophagosomes ↑, as well as autolysosome ↑ | ||
| 50 μM | A549 and SK-MES-1 cells WB | Cleaved caspase3 ↑, LC3-II ↑, p-AMPK/AMPK ↑, p-P53/P53 ↑, PUMA ↑, p21 ↑, p27 ↑, and Bcl-2 ↓ | ||
| Anti-colitis (through promoting AMPK activation and inhibition of TNF-α mediated inflammation) | pre-treated with mogrol (1 or 10 μM) | NCM460 human intestinal epithelial cells stimulated through TNF-α (metformin (the positive control drug), 2 mM used as control in the study) | Occludin ↑, ZO-1 ↑, bcl-2/bax ↑, TNF-α ↓, and p-AMPK/AMPK ↑ at (10 μM of mogrol) | [37] |
| 10 μM | THP-1 cells were stimulated by PMA to macrophages and stimulated by LPS and ATP (metformin as positive control drug used in the study) | Cleaved-caspase1/pro-caspase1 ↓, and IL-1β ↓ | ||
| Antifibrotic activity (through activation of AMPK-mediated signaling pathways and inhibition of NF-κB signaling pathways) | 1, 5, and 10 μM of mogrol | TGF-β1-treated mouse type II alveolar epithelial cells (MLE-12 cell line) Expression analysis through WB | E-cadherin ↑, α-SMA ↓, type Col I ↓, and Vimentin ↓ | [26] |
| 1, 5, and 10 μM of mogrol | PLFs cells expression analysis through WB | α-SMA ↓, (LOXL2 ↓), collagen I ↓, and phosphorylation forms of Smad2/3 ↓ and increased p-AMPK ↑ | ||
| 10 μM of mogrol | The protein expression was measured by immunofluorescence staining | NOX4 ↓ protein expression in TGF-β1-treated PLFs | ||
| Anti-osteoporosis (inhibition of TRAF6/MAPK/NF-κB signaling pathway) | (0, 5, 10, 20 μM) | BMM stimulated with RANKL | Osteoclasts ↓ (236.67 ± 37.07 to 20.0 ± 6.08) at 20 μM mogrol. | [25] |
| 0, 5, 10, 20 μM | Bone resorption in bovine bone slices through SEM | Bone resorption ↓ | ||
| 20 μM | RNA-Seq | lilrb4a ↑ and Fcgr3 ↑ osteoclastogenesis suppressive genes) MMP9 ↓, OSCAR ↓, and ACP5 ↓ (osteoclastogenesis genes) | ||
| 20 μM | RT–PCR was utilized to confirm osteoclastogenesis marker genes expression | MMP9 ↓, CTSK ↓, ATP6v0d2 ↓, ACP5 ↓, and DCSTAMP ↓ | ||
| 20 μM | WB | Phosphorylation of JNK ↓, ERK ↓, P65 ↓, and P38 ↓ Expression of c-FOS ↓, NFATc1 ↓, TRAF6 ↓, and Siglec-15 ↓ Suppressed the degradation of IκBα ↓ | ||
| 20 μM | Immunofluorescence staining | Mogrol efficiently blocked P65 nuclear translocation, which is required for NF-κB activation |
| Activity (and Probable Mechanism) | Model | Dose | Method | Results | Ref. |
|---|---|---|---|---|---|
| Anticancer (activating AMPK signaling and p53 pathway) | Male thymus-deficient mice (BALB/C) | 10 mg/Kg three times a week for 2 weeks | Mogrol injected intraperitoneally into the mice | The weight and volume of tumor decreased (by 69.18% and 66.22%, respectively | [36] |
| Anti-UC (through promoting AMPK activation and inhibition of inflammation) | Female C57BL/6 mice DSS-induced mouse UC model | Mogrol (1 or 5 mg/kg) for 7 days orally | Weight monitoring, DAI, and colon length calculation (metformin as positive control drug used in the study) | Weight loss ↓, DAI ↓, and colon shortening ↓ | [37] |
| Histopathological examination (metformin as positive control drug used in the study) | Inflammatory infiltration in colonic tissues ↓ | ||||
| RT-PCR | IL-10 ↑, IL-17 ↓, NLRP3 ↓, IL-1β ↓, occludin ↑, SIRT1 ↑, and ZO-1 ↑ | ||||
| WB | IκBα ↑, occludin ↑, ZO-1 ↑, and p-AMPK/AMPK ↑ | ||||
| Anti-PF (through activation of AMPK-mediated signaling pathways and inhibition of NF-κB signaling pathways) | Male C57BL/6 mice | 1, 5, and 10 mg/kg | The lung was used for measuring pulmonary index | Positive drug nintedanib Pulmonary index ↓ and weight loss ↓ | [26] |
| 1, 5, and 10 mg/kg | Histology of lungs (Nintedanib used as positive drug group) | Alveolar wall thickening ↓, neutrophil infiltration ↓, edema ↓, high collagen production ↓, area of collagen fibrils ↓ and inflammatory degree ↓ | |||
| 1, 5, and 10 mg/kg | Expression analysis of lung tissues through RT-PCR and WB | α-SMA ↓, Col I ↓, and TGF-β1 ↓ | |||
| 10 μM of mogrol | Expression analysis in lung tissues through WB | LOXL2 ↓, NOX4 ↓, deacetylase Sirt1 ↓, Smad2/3 phosphorylation ↓, and p-AMPK ↑ in lung tissues | |||
| Antiosteoporosis (inhibition of TRAF6/MAPK/NF-κB signaling pathway) | Female C5BL/6J mice ovaries were removed | 10 mg/kg mogrol intraperitoneally every second day for 42 days | Micro-CT 3D reconstructions indicated that mogrol decreased bone loss of femurs in OVX mice | BV/TV ↑, Tb. Th ↑, Tb. N ↑ and Cs. Th ↑ of the mogrol group were considerably higher than the vehicle group; BS/BV ↓ was inversely lower | [25] |
| Histological assessment | BV/TV ↑ of the mogrol group was dramatically higher than that of the vehicle group | ||||
| Immunohistochemistry TRAP staining of femurs | Oc. S/BS ↓ and N. Oc/B ↓ of the mogrol group were substantially lower than those of the vehicle group | ||||
| Neuroprotective (suppression of NF-κB mediated inflammation) | Male ICR mice AB-induced memory impairment | 20, 40, 80 mg/kg intragastric administration | Morris water maze test and Y-maze test (2 mg/kg donepezil as positive control drug used in the study) | At all doses, significantly improved memory impairment | [41] |
| Immunohistochemical analyses (donepezil as positive control drug used in the study) | Iba1-positive cells↓ | ||||
| Hoechst assay | Number of Hoechst-positive cells of the DG ↓ | ||||
| WB | NF-κB p65 ↓ IL-1β ↓, IL-6 ↓, TNF-α ↓, cleaved-caspase3/pro-caspase-3 ↓ and the Bcl-2/Bax ↑ | ||||
| Male ICR mice LPS-induced memory impairment | 20, 40, 80 mg/kg intragastric administration | Morris water maze test, Y-maze test, and novel object recognition test | In all the tests, mogrol improved memory impairment | [42] | |
| Immunohistochemical analyses | Iba1-positive cells ↓ | ||||
| WB of mouse in the mouse hippocampus and frontal cortex | NF-κB p65 ↓ IL-1β ↓, IL-6 ↓, and TNF-α ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, V.; Lee, H.-J. Pharmacological Activities of Mogrol: Potential Phytochemical against Different Diseases. Life 2023, 13, 555. https://doi.org/10.3390/life13020555
Jaiswal V, Lee H-J. Pharmacological Activities of Mogrol: Potential Phytochemical against Different Diseases. Life. 2023; 13(2):555. https://doi.org/10.3390/life13020555
Chicago/Turabian StyleJaiswal, Varun, and Hae-Jeung Lee. 2023. "Pharmacological Activities of Mogrol: Potential Phytochemical against Different Diseases" Life 13, no. 2: 555. https://doi.org/10.3390/life13020555
APA StyleJaiswal, V., & Lee, H. -J. (2023). Pharmacological Activities of Mogrol: Potential Phytochemical against Different Diseases. Life, 13(2), 555. https://doi.org/10.3390/life13020555









