Therapeutic Potential of Gut Microbiota and Its Metabolite Short-Chain Fatty Acids in Neonatal Necrotizing Enterocolitis
Abstract
:1. Introduction
2. Methods
3. Epigenetics and Inflammatory Biomarkers in NEC
4. Insights into the SCFA-Producing Bacteria in Preterm Infants
5. Effects of Feeding Types on Gut Microbiota and Its Metabolite SCFAs in Preterm Infants
5.1. Prospective Cohort Studies
5.2. RCTs
6. Role of Gut Microbiota and Its Metabolite SCFAs as Therapeutic Potential Agents in NEC
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015, 17, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Mesa, M.D.; Loureiro, B.; Iglesia, I.; Fernandez Gonzalez, S.; Olivé, E.L.; Garcia-Algar, O.; Solana, M.J.; Perez, M.J.C.; Sainz, T.; Martinez, L.; et al. The evolving microbiome from pregnancy to early infancy: A comprehensive review. Nutrients 2020, 12, 133. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The role of microbiota in infant health: From early life to adulthood. Front. Immunol. 2021, 12, 708472. [Google Scholar] [CrossRef]
- Oyedemi, O.T.; Shaw, S.; Martin, J.C.; Ayeni, F.A.; Scott, K.P. Changes in the gut microbiota of Nigerian infants within the first year of life. PLoS ONE 2022, 17, e0265123. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; Collado, M.C.; Wopereis, H.; Salminen, S.; Knol, J.; Roeselers, G. The bifidogenic effect revisited-ecology and health perspectives of bifidobacterial colonization in early life. Microorganisms 2020, 8, 1855. [Google Scholar] [CrossRef]
- Stuivenberg, G.A.; Burton, J.P.; Bron, P.A.; Reid, G. Why are bifidobacteria important for infants? Microorganisms 2022, 10, 278. [Google Scholar] [CrossRef]
- Di Profio, E.; Magenes, V.C.; Fiore, G.; Agostinelli, M.; La Mendola, A.; Acunzo, M.; Francavilla, R.; Indrio, F.; Bosetti, A.; D’Auria, N.; et al. Special diets in infants and children and impact on gut microbioma. Nutrients 2022, 14, 3198. [Google Scholar] [CrossRef]
- Lin, C.; Lin, Y.; Zhang, H.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Intestinal ‘infant-type’ bifidobacteria mediate immune system development in the first 1000 days of life. Nutrients 2022, 14, 1498. [Google Scholar] [CrossRef]
- Gregory, K.E.; Deforge, C.E.; Natale, K.M.; Phillips, M.; Van Marter, L.J. Necrotizing enterocolitis in the premature infant: Neonatal nursing assessment, disease pathogenesis, and clinical presentation. Adv. Neonatal Care 2011, 11, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.K.; Shah, J.S. Necrotizing enterocolitis in very low birth weight infants: A systemic review. ISRN Gastroenterol. 2012, 2012, 562594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, A.T.; Patel, R.M. A critical analysis of risk factors for NEC. Semin. Fetal Neonatal Med. 2018, 23, 374–379. [Google Scholar] [CrossRef]
- Torrazza, R.M.; Ukhanova, M.; Wang, X.; Sharma, R.; Hudak, M.L.; Neu, J.; Mai, V. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PLoS ONE 2013, 8, e83304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, S.; Stintzi, A.; Shah, P.; Mack, D.; O’Connor, D.L. Gut microbiota of the very-low-birth-weight infant. Pediatr. Res. 2015, 77, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Underwood, M.A.; Mukhopadhyay, S.; Lakshminrusimha, S.; Bevins, C.L. Neonatal intestinal dysbiosis. J. Perinatol. 2020, 40, 1597–1608. [Google Scholar] [CrossRef]
- He, Y.; Du, W.; Xiao, S.; Zeng, B.; She, X.; Liu, D.; Du, H.; Li, L.; Li, F.; Ai, Q.; et al. Colonization of fecal microbiota from patients with neonatal necrotizing enterocolitis exacerbates intestinal injury in germfree mice subjected to necrotizing enterocolitis-induction protocol via alterations in butyrate and regulatory T cells. J. Transl. Med. 2021, 19, 510. [Google Scholar] [CrossRef]
- Liu, X.-C.; Du, T.-T.; Gao, X.; Zhao, W.-J.; Wang, Z.-L.; He, Y.; Bao, L.; Li, L.-Q. Gut microbiota and short-chain fatty acids may be new biomarkers for predicting neonatal necrotizing enterocolitis: A pilot study. Front. Microbiol. 2022, 13, 969656. [Google Scholar] [CrossRef]
- Lemme-Dumit, J.M.; Song, Y.; Lwin, H.W.; Hernandez-Chavez, C.; Sundararajan, S.; Viscardi, R.M.; Ravel, J.; Pasetti, M.F.; Ma, B. Altered gut microbiome and fecal immune phenotype in early preterm infants with leaky gut. Front. Immunol. 2022, 13, 815046. [Google Scholar] [CrossRef]
- Alsharairi, N.A. The infant gut microbiota and risk of asthma: The effect of maternal nutrition during pregnancy and lactation. Microorganisms 2020, 8, 1119. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. The role of short-chain fatty acids in mediating very low-calorie ketogenic diet-infant gut microbiota relationships and its therapeutic potential in obesity. Nutrients 2021, 13, 3702. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. The role of short-chain fatty acids in the interplay between a very low-calorie ketogenic diet and the infant gut microbiota and its therapeutic implications for reducing asthma. Int. J. Mol. Sci. 2020, 21, 9580. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Gao, Y.; Zhu, W.; Meng, D.; Walker, W.A. Short chain fatty acids produced by colonizing intestinal commensal bacterial interaction with expressed breast milk are anti-inflammatory in human immature enterocytes. PLoS ONE 2020, 15, e0229283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziętek, M.; Celewicz, Z.; Szczuko, M. Short-chain fatty acids, maternal microbiota and metabolism in pregnancy. Nutrients 2021, 13, 1244. [Google Scholar] [CrossRef] [PubMed]
- Łoniewski, I.; Skonieczna-Żydecka, K.; Stachowska, L.; Fraszczyk-Tousty, M.; Tousty, P.; Łoniewska, B. Breastfeeding affects concentration of faecal short chain fatty acids during the first year of life: Results of the systematic review and meta-analysis. Front. Nutr. 2022, 9, 939194. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Toldi, G. The impact of short-chain fatty acids on neonatal regulatory T cells. Nutrients 2022, 14, 3670. [Google Scholar] [CrossRef]
- Guo, J.; Ren, C.; Han, X.; Huang, W.; You, Y.; Zhan, J. Role of IgA in the early-life establishment of the gut microbiota and immunity: Implications for constructing a healthy start. Gut Microbes. 2021, 13, 1908101. [Google Scholar] [CrossRef]
- McKeen, S.; Young, W.; Mullaney, J.; Fraser, K.; McNabb, W.C.; Roy, N.C. Infant complementary feeding of prebiotics for the microbiome and immunity. Nutrients 2019, 11, 364. [Google Scholar] [CrossRef] [Green Version]
- Differding, M.K.; Benjamin-Neelon, S.E.; Hoyo, C.; Østbye, T.; Mueller, N.T. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. 2020, 20, 56. [Google Scholar] [CrossRef] [Green Version]
- Fabiano, V.; Indrio, F.; Verduci, E.; Calcaterra, V.; Pop, T.L.; Mari, A.; Zuccotti, G.V.; Cokugras, F.C.; Pettoello-Mantovani, M.; Goulet, O. Term infant formulas influencing gut microbiota: An overview. Nutrients 2021, 13, 4200. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.; Lyttle, A.; Amin, H.; Shaireen, H.; Robertson, H.L.; Lodha, A.K. Arginine supplementation in prevention of necrotizing enterocolitis in the premature infant: An updated systematic review. BMC Pediatr. 2014, 14, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.S.; Shah, V.S.; Kelly, L.E. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2017, 4, CD004339. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2020, 3, CD007137. [Google Scholar] [CrossRef]
- Al-Alaiyan, S.; Abdulaziz, N.; Alkohlani, A.; Almairi, S.O.; Al Hazzani, F.; Binmanee, A.; Alfattani, A. Effects of probiotics and lactoferrin on necrotizing enterocolitis in preterm infants. Cureus 2021, 13, e18256. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Y.; Ma, L.-Y.; Lin, H.-C. The role of immunonutrients in the prevention of necrotizing enterocolitis in preterm very low birth weight infants. Nutrients 2015, 7, 7256–7270. [Google Scholar] [CrossRef]
- Johnson-Henry, K.C.; Abrahamsson, T.R.; Wu, R.Y.; Sherman, P.M. Probiotics, prebiotics, and synbiotics for the prevention of necrotizing enterocolitis. Adv. Nutr. 2016, 7, 928–937. [Google Scholar] [CrossRef] [Green Version]
- Nolan, L.S.; Rimer, J.M.; Good, M. The role of human milk oligosaccharides and probiotics on the neonatal microbiome and risk of necrotizing enterocolitis: A narrative review. Nutrients 2020, 12, 3052. [Google Scholar] [CrossRef]
- Campos-Martinez, A.M.; Expósito-Herrera, J.; Gonzalez-Bolívar, M.; Fernández-Marin, E.; Uberos, J. Evaluation of risk and preventive factors for necrotizing enterocolitis in premature newborns. A systematic review of the literature. Front. Pediatr. 2022, 10, 874976. [Google Scholar] [CrossRef]
- Xu, W.; Judge, M.P.; Maas, K.; Hussain, N.; McGrath, J.M.; Henderson, W.A.; Cong, X. Systematic review of the effect of enteral feeding on gut microbiota in preterm infants. J. Obstet. Gynecol. Neonatal Nurs. 2018, 47, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.A.; Baumgartel, K.; Morowitz, M.J.; Giangrasso, V.; Demirci, J.R. The role of human milk in decreasing necrotizing enterocolitis through modulation of the infant gut microbiome: A scoping review. J. Hum. Lact. 2020, 36, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. The therapeutic role of short-chain fatty acids mediated very low-calorie ketogenic diet-gut microbiota relationships in paediatric inflammatory bowel diseases. Nutrients 2022, 14, 4113. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Claud, E.C. Intrauterine inflammation, epigenetics, and microbiome influences on preterm infant health. Curr. Pathobiol. Rep. 2018, 6, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, J.; Maheshwari, A. Epigenetics in necrotizing enterocolitis. Curr. Pediatr. Rev. 2021, 17, 172–184. [Google Scholar] [CrossRef]
- Good, M.; Chu, T.; Shaw, P.; McClain, L.; Chamberlain, A.; Castro, C.; Rimer, J.M.; Mihi, B.; Gong, Q.; Nolan, L.S.; et al. Global hypermethylation of intestinal epithelial cells is a hallmark feature of neonatal surgical necrotizing enterocolitis. Clin. Epigenet. 2020, 12, 190. [Google Scholar] [CrossRef]
- Good, M.; Chu, T.; Shaw, P.; Nolan, L.S.; McClain, L.; Chamberlain, A.; Castro, C.; Gong, Q.; Cooksey, K.; Linneman, L.; et al. Neonatal necrotizing enterocolitis-associated DNA methylation signatures in the colon are evident in stool samples of affected individuals. Epigenomics 2021, 13, 829–844. [Google Scholar] [CrossRef]
- Good, M.; Chu, T.; Shaw, P.; Nolan, L.S.; Wrobleski, J.; Castro, C.; Gong, Q.; DeWitt, O.; Finegold, D.N.; Peters, D. Selective hypermethylation is evident in small intestine samples from infants with necrotizing enterocolitis. Clin. Epigenet. 2022, 14, 49. [Google Scholar] [CrossRef]
- Lu, L.; Fan, J.; Xu, W.; Cui, X.; Hu, S.; Guo, T.; Lv, Z. DNA methylome mapping identifies epigenetic abnormalities in intestinal lymphocyte regulation in human necrotizing enterocolitis. Dig. Dis. Sci. 2022, 67, 4434–4443. [Google Scholar] [CrossRef]
- Klerk, D.H.; Plösch, T.; Verkaik-Schakel, R.N.; Hulscher, J.B.F.; Kooi, E.M.W.; Bos, A.F. DNA methylation of TLR4, VEGFA, and DEFA5 is associated with necrotizing enterocolitis in preterm infants. Front. Pediatr. 2021, 9, 630817. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Chan, K.Y.Y.; Leung, K.T.; Lam, H.S.; Tam, Y.H.; Lee, K.H.; Li, K.; Ng, P.C. Dysregulation of miR-431 and target gene FOXA1 in intestinal tissues of infants with necrotizing enterocolitis. FASEB J. 2019, 33, 5143–5152. [Google Scholar] [CrossRef]
- Chen, W.; Yan, X.; Tian, T.; Yan, R.; Wang, X.; Yu, Z.; Li, Y.; Zhang, L.; Han, S. Integrated analysis of a lncRNA-mRNA network reveals a potential mechanism underlying necrotizing enterocolitis. Mol. Med. Rep. 2020, 22, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, C.J.; Upperman, J.S.; Ford, H.R.; Camerini, V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr. Res. 2008, 63, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.L.; Jilling, T.; Caplan, M.S. The importance of pro-inflammatory signaling in neonatal NEC. Semin. Perinatol. 2008, 32, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Plaen, I.G. Inflammatory signaling in Necrotizing enterocolitis. Clin. Perinatol. 2013, 40, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Hunter, C.J.; De Plaen, I.G. Inflammatory signaling in NEC. Role of NFKB and cytokines. Pathophysiology 2014, 21, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Nino, D.F.; Sodhi, C.P.; Hackam, D.J. Necrotizing enterocolitis: New insights into pathogenesis and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Mohankumar, K.; Namachivayam, K.; Ho, T.T.; Torres, B.A.; Ohls, R.K.; Maheshwari, A. Cytokines and growth factors in the developing intestine and during necrotizing enterocolitis. Semin. Perinatol. 2017, 41, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Niemarkt, H.J.; De Meij, T.G.; van Ganzewinkel, C.-J.; de Boer, N.K.H.; Andriessen, P.; Hütten, M.C.; Kramer, B.W. Necrotizing enterocolitis, gut microbiota, and brain development: Role of the brain-gut axis. Neonatology 2019, 115, 423–431. [Google Scholar] [CrossRef]
- Nanthakumar, N.; Meng, D.; Goldstein, A.M.; Zhu, W.; Lu, L.; Uauy, R.; Llanos, A.; Claud, E.C.; and Walker, W.A. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: An immature innate immune response. PLoS ONE 2011, 6, e17776. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.X.; Rudloff, I.; Lao, J.C.; Pang, M.A.; Goldberg, R.; Bui, C.B.; McLean, C.A.; Stock, M.; Klassert, T.E.; Slevogt, H.; et al. Characterization of the pathoimmunology of necrotizing enterocolitis reveals novel therapeutic opportunities. Nat. Commun. 2020, 11, 5794. [Google Scholar] [CrossRef]
- Tremblay, E.; Ferretti, E.; Babakissa, C.; Burghardt, K.M.; Levy, E.; Beaulieu, J.-F. IL-17-related signature genes linked to human necrotizing enterocolitis. BMC Res. Notes 2021, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sheng, L. Significance of dynamic evolution of TNF-α, IL-6 and intestinal fatty acid-binding protein levels in neonatal necrotizing enterocolitis. Exp. Ther. Med. 2018, 15, 1289–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J. Too much short chain fatty acids cause neonatal necrotizing enterocolitis. Med. Hypotheses 2004, 62, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Waligora-Dupriet, A.-J.; Dugay, A.; Auzeil, N.; Huerre, M.; Butel, M.J. Evidence for clostridial implication in necrotizing enterocolitis through bacterial fermentation in a gnotobiotic quail model. Pediatr. Res. 2005, 58, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Waligora-Dupriet, A.J.; Dugay, A.; Auzeil, N.; Nicolis, I.; Rabot, S.; Huerre, M.R.; Butel, M.J. Short-chain fatty acids and polyamines in the pathogenesis of necrotizing enterocolitis: Kinetics aspects in gnotobiotic quails. Anaerobe 2009, 15, 138–144. [Google Scholar] [CrossRef]
- Rajili’c-Stojanovi´c, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Wong, C.B.; Odamaki, T.; Xiao, J. Insights into the reason of Human-Residential Bifidobacteria (HRB) being the natural inhabitants of the human gut and their potential health-promoting benefits. FEMS Microbiol. Rev. 2020, 44, 369–385. [Google Scholar] [CrossRef]
- Kozak, K.; Charbonneau, D.; Sanozky-Dawes, R.; Klaenhammer, T. Characterization of bacterial isolates from the microbiota of mothers breast milk and their infants. Gut Microbes 2015, 6, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Solis, G.; Delosreyes-Gavilan, C.G.; Fernandez, N.; Margolles, A.; Gueimonde, M. Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast-milk and the infant gut. Anaerobe 2010, 16, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Kitaoka, M. Bifidobacterial enzymes involved in the metabolism of human milk oligosaccharides. Adv. Nutr. 2012, 3, 422S–429S. [Google Scholar] [CrossRef] [Green Version]
- Pokusaeva, K.; Fitzgerald, G.F.; Van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OCaliaghan, A.; Van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Moyano, S.; Totten, S.M.; Garrido, D.A.; Smilowitz, J.T.; German, J.B.; Lebrilia, C.B.; Mills, D.A. Variation in consumption of human milk oligosaccharides by infant gut-associated srains of bifidobacterium breve. Appl. Envion. Microbiol. 2013, 79, 6040–6049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotoh, A.; Katoh, T.; Sakanaka, M.; Ling, Y.; Yamada, C.; Asakuma, S.; Urashima, T.; Tomabechi, Y.; Katayama-Ikegami, A.; Kurihara, S.; et al. Sharing of human milk oligosaccharides degrades within bifodobacterial communities in faecal cultures supplemented with bifidobacterium. Sci. Rep. 2018, 8, 13958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuniga, M.; Monedero, V.; Yebra, M.J. Utilization of host-derived glycans by intestinal lactobacillus and bifidobacterium species. Front. Microbiol. 2018, 9, 1917. [Google Scholar] [CrossRef] [Green Version]
- Barile, D.; Rastall, R.A. Human milk and related oligosaccharides as prebiotics. Curr. Opin. Biotechnol. 2013, 19, 9–16. [Google Scholar] [CrossRef]
- Arboleya, S.; Salazar, N.; Solís, G.; Fernández, N.; Hernández-Barranco, A.M.; Cuesta, I.; Gueimonde, M.; de los Reyes-Gavilán, C.G. Assessment of intestinal microbiota modulation ability of Bifidobacterium strains in in vitro fecal batch cultures from preterm neonates. Anaerobe 2013, 19, 9–16. [Google Scholar] [CrossRef]
- Gopalsamy, G.; Mortimer, E.; Greenfield, P.; Bird, A.R.; Young, G.P.; Christophersen, C.T. Resistant starch is actively fermented by infant faecal microbiota and increases microbial diversity. Nutrients 2019, 11, 1345. [Google Scholar] [CrossRef] [Green Version]
- Bergström, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Mølgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of intestinal microbiota during early life: A longitudinal, explorative study of a large cohort of Danish infants. Appl. Environ. Microbiol. 2014, 80, 2889–2900. [Google Scholar] [CrossRef] [Green Version]
- Moles, L.; Gómez, M.; Heilig, H.; Bustos, G.; Fuentes, S.; de Vos, W.; Fernández, L.; Rodríguez, J.M.; Jiménez, E. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS ONE 2013, 8, e66986. [Google Scholar] [CrossRef] [Green Version]
- Morais, J.; Marques, C.; Teixeira, D.; Durão, C.; Faria, A.; Brito, S.; Cardoso, M.; Macedo, I.; Pereira, E.; Tomé, T.; et al. Extremely preterm neonates have more Lactobacillus in meconium than very preterm neonates—The in utero microbial colonization hypothesis. Gut Microbes 2020, 12, 1785804. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, E.; Fernandez, L.; Maldondado, A.; Martin, R.; Olivares, M.; Xaus, J.; Rodriguez, J.M. Oral administration of lactobacillus strains isolated from breast milk as an alternative for the treatment of infectious mastitis during lactation. Appl. Environ. Microbiol. 2008, 74, 4650–4655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, A.; Martin, V.; Jimenez, E.; Mader, I.; Rodtiguez, J.M.; Fernandez, L. Lacobacilli and bifidobacteria in human breast milk: Influence of antibiotherapy and other host clinical factors. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 78–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirtzalidou, E.; Pramateftaki, P.; Kotsou, M.; Kyriacou, A. Screening for lactobacilli with probiotic properties in the infant gut microbiota. Anaerobe 2011, 17, 440–443. [Google Scholar] [CrossRef]
- Ahrne, S.; Lonnermark, E.; Wold, A.E.; Aberg, N.; Hesselmar, B.; Saalman, R.; Strannegard, I.; Molin, G.; Adlerberth, I. Lcatobacilli in the intestinal microbiota of Swedish infants. Microbes Infect. 2005, 7, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Coulson, S.; Thomsen, M.; Nguyen, T.; Hall, S. Probiotics D-lactic acidosis, oxidative stress and strain specificity. Gut Microbes 2017, 8, 311–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Markowiak-kopec, P.; Slizewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [Green Version]
- Wandro, S.; Osborne, S.; Enriquez, C.; Bixby, C.; Arrieta, A.; Whiteson, K. The microbiome and metabolome of preterm infant stool are personalized and not driven by health outcomes, including necrotizing enterocolitis and late-onset sepsis. mSphere 2018, 3, e00104-18. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Yu, X.; Chang, Y.; You, Y.; Chen, Z.; Wang, Y.; Liu, B.; Chen, L.; Ma, D.; Xing, Y.; et al. Dynamic changes of the gut microbiota in preterm infants with different gestational age. Front. Microbiol. 2022, 13, 923273. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Chen, C.; Newburg, D.S. Utilization of major fucosylated and sialylated human milk oligosaccharides isolated human gut microbes. Glycobiology 2013, 23, 1281–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.K.; Kumar, S.S.; Ghosh, A.R. Probiotics enterococcus faecalis effectively assimilates cholesterol and produces fatty acids including propionate. FEMS Microbiol. Lett. 2019, 366, FNZ039. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhi, X.; Li, H.; Klenk, H.; Li, W. Comparative genomics of the bacterial genus Streptococcus illuminates evolutionary implications of species groups. PLoS ONE 2014, 9, e101229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Gregory, K.E.; LaPlante, R.D.; Shan, G.; Kumar, D.V.; Gregas, M. Mode of birth influences preterm infant intestinal colonization with bacteroides over the early neonatal period. Adv. Neonatal Care 2015, 15, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.; Firek, B.A.; Miller, C.S.; Sharon, I.; Thomas, B.C.; Baker, R.; Morowitz, M.J.; Banfield, J.F. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome 2014, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Yap, P.S.X.; Chong, C.W.; Kamar, A.A.; Yap, I.K.S.; Choo, Y.M.; Lai, N.M.; Teh, C.S.J. Neonatal intensive care unit (NICU) exposures exert a sustained influence on the progression of gut microbiota and metabolome in the first year of life. Sci. Rep. 2021, 11, 1353. [Google Scholar] [CrossRef]
- Lawley, B.; Otal, A.; Moloney-Geany, K.; Diana, A.; Houghton, L.; Hearth, A.M.; Taylor, R.W.; Tannock, G.W. Fecal microbiotas of Indonesian and New Zealand children differ in complexity and bifidobacteria taxa during the first year of life. Appl. Environ. Microbiol. 2019, 85, e01105-19. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, M.; Wu, S.; Lebrilia, C.B.; Chapkin, R.S.; Ivanov, I.; Donovan, S.M. Fecal microbiota composition breast-fed infants is correlated with human milk oligosaccharides consumed. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Tannock, G.W.; Lawley, B.; Munro, K.; Pathmanathan, S.G.; Zhou, S.J.; Makrides, M.; Gibson, R.A.; Sullivan, T.; Prosser, C.G.; Lowery, D. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl. Environ. Microbiol. 2013, 79, 3040–3048. [Google Scholar] [CrossRef] [Green Version]
- Talford, L.E.; Crost, E.H.; Kavanaugh, D.; Juge, N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 2005, 6, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcobal, A.; Barboza, M.; Froehlich, J.W. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcobal, A.; Barboza, M.; Sonnenburg, E.D.; Pudlo, N.; Martens, E.C.; Desal, P.; Lebrilia, C.B.; Weimer, B.C.; Mills, D.A.; German, J.B.; et al. Bacteroides in the infant gut consume milk oligosaccharidsvia mucus-utilization pathways. Cell Host Microbe 2011, 10, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcobal, A.; Sonnenburg, J.L. Human milk oligosaccharide consumption by intestinal microbiota. Clin. Microbiol. Infect. 2012, 18, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Feichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; Mcwilliam, L.C.; Scott, K.P.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [Green Version]
- Rios-Covian, D.; Salazar, N.; Gueimonde, M.G.; De Los Reyes-Gavilan, C. Shaping the metabolism of intestinal bacteriods population through diet to improve human health. Front. Microbiol. 2017, 8, 376. [Google Scholar] [CrossRef] [Green Version]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbioame major fermentation by-products and their impact on host health. Microbiome 2018, 7, 91. [Google Scholar] [CrossRef]
- Pourcyrous, M.; Nolan, V.G.; Goodwin, A.; Davis, S.L.; Buddington, R.K. Fecal short-chain fatty acids of very-low-birth-weight preterm infants fed expressed breast milk or formula. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 725–731. [Google Scholar] [CrossRef]
- Chen, C.; Yin, Q.; Wu, H.; Cheng, L.; Kwon, J.-I.; Jin, J.; Han, T.; Che, H. Different effects of premature infant formula and breast milk on intestinal microecological development in premature infants. Front. Microbiol. 2019, 10, 3020. [Google Scholar] [CrossRef]
- Alcon-Giner, C.; Dalby, M.J.; Caim, S.; Ketskemety, J.; Shaw, A.; Sim, K.; Lawson, M.A.E.; Kiu, R.; Leclaire, C.; Chalklen, L.; et al. Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome: An observational study. Cell Rep. Med. 2020, 1, 100077. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.C.; Masi, A.C.; Young, G.R.; Vatanen, T.; Lamb, C.A.; Smith, R.; Coxhead, J.; Butler, A.; Marsland, B.J.; Embleton, N.D.; et al. Strain-specific impacts of probiotics are a significant driver of gut microbiome development in very preterm infants. Nat. Microbiol. 2022, 7, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Westaway, J.A.F.; Huerlimann, R.; Kandasamy, Y.; Miller, C.M.; Norton, R.; Staunton, K.M.; Watson, D.; Rudd, D. The bacterial gut microbiome of probiotic-treated very-preterm infants: Changes from admission to discharge. Pediatr. Res. 2022, 92, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Fan, Y.; Li, C.; Li, Y.; Guo, S.; Li, T.; Buys, N.; Clifton, V.L.; Colditz, P.B.; Yin, C.; et al. Early gut microbiota colonisation of premature infants fed with breastmilk or formula with or without probiotics: A cohort study. Nutrients 2021, 13, 4068. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Holdbrooks, H.; Mishra, P.; Abrantes, M.A.; Eskew, S.; Garma, M.; Oca, C.-G.; McGuckin, C.; Hein, C.B.; Mitchell, R.D.; et al. Impact of probiotic B. infantis EVC001 feeding in premature infants on the gut microbiome, nosocomially acquired antibiotic resistance, and enteric inflammation. Front. Pediatr. 2021, 9, 618009. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Saturio, S.; Suárez, M.; Fernández, N.; Mancabelli, L.; de Los Reyes-Gavilán, C.G.; Ventura, M.; Solís, G.; Gueimonde, M. Donated human milk as a determinant factor for the gut bifidobacterial ecology in premature babies. Microorganisms 2020, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.; Marques, C.; Faria, A.; Teixeira, D.; Barreiros-Mota, I.; Durão, C.; Araújo, J.; Ismael, S.; Brito, S.; Cardoso, M.; et al. Influence of human milk on very preterms’ gut microbiota and alkaline phosphatase activity. Nutrients 2021, 13, 1564. [Google Scholar] [CrossRef]
- Cong, X.; Xu, W.; Janton, S.; Henderson, W.A.; Matson, A.; McGrath, J.M.; Maas, K.; Graf, J. Gut microbiome developmental patterns in early life of preterm infants: Impacts of feeding and gender. PLoS ONE 2016, 11, e0152751. [Google Scholar] [CrossRef] [Green Version]
- Horigome, A.; Hisata, K.; Odamaki, T.; Iwabuchi, N.; Xiao, J.-Z.; Shimizu, T. Colonization of supplemented bifidobacterium breve M-16V in low birth weight infants and its effects on their gut microbiota weeks post-administration. Front. Microbiol. 2021, 12, 610080. [Google Scholar] [CrossRef]
- Ishizeki, S.; Sugita, M.; Takata, M.; Yaeshima, T. Effect of administration of bifidobacteria on intestinal microbiota in low-birth-weight infants and transition of administrated bifidobacteria. A comparison between one-species and three-species administration. Anaerobe 2013, 23, 38–44. [Google Scholar] [CrossRef]
- Esaiassen, E.; Hjerde, E.; Cavanagh, J.P.; Pedersen, T.; Andresen, J.H.; Rettedal, S.I.; Støen, R.; Nakstad, B.; Willassen, N.P.; Klingenberg, C. Effects of probiotic supplementation on the gut microbiota and antibiotic resistance development in preterm infants. Front. Pediatr. 2018, 6, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurath-Koller, S.; Neumann, C.; Moissl-Eichinger, C.; Kraschl, R.; Kanduth, C.; Hopfer, B.; Pausan, M.-R.; Urlesberger, B.; Resch, B. Hospital regimens including probiotics guide the individual development of the gut microbiome of very low birth weight infants in the first two weeks of life. Nutrients 2020, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, E.I.; Carvalho, M.; Dizzell, S.E.; Kim, S.; Gunn, E.; Twiss, J.; Giglia, L.; Stuart, C.; Hutton, E.K. Persistence of suspected probiotic organisms in preterm infant gut microbiota weeks after probiotic supplementation in the NICU. Front. Microbiol. 2020, 11, 574137. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadir, B.; Nelson, A.; Skeath, T.; Marrs, E.C.L.; Perry, J.D.; Cummings, S.P.; Embleton, N.D.; Berrington, J.E.; Stewart, C.J. Routine use of probiotics in preterm infants: Longitudinal impact on the microbiome and metabolome. Neonatology 2016, 109, 239–247. [Google Scholar] [CrossRef]
- Larke, J.A.; Kuhn-Riordon, K.; Taft, D.H.; Sohn, K.; Iqbal, S.; Underwood, M.A.; Mills, D.A.; Slupsky, C.M. Preterm infant fecal microbiota and metabolite profiles are modulated in a probiotic specific manner. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 535–542. [Google Scholar] [CrossRef]
- Hui, Y.; Smith, B.; Mortensen, M.S.; Krych, L.; Sørensen, S.J.; Greisen, G.; Krogfelt, K.A.; Nielsen, D.S. The effect of early probiotic exposure on the preterm infant gut microbiome development. Gut Microbes 2021, 13, 1951113. [Google Scholar] [CrossRef]
- Chang, C.-M.; Tsai, M.-H.; Liao, W.-C.; Yang, P.-H.; Li, S.-W.; Chu, S.-M.; Huang, H.-R.; Chiang, M.-C.; Hsu, J.-F. Effects of probiotics on gut microbiomes of extremely preterm infants in the neonatal intensive care unit: A prospective cohort study. Nutrients 2022, 14, 3239. [Google Scholar] [CrossRef]
- Underwood, M.A.; Salzman, N.H.; Bennett, S.H.; Barman, M.; Mills, D.A.; Marcobal, A.; Tancredi, D.J.; Bevins, C.L.; Sherman, M.O. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: Impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Athalye-Jape, G.; Esvaran, M.; Patole, S.; Simmer, K.; Nathan, E.; Doherty, D.; Keil, A.; Rao, S.; Chen, L.; Chandrasekaran, L.; et al. Effect of single versus multistrain probiotic in extremely preterm infants: A randomised trial. BMJ Open Gastroenterol. 2022, 9, e000811. [Google Scholar] [CrossRef]
- Oshiro, T.; Nagata, S.; Wang, C.; Takahashi, T.; Tsuji, H.; Asahara, T.; Nomoto, K.; Takei, H.; Nittono, H.; Yamashiro, Y. Bifidobacterium supplementation of colostrum and breast milk enhances weight gain and metabolic responses associated with microbiota establishment in very-preterm infants. Biomed. Hub 2019, 4, 1–10. [Google Scholar] [CrossRef]
- Boehm, G.; Lidestri, M.; Casetta, P.; Jelinek, J.; Negretti, F.; Stahl, B.; Marini, A. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 86, F178–F181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plummer, E.L.; Bulach, D.M.; Murray, G.L.; Jacobs, S.E.; Tabrizi, S.N.; Garland, S.M.; ProPrems Study Group. Gut microbiota of preterm infants supplemented with probiotics: Sub-study of the ProPrems trial. BMC Microbiol. 2018, 18, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, R.; Koebnick, C.; Schildt, J.; Schmidt, S.; Mueller, M.; Possner, M.; Radke, M.; Blaut, M. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: A double-blind, placebo-controlled, randomized study. J. Clin. Microbiol. 2006, 44, 4025–4031. [Google Scholar] [CrossRef] [Green Version]
- Martí, M.; Spreckels, J.E.; Ranasinghe, P.D.; Wejryd, I.; Marchini, G.; Sverremark-Ekström, E.; Jenmalm, M.C.; Abrahamsson, T. Effects of Lactobacillus reuteri supplementation on the gut microbiota in extremely preterm infants in a randomized placebo-controlled trial. Cell Rep. Med. 2021, 2, 100206. [Google Scholar] [CrossRef] [PubMed]
- Plummer, E.L.; Danielewski, J.A.; Garland, S.M.; Su, J.; Jacobs, S.E.; Murray, J.L. The effect of probiotic supplementation on the gut microbiota of preterm infants. J. Med. Microbiol. 2021, 70, 001403. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Davis, B.; Zhu, W.; Zheng, N.; Meng, D.; Walker, W.A. Short-chain fatty acid butyrate, a breast milk metabolite, enhances immature intestinal barrier function genes in response to inflammation in vitro and in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G521–G530. [Google Scholar] [CrossRef]
- Huang, S.; Gao, Y.; Wang, Z.; Yang, X.; Wang, J.; Zheng, N. Anti-inflammatory actions of acetate, propionate, and butyrate in fetal mouse jejunum cultures ex vivo and immature small intestinal cells in vitro. Food Sci. Nutr. 2022, 10, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Nastasi, C.; Candela, M.; Bonefeld, C.M.; Geisler, C.; Hansen, M.; Krejsgaard, T.; Biagi, E.; Andersen, M.H.; Brigidi, P.; Ødum, N.; et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015, 5, 16148. [Google Scholar] [CrossRef] [Green Version]
- Moylan, H.E.C.; Nguyen-Ngo, C.; Lim, R.; Lappas, M. The short-chain fatty acids butyrate and propionate protect against inflammation-induced activation of mediators involved in active labor: Implications for preterm birth. Mol. Hum. Reprod. 2020, 26, 452–468. [Google Scholar] [CrossRef]
- Ganguli, K.; Collado, M.C.; Rautava, J.; Lu, L.; Satokari, R.; Von Ossowski, I.; Reunaene, J.; De vos, W.M.; Palva, A.; Isolauri, E.; et al. Lactobacillius rhamnosus GG and its spac pilus adhesion modulate inflammatory responsiveness and TLR-related gene expression in the fetal human gut. Pediatr. Res. 2015, 77, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Ganguli, K.; Meng, D.; Rautava, S.; Lu, L.; Walker, W.A.; Nanthakumar, N. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G132–G141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, D.; Zhu, W.; Ganguli, K.; Shi, H.N.; Walker, W.A. Anti-inflammatory effects of Bifidobacterium longum subsp infantis secretions on fetal human enterocytes are mediated by TLR-4 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G744–G753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Cho, K.Y.; Meng, D.; Walker, W.A. The impact of indole-3-lactic acid on immature intestinal innate immunity and development: A transcriptomic analysis. Sci. Rep. 2021, 11, 8088. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, Y.; Ergun, A.; Lu, L.; Walker, W.A.; Ganguli, K. Secreted metabolites of Bifidobacterium infantis and Lactobacillus acidophilus protect immature human enterocytes from IL-1β-induced inflammation: A transcription profiling analysis. PLoS ONE 2015, 10, e0124549. [Google Scholar] [CrossRef]
- Jiang, F.; Memg, D.; Weng, M.; Zhu, W.; Wu, W.; Kasper, D.; Walker, W.A. The symbiotic bacterial surface factor polysaccharide A on bacteroides fragilis inhibits IL-B-induced inflammation in human fetal enterocytes via toll receptors 2 and 4. PLoS ONE 2017, 12, E0172738. [Google Scholar] [CrossRef] [Green Version]
- Gorreja, F.; Rush, S.T.A.; Kasper, D.L.; Meng, D.; Walker, W.A. The developmentally regulated fetal enterocyte gene, ZP4, mediates anti-inflammation by the symbiotic bacterial surface factor polysaccharide A on Bacteroides fragilis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G398–G407. [Google Scholar] [CrossRef]
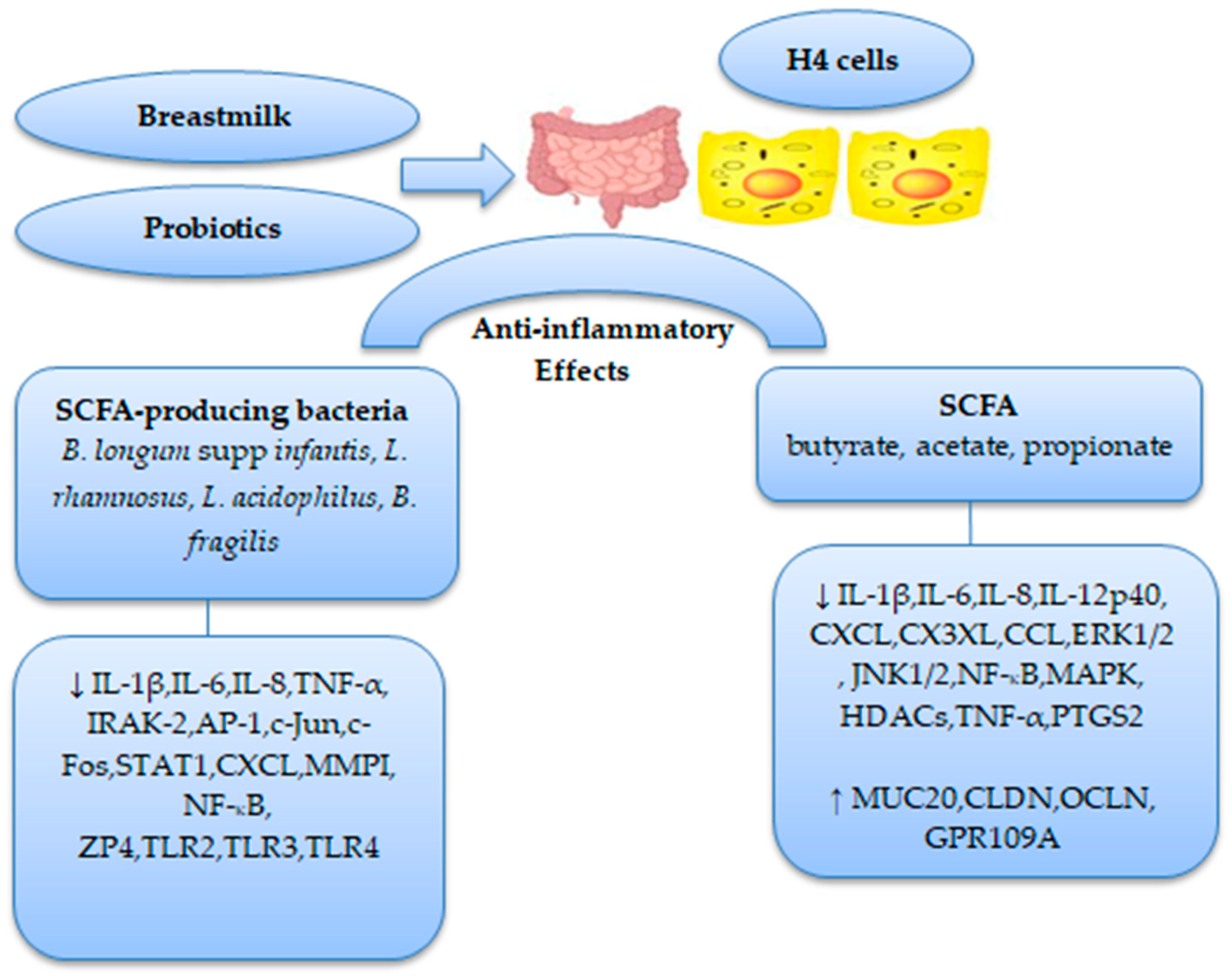
| Study Design | Sample Characteristics | Feeding Types | SCFAs/Microorganisms | References |
|---|---|---|---|---|
| Prospective cohort study | 32 preterm infants delivered at <32 weeks of gestation over 1-year period | breastmilk with bovine milk-based fortifier OR Similac advanced special care formula | Breastmilk = acetate, propionate ↑ Formula = butyrate ↑ | [109] |
| Prospective cohort study | 60 preterm infants delivered at ≤36 weeks of gestation/birth weight (<1500 g) over 1-year period | Breastmilk OR formula | Breastmilk = propionate ↑ Formula = butyrate, acetate ↑ | [110] |
| Prospective cohort study | 234 preterm infants = 101 infants orally supplemented with probiotics over 100 day-period and 133 infants not supplemented | Probiotics Bifidobacterium and Lactobacillus (Bif/Lacto) | Bif/Lacto group = lactate, acetate, B. bifidum, B. breve ↑ | [111] |
| Prospective cohort study | 95 preterm infants delivered at <32 weeks of gestation who did not develop a disease over a study period of 10 years received probiotics and 28 infants not supplemented | Breastmilk, probiotic Infloran (B. bifidum, L. acidophilus), probiotic Labinic (B. bifidum, B. longum subsp. infantis, L. acidophilus) | Breastmilk = Bifidobacterium spp. ↑, Staphylococci ↓ Infloran probiotic= B. breve, B. bifidum, E. faecium ↑, Veillonella parvula, Propionibacterium acnes ↓ Labinic probiotic = B. animalis ↑ No probiotic = Klebsiella spp. ↑ | [112] |
| Prospective cohort study | 134 preterm infants delivered at <32 weeks of gestation/birth weight (<1500 g) over 1-year period | Breastmilk, OR probiotic formula (L. acidophilus, L. bifidus, B. bifidum) | Breastmilk = Bifidobacterium spp. ↑, Veillonella ↓ Probiotic formula = Lactobacillus ↓ | [113] |
| Prospective cohort study | 90 preterm infants delivered at <37 weeks of gestation/birth weight (<2500 g) fed with breastmilk or probiotic formula over 3 months and 48 infants received no probiotic | Breastmilk OR probiotic formula (B. lactis) | Probiotic = Bifidobacterium, Lactobacillus ↑ No probiotic = Enterococcus, Streptococcus, Klebsiella ↑ | [114] |
| Prospective cohort study | 31 preterm infants delivered at <32 weeks of gestation/birth weight (<1500 g) received probiotics over 5 month-period and 46 infants non-supplemented | Probiotic B. infantis EVC001 | Probiotic group = Bifidobacteriaceae ↑, Staphylococcaceae, Enterobacteriaceae ↓ | [115] |
| Prospective cohort study | 42 preterm infants over 3 month-period | Breastmilk OR donor human milk | Breastmilk = B. longum, B. breve, B. pseudolongum spp. globosum, B. longum spp. infantis, B. animalis spp. lactis, B. adolescentis, B. bifidum, B. dentium ↑ Human donor milk = B. bifidum, B. longum spp. longum, B. reuteri, B. vansinderenii, B. pseudolongum spp. pseudolongum, B. animalis spp. lactis, B. longum spp. suis ↑ | [116] |
| Prospective cohort study | 117 preterm infants delivered at ≤32 weeks of gestation over 26-day period | Breastmilk, donor human milk OR formula | Breastmilk = Bifidobacterium, Lactobacillus, Bacteroides, Bacteroidetes, Enterococcus ↑ | [117] |
| Prospective cohort study | 29 preterm infants over 30-day period | Breastmilk | Clostridiales, Lactobacillales ↑ | [118] |
| Prospective cohort study | 12 preterm infants supplemented with a probiotic over 10 weeks-period and 10 infants not supplemented | Probiotic B.breve M-16V | Probiotic group = Bifidobacterium, Enterococcus ↑, Lactococcus, Klebsiella, Rothia ↓ | [119] |
| Prospective cohort study | 28 preterm infants supplemented with single or mixture probiotics over 6 month-period and 16 infants not supplemented | Single-strain probiotic B.breve M-16V Multiple-strain probiotics (B. longum subsp. infantis M-63, B. breve M-16V and B. longum subsp. longum BB536) | Single-strain probiotic = Clostridium ↓ Multiple-strain probiotics = bifidobacteria ↑, Enterobacteriaceae ↓ | [120] |
| Prospective cohort study | 31 preterm infants delivered at <32 weeks of gestation received probiotics over 4-month period, 35 preterm infants not received probiotics and 10 healthy full-term infants | Probiotic Infloran (L. acidophilus ATCC 4356, B. longum subspecies infantis ATCC 15697 | Probiotic = Bifidobacterium, Lactobacillus ↑ | [121] |
| Prospective cohort study | 36 preterm infants delivered at birth weight (<1500 g) received two different probiotics over 5-month period, and 18 infants did not received probiotics | Probiotic L. rhamnosus Probiotics B. infantis and L. acidophilus | Probiotic groups = Bifidobacterium, Lactobacillus ↑ | [122] |
| Prospective cohort study | 8 preterm infants received a probiotic formula over 5-month period, and 14 infants not received a probiotic | Probiotic formula (B. breve HA-129, B. bifidum HA-132, B. longum subsp. longum HA-135, B. longum subsp. infantis HA-116, L. rhamnosus HA-111) | Probiotic group = Bifidobacterium, Lactobacillus ↑ | [123] |
| Prospective cohort study | 7 preterm infants delivered at <32 weeks of gestation received a probiotic formula over 1-month period and 3 preterm infants not received a probiotic | Probiotic Infloran (B. bifidum ATCC15696 and L. acidophilus NCIMB701748) | Probiotic group = Bifidobacterium, Lactobacillus ↑ | [124] |
| Prospective cohort study | 10 Preterm infants at delivered at <32 weeks of gestation received two different probiotics over 1-month period | Probiotics B. longum subsp. infantis OR L. reuteri | Probiotic B. longum subsp. Infantis = Bifidobacterium ↑ Probiotic L. reuteri = HMO ↑ | [125] |
| Prospective cohort study | 87 preterm infants at delivered at <30 weeks of gestation received a probiotic formula over 1-month period, and 165 infants did not receive a probiotic | Probiotic formula (L. rhamnosus GG and B. animalis ssp. lactis BB-12) | Probiotic group = Firmicutes, Actinobacteria ↑, Weissella, Veillonella, Klebsiella ↓ | [126] |
| Prospective cohort study | 70 preterm infants at delivered at ≤28 weeks of gestation received a probiotic formula over 1-month period, and 50 infants did not receive a probiotic | Probiotic Infloran (L. acidophilus and B. bifidum) | Probiotic group = Lactobacillus spp., E. faecium ↑, Yersiniaceae, Staphylococcus, Klebsiella spp. ↓ | [127] |
| RCT | 90 preterm infants delivered at <35 weeks of gestation supplemented with either probiotic species (CUL) or prebiotic/probiotic combinations (PBP) 29 preterm infants received Pregestamil formula (placebo) | CUL probiotics = Two lactobacillus spp. PBP probiotics = Several lactobacillus and Bifidobacterium spp. plus fructo-oligosaccharides | PBP group = acetate, bifidobacteria ↑ Placebo = bifidobacteria ↓ | [128] |
| RCT | 173 preterm infants delivered at <28 weeks of gestation supplemented with either single (SS) or triple-strain (TS) probiotics 29 preterm infants (no probiotics, placebo) | SS probiotic = B. breve M-16V TS probiotics = B. breve M-16V, B. longum subsp. infantis M-63 and B. longum subsp. longum BB536 | SS group = propionate, B.breve, B.bifidum ↑, Clostridium ↓ TS group = butyrate, propionate, B. longum, B. reuteri, B. longum subsp. infantis, B. longum subsp. longum ↑, Clostridium ↓ Placebo = Clostridium butyricum, Streptococcus salivarius, S. thermophilus ↑ | [129] |
| RCT | 17 preterm infants delivered at <31 weeks of gestation/birth weight (<1500 g) supplemented with probiotic (Bifid) 18 preterm infants received vehicle supplement only (placebo) | Probiotic B. breve BBG-01 | Bifid group = Bifidobacterium ↑ | [130] |
| RCT | 15 preterm infants delivered at <32 weeks of gestation supplemented with prebiotic formula 15 preterm infants supplemented with maltodextrin (placebo) | Prebiotic formula (mixture of fructo-oligosaccharides and galacto-oligosaccharides) | Prebiotic group = bifidobacteria ↑ | [131] |
| RCT | 38 preterm infants delivered at <32 weeks of gestation/birth weight (<1500 g) supplemented with probiotics mixture 28 preterm infants supplemented with maltodextrin (placebo) | Probiotics mixture (S. thermophilus TH-4, B. longum subsp. infantis BB-02 and B. animalis subsp. lactis BB-12) | Probiotics group = Bifidobacterium ↑ | [132] |
| RCT | 37 preterm infants delivered at <37 weeks of gestation received a probiotic 32 preterm infants received Nestle ’ FM 2000B formula (placebo) | Probiotic B. lactis Bb12 | Probiotic group = bifidobacteria ↑, Clostridia, Enterobacteria spp. ↓ | [133] |
| RCT | 54 preterm infants received a probiotic, and 54 infants no received any probiotic (placebo) | Probiotic L. reuteri DSM 17938 | Probiotic group = Lactobacillus ↑, Clostridium, Enterobacteriaceae, Staphylococcaceae ↓ | [134] |
| RCT | 229 preterm infants delivered at <32 weeks of gestation received a probiotic formula, and 230 infants did not receive a probiotic (placebo) | Probiotic formula (S. thermophilus TH-4, B. animalis subsp. lactis BB-12 and B. longum subsp. infantis BB-02) | Probiotic group = B. longum subsp. infantis, B. animalis subsp. lactis, S. thermophilus ↑ | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsharairi, N.A. Therapeutic Potential of Gut Microbiota and Its Metabolite Short-Chain Fatty Acids in Neonatal Necrotizing Enterocolitis. Life 2023, 13, 561. https://doi.org/10.3390/life13020561
Alsharairi NA. Therapeutic Potential of Gut Microbiota and Its Metabolite Short-Chain Fatty Acids in Neonatal Necrotizing Enterocolitis. Life. 2023; 13(2):561. https://doi.org/10.3390/life13020561
Chicago/Turabian StyleAlsharairi, Naser A. 2023. "Therapeutic Potential of Gut Microbiota and Its Metabolite Short-Chain Fatty Acids in Neonatal Necrotizing Enterocolitis" Life 13, no. 2: 561. https://doi.org/10.3390/life13020561
APA StyleAlsharairi, N. A. (2023). Therapeutic Potential of Gut Microbiota and Its Metabolite Short-Chain Fatty Acids in Neonatal Necrotizing Enterocolitis. Life, 13(2), 561. https://doi.org/10.3390/life13020561







