Altered Language-Related Effective Connectivity in Patients with Benign Childhood Epilepsy with Centrotemporal Spikes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Psychometric Tests
2.3. Image Data Acquisition
2.4. MRI Date Preprocessing
2.5. Granger Causality Analysis
2.6. Statistical Analysis
3. Results
3.1. Demographic Characteristics and Neuropsychological Tests
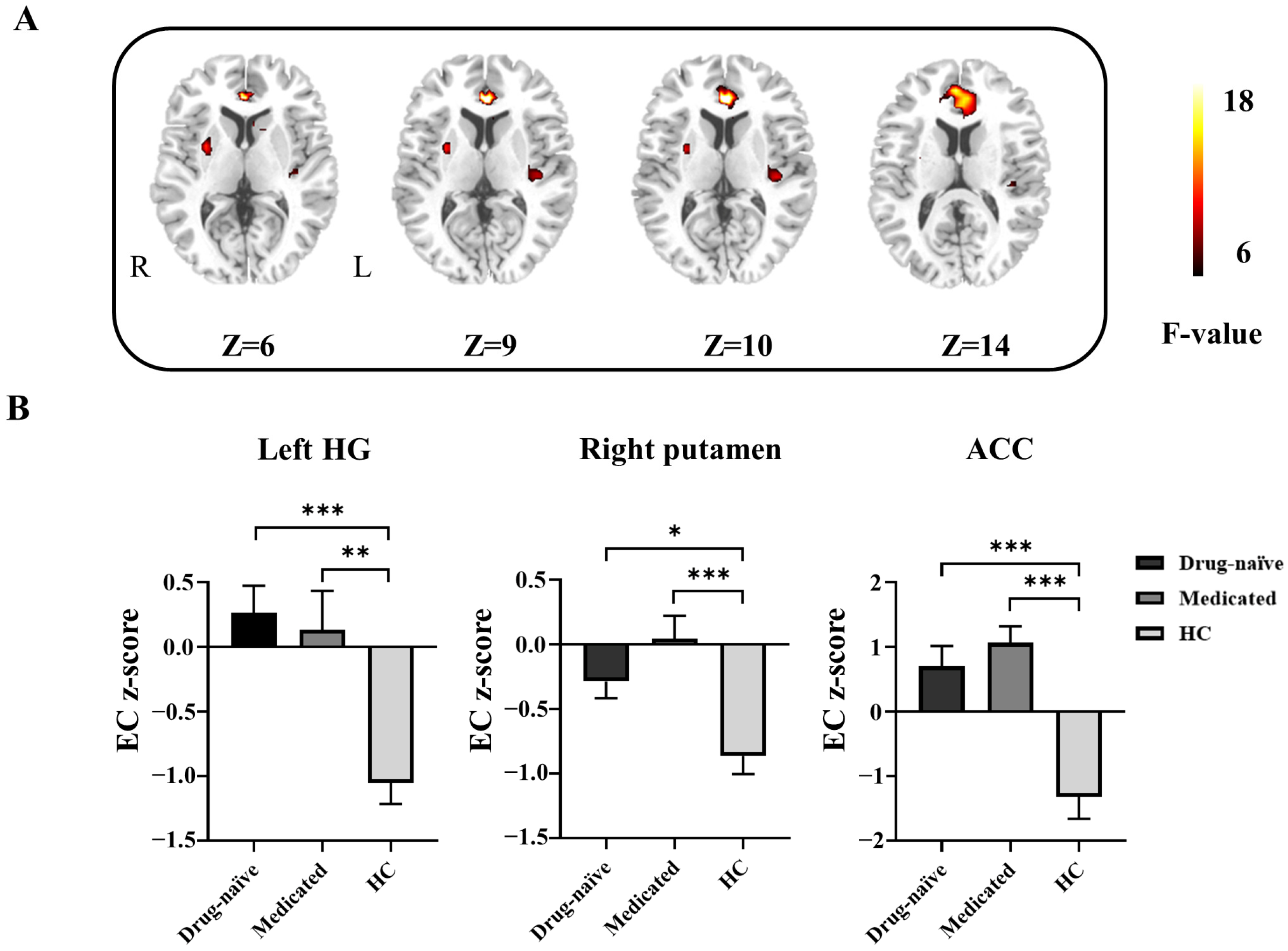
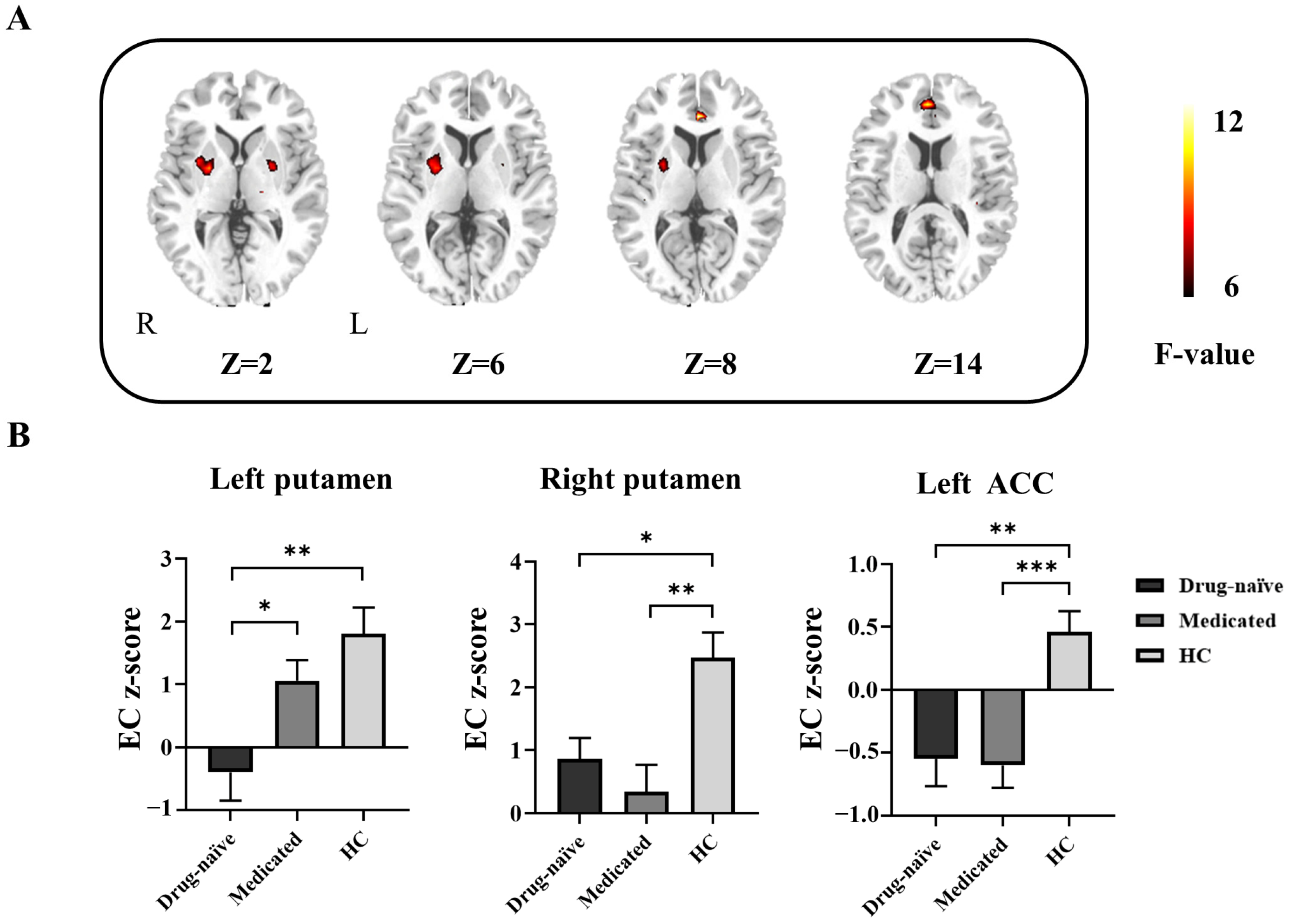
3.2. Differences in GCA among Drug-Naïve, Medicated, and HCs
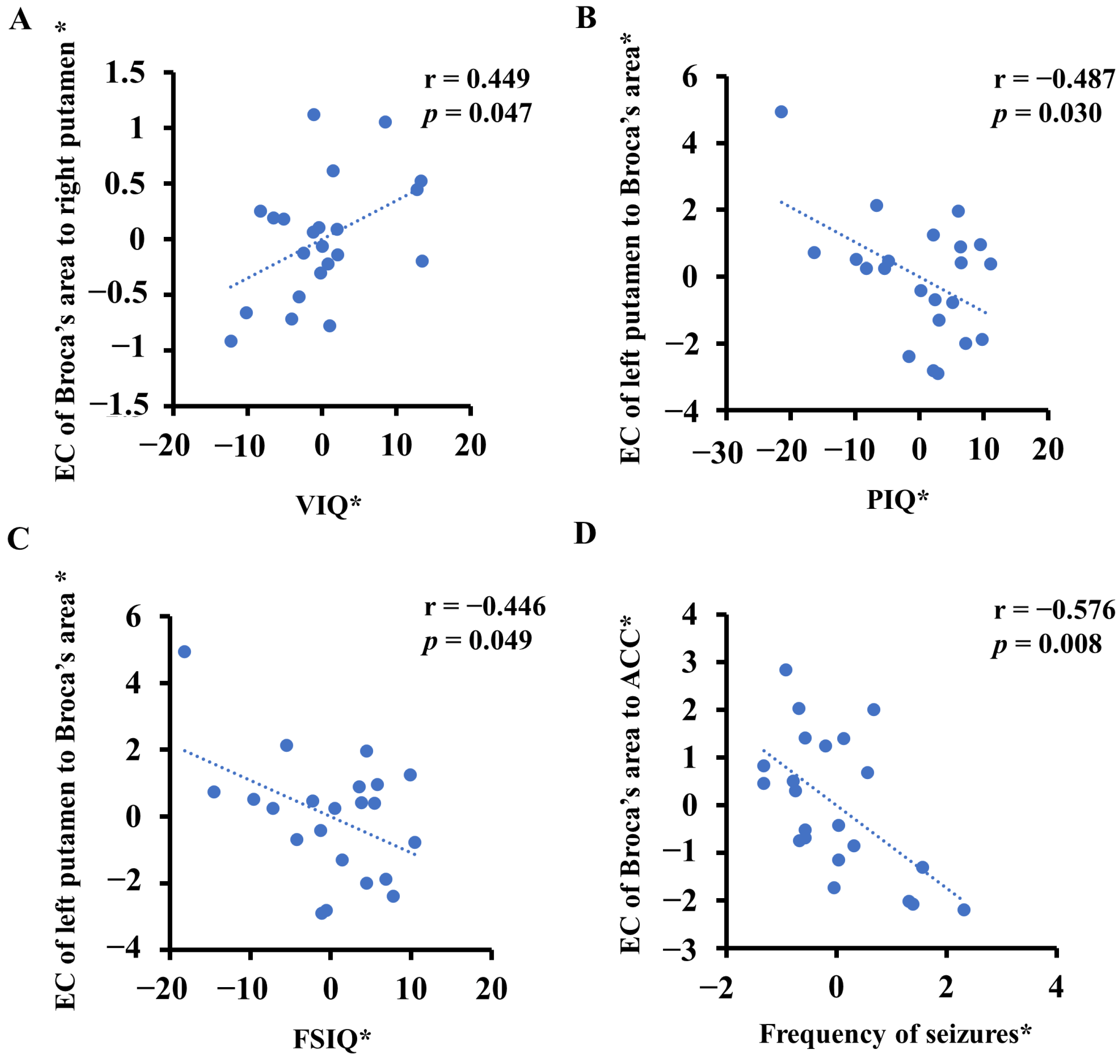
3.3. Abnormal ECs Was Correlated with Some Clinical Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, S.; Luo, C.; Huang, Y.; Li, Z.; Chen, Y.; Li, X.; Pei, H.; Wang, P.; Wang, X.; Yao, D. Altered Static and Dynamic Spontaneous Neural Activity in Drug-Naïve and Drug-Receiving Benign Childhood Epilepsy with Centrotemporal Spikes. Front. Hum. Neurosci. 2020, 14, 361. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, T.; Lv, F.; Li, S.; Liu, H.; Zhang, Z.; Luo, T. Structural Covariance Network of Cortical Gyrification in Benign Childhood Epilepsy with Centrotemporal Spikes. Front. Neurol. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessi, M.; Yan, F.; Pan, L.; Chen, B.; Olatoutou, E.; Li, D.; He, F.; Rugambwa, T.; Yang, L.; Peng, J.; et al. Treatment for the Benign Childhood Epilepsy with Centrotemporal Spikes: A Monocentric Study. Front. Neurol. 2021, 12, 670958. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, J.; Petermann, F. Cognitive Deficits in Children with Benign Rolandic Epilepsy of Childhood or Rolandic Discharges: A Study of Children between 4 and 7 Years of Age with and without Seizures Compared with Healthy Controls. Epilepsy Behav. 2009, 16, 646–651. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Zhang, T.; Shi, Q.; Sun, J.; Xiang, J.; Chen, Q.; Hu, Z.; Wang, X. The Relationship between Epilepsy and Cognitive Function in Benign Childhood Epilepsy with Centrotemporal Spikes. Brain Behav. 2020, 10, e01854. [Google Scholar] [CrossRef] [PubMed]
- McGinnity, C.J.; Smith, A.B.; Yaakub, S.N.; Gerbase, S.W.; Gammerman, A.; Tyson, A.L.; Bell, T.K.; Elmasri, M.; Barker, G.J.; Richardson, M.P.; et al. Decreased Functional Connectivity within a Language Subnetwork in Benign Epilepsy with Centrotemporal Spikes. Epilepsia Open 2017, 2, 214–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monjauze, C.; Tuller, L.; Hommet, C.; Barthez, M.A.; Khomsi, A. Language in Benign Childhood Epilepsy with Centro-Temporal Spikes Abbreviated Form: Rolandic Epilepsy and Language. Brain Lang. 2005, 92, 300–308. [Google Scholar] [CrossRef]
- Georgiopoulos, C.; Witt, S.T.; Haller, S.; Dizdar, N.; Zachrisson, H.; Engstrom, M.; Larsson, E.M. A Study of Neural Activity and Functional Connectivity within the Olfactory Brain Network in Parkinson’s Disease. Neuroimage Clin. 2019, 23, 101946. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, L.; Li, X.; Zhang, Y.; Chen, Y.; Jiang, S.; Hou, C.; Yao, D.; Wang, X.; Luo, C. Dysfunctional White-Matter Networks in Medicated and Unmedicated Benign Epilepsy with Centrotemporal Spikes. Hum. Brain Mapp. 2019, 40, 3113–3124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, Y.; Fu, Z.; Mao, C.; Falahpour, M.; Gollub, R.L.; Park, J.; Wilson, G.; Napadow, V.; Gerber, J.; Chan, S.T.; et al. Distinct Thalamocortical Network Dynamics Are Associated with the Pathophysiology of Chronic Low Back Pain. Nat. Commun. 2020, 11, 3948. [Google Scholar] [CrossRef]
- Tracy, J.I.; Osipowicz, K.; Spechler, P.; Sharan, A.; Skidmore, C.; Doucet, G.; Sperling, M.R. Functional Connectivity Evidence of Cortico-Cortico Inhibition in Temporal Lobe Epilepsy. Hum. Brain Mapp. 2014, 35, 353–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Calhoun, V.D.; Phlypo, R.; Adali, T. Dynamic Changes of Spatial Functional Network Connectivity in Healthy Individuals and Schizophrenia Patients Using Independent Vector Analysis. Neuroimage 2014, 90, 196–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koelewijn, L.; Hamandi, K.; Brindley, L.M.; Brookes, M.J.; Routley, B.C.; Muthukumaraswamy, S.D.; Williams, N.; Thomas, M.A.; Kirby, A.; Naude, J.T.W.; et al. Resting-State Oscillatory Dynamics in Sensorimotor Cortex in Benign Epilepsy with Centro-Temporal Spikes and Typical Brain Development. Hum. Brain Mapp. 2015, 36, 3935–3949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ofer, I.; Jacobs, J.; Jaiser, N.; Akin, B.; Hennig, J.; Schulze-Bonhage, A.; LeVan, P. Cognitive and Behavioral Comorbidities in Rolandic Epilepsy and Their Relation with Default Mode Network’s Functional Connectivity and Organization. Epilepsy Behav. 2018, 78, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, L.; An, D.; Lei, D.; Tang, Y.; Yang, T.; Ren, J.; Chen, S.; Huang, X.; Gong, Q.; et al. Altered Attention Networks in Benign Childhood Epilepsy with Centrotemporal Spikes (Bects): A Resting-State Fmri Study. Epilepsy Behav. 2015, 45, 234–241. [Google Scholar] [CrossRef]
- Fang, J.; Chen, S.; Luo, C.; Gong, Q.; An, D.; Zhou, D. Altered Language Network in Benign Childhood Epilepsy Patients with Spikes from Non-Dominant Side: A Resting-State Fmri Study. Epilepsy Res. 2017, 136, 109–114. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H.; Park, C.H.; Hong, H.S.; Choi, Y.S.; Yoo, J.H.; Lee, H.W. Role of Language-Related Functional Connectivity in Patients with Benign Childhood Epilepsy with Centrotemporal Spikes. J. Clin. Neurol. 2018, 14, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Besseling, R.M.; Jansen, J.F.; Overvliet, G.M.; van der Kruijs, S.J.; Vles, J.S.; Ebus, S.C.; Hofman, P.A.; Louw, A.; Aldenkamp, A.P.; Backes, W.H. Reduced Functional Integration of the Sensorimotor and Language Network in Rolandic Epilepsy. Neuroimage Clin. 2013, 2, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Besseling, R.M.; Overvliet, G.M.; Jansen, J.F.; van der Kruijs, S.J.; Vles, J.S.; Ebus, S.C.; Hofman, P.A.; de Louw, A.J.; Aldenkamp, A.P.; Backes, W.H. Aberrant Functional Connectivity between Motor and Language Networks in Rolandic Epilepsy. Epilepsy Res. 2013, 107, 253–262. [Google Scholar] [CrossRef]
- Oser, N.; Hubacher, M.; Specht, K.; Datta, A.N.; Weber, P.; Penner, I.K. Default Mode Network Alterations During Language Task Performance in Children with Benign Epilepsy with Centrotemporal Spikes (Bects). Epilepsy Behav. 2014, 33, 12–17. [Google Scholar] [CrossRef]
- Park, C.H.; Choi, Y.S.; Kim, H.J.; Chung, H.K.; Jung, A.R.; Yoo, J.H.; Lee, H.W. Interactive Effects of Seizure Frequency and Lateralization on Intratemporal Effective Connectivity in Temporal Lobe Epilepsy. Epilepsia 2018, 59, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Ewen, J.B.; Lakshmanan, B.M.; Hallett, M.; Mostofsky, S.H.; Crone, N.E.; Korzeniewska, A. Dynamics of Functional and Effective Connectivity within Human Cortical Motor Control Networks. Clin. Neurophysiol. 2015, 126, 987–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Yan, H.; Shan, Y.; Shang, K.; Wang, X.; Wang, P.; Shan, Y.; Lu, J.; Zhao, G. Distinctive Structural and Effective Connectivity Changes of Semantic Cognition Network across Left and Right Mesial Temporal Lobe Epilepsy Patients. Neural. Plast 2016, 2016, 8583420. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Ma, X.; Liu, H.; Wang, J.; Zhang, J.; Zhang, G.; Li, S.; Zhang, T. Aberrant Dynamics of Regional Coherence Measured by Resting-State Fmri in Children with Benign Epilepsy with Centrotemporal Spikes (Bects). Front. Neurol. 2021, 12, 712071. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, B.; Amunts, K.; Bhattacharyya, R.; Patkin, D.; Jeffries, K.; Zilles, K.; Braun, A.R. Activation of Broca’s Area During the Production of Spoken and Signed Language: A Combined Cytoarchitectonic Mapping and Pet Analysis. Neuropsychologia 2003, 41, 1868–1876. [Google Scholar] [CrossRef]
- Proposal for Revised Classification of Epilepsies and Epileptic Syndromes. Commission on Classification and Terminology of the International League against Epilepsy. Epilepsia 1989, 30, 389–399. [Google Scholar]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshe, S.L.; et al. Ilae Classification of the Epilepsies: Position Paper of the Ilae Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.H.; Hara, K.; Solo, V.; Vangel, M.; Belliveau, J.W.; Stufflebeam, S.M.; Hamalainen, M.S. Dynamic Granger-Geweke Causality Modeling with Application to Interictal Spike Propagation. Hum. Brain Mapp. 2009, 30, 1877–1886. [Google Scholar] [CrossRef]
- Chen, S.; Fang, J.; An, D.; Xiao, F.; Chen, D.; Chen, T.; Zhou, D.; Liu, L. The Focal Alteration and Causal Connectivity in Children with New-Onset Benign Epilepsy with Centrotemporal Spikes. Sci. Rep. 2018, 8, 5689. [Google Scholar] [CrossRef] [Green Version]
- Bassett, D.S.; Wymbs, N.F.; Rombach, M.P.; Porter, M.A.; Mucha, P.J.; Grafton, S.T. Task-Based Core-Periphery Organization of Human Brain Dynamics. PLoS Comput Biol 2013, 9, e1003171. [Google Scholar] [CrossRef]
- Fedorenko, E. The Role of Domain-General Cognitive Control in Language Comprehension. Front. Psychol. 2014, 5, 335. [Google Scholar] [CrossRef]
- Wallace, M.P.; Lee, K. Examining Second Language Listening, Vocabulary, and Executive Functioning. Front. Psychol. 2020, 11, 1122. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Aisikaer, A.; Chen, Z.; Yu, Q.; Yin, J.; Yang, W. Different Functional Network Connectivity Patterns in Epilepsy: A Rest-State Fmri Study on Mesial Temporal Lobe Epilepsy and Benign Epilepsy with Centrotemporal Spike. Front. Neurol. 2021, 12, 668856. [Google Scholar] [CrossRef] [PubMed]
- Fratantoni, J.M.; DeLaRosa, B.L.; Didehbani, N.; Hart, J., Jr.; Kraut, M.A. Electrophysiological Correlates of Word Retrieval in Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1017–1021. [Google Scholar] [CrossRef]
- Choi, H.S.; Chung, Y.G.; Choi, S.A.; Ahn, S.; Kim, H.; Yoon, S.; Hwang, H.; Kim, K.J. Electroencephalographic Resting-State Functional Connectivity of Benign Epilepsy with Centrotemporal Spikes. J. Clin. Neurol. 2019, 15, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Zhang, Z.; Pan, Z.; Mantini, D.; Ding, J.; Duan, X.; Luo, C.; Wang, Z.; Tan, Q.; Lu, G.; et al. Default Mode Network Abnormalities in Mesial Temporal Lobe Epilepsy: A Study Combining Fmri and Dti. Hum. Brain Mapp. 2011, 32, 883–895. [Google Scholar] [CrossRef]
- Ciumas, C.; Montavont, A.; Ilski, F.; Laurent, A.; Saignavongs, M.; Lachaux, J.P.; de Bellescize, J.; Panagiotakaki, E.; Ostrowsky-Coste, K.; Herbillon, V.; et al. Neural Correlates of Verbal Working Memory in Children with Epilepsy with Centro-Temporal Spikes. Neuroimage Clin. 2020, 28, 102392. [Google Scholar] [CrossRef]
- Cadena, E.J.; White, D.M.; Kraguljac, N.V.; Reid, M.A.; Maximo, J.O.; Nelson, E.A.; Gawronski, B.A.; Lahti, A.C. A Longitudinal Multimodal Neuroimaging Study to Examine Relationships between Resting State Glutamate and Task Related Bold Response in Schizophrenia. Front. Psychiatry 2018, 9, 632. [Google Scholar] [CrossRef] [Green Version]
- Burhan, A.M.; Marlatt, N.M.; Palaniyappan, L.; Anazodo, U.C.; Prato, F.S. Role of Hybrid Brain Imaging in Neuropsychiatric Disorders. Diagnostics 2015, 5, 577–614. [Google Scholar] [CrossRef] [Green Version]
- Thothathiri, M.; Rattinger, M.; Trivedi, B. Cognitive Control During Sentence Generation. Cogn. Neurosci. 2017, 8, 39–49. [Google Scholar] [CrossRef]
- Weber, K.; Micheli, C.; Ruigendijk, E.; Rieger, J.W. Sentence Processing Is Modulated by the Current Linguistic Environment and a Priori Information: An Fmri Study. Brain Behav. 2019, 9, e01308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Zhang, Y.; Cao, W.; Huang, Y.; Yang, F.; Wang, J.; Tu, S.; Wang, X.; Yao, D. Altered Structural and Functional Feature of Striato-Cortical Circuit in Benign Epilepsy with Centrotemporal Spikes. Int. J. Neural. Syst. 2015, 25, 1550027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grillner, S.; Hellgren, J.; Menard, A.; Saitoh, K.; Wikstrom, M.A. Mechanisms for Selection of Basic Motor Programs--Roles for the Striatum and Pallidum. Trends Neurosci. 2005, 28, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Badawy, R.A.; Lai, A.; Vogrin, S.J.; Cook, M.J. Subcortical Epilepsy? Neurology 2013, 80, 1901–1907. [Google Scholar] [CrossRef]

| Clinical Features | Drug-Naïve (n = 22) | Medicated (n = 22) | HCs (n = 23) | p Value |
|---|---|---|---|---|
| Age (year) | 9.11 ± 1.86 | 9.68 ± 1.73 | 10.09 ± 2.86 | 0.343 a |
| Sex (male/female) | 11:11 | 16:6 | 16:7 | 0.233 b |
| Age at onset (year) | 8.64 ± 1.82 | 8.10 ± 2.06 | - | 0.350 c |
| Duration (month) | 6.58 ± 7.61 | 20.82 ± 11.82 | - | <0.05 c |
| EEG lateralization | 9 L/3 R/10 B | 6 L/7 R/9 B | - | 0.324 b |
| Number of seizures (count) | 2.86 ± 1.35 | 4.14 ± 2.10 | - | <0.05 c |
| Frequency of seizures (counts/year) | 2.55 ± 1.01 | 2.89 ± 1.50 | - | 0.381 c |
| VIQ | 84.00 ±7.35 | 82.41 ± 7.43 | 98.17 ± 6.04 | <0.001 a |
| PIQ | 95.00 ± 10.71 | 93.18 ± 12.12 | 110.87 ±7.23 | <0.001 a |
| FSIQ | 88.09 ± 8.66 | 86.13 ± 9.41 | 105.39 ± 6.04 | <0.001 a |
| Brain Region | MNI Coordinates (X, Y, Z) | BA | Cluster Size (Voxel) | F Value | EC Z-Score (Mean ± SD) | ||
|---|---|---|---|---|---|---|---|
| Drug-Naïve | Medicated | HCs | |||||
| Left HG | −39, −24, 9 | 48 | 47 | 9.75 | 0.26 ± 0.99 c | 0.13 ± 1.40 b | −1.05 ± 0.77 |
| Right putamen | 30, 0, 6 | - | 39 | 10.58 | −0.29 ± 0.61 a | 0.04 ± 0.83 c | −0.86 ± 0.68 |
| ACC | 0, 36, 9 | 32 | 155 | 15.83 | 0.70 ± 1.47 c | 1.07 ± 1.18 c | −1.32 ± 1.64 |
| Brain Region | MNI Coordinates (X, Y, Z) | BA | Cluster Size (Voxel) | F Value | EC Z-Score (Mean ± SD) | ||
|---|---|---|---|---|---|---|---|
| Drug-Naïve | Medicated | HCs | |||||
| Left putamen | −18, 6, −6 | - | 33 | 8.20 | −0.39 ± 2.12 b | 1.06 ± 1.55 d | 1.8 ± 2.02 |
| Right putamen | 24, −3, 3 | - | 48 | 8.93 | 0.86 ± 1.56 a | 0.34 ± 1.99 b | 2.47 ± 1.91 |
| Left ACC | 0, 36, 6 | 32 | 37 | 11.24 | −0.55 ± 1.01 b | −0.60 ± 0.84 c | 0.46 ± 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Tan, J.; Huang, Y.; Xiao, R.; Wang, X.; Han, Y. Altered Language-Related Effective Connectivity in Patients with Benign Childhood Epilepsy with Centrotemporal Spikes. Life 2023, 13, 590. https://doi.org/10.3390/life13020590
Yang F, Tan J, Huang Y, Xiao R, Wang X, Han Y. Altered Language-Related Effective Connectivity in Patients with Benign Childhood Epilepsy with Centrotemporal Spikes. Life. 2023; 13(2):590. https://doi.org/10.3390/life13020590
Chicago/Turabian StyleYang, Fei, Juan Tan, Yue Huang, Ruhui Xiao, Xiaoming Wang, and Yanbing Han. 2023. "Altered Language-Related Effective Connectivity in Patients with Benign Childhood Epilepsy with Centrotemporal Spikes" Life 13, no. 2: 590. https://doi.org/10.3390/life13020590






