Learning Curve of Transperineal MRI/US Fusion Prostate Biopsy: 4-Year Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI Protocol
2.3. Biopsy Protocol
2.4. Histopathological Analysis
2.5. Outcome Measures and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Hilscher, M.; Røder, A.; Helgstrand, J.T.; Klemann, N.; Brasso, K.; Vickers, A.J.; Stroomberg, H.V. Risk of prostate cancer and death after benign transurethral resection of the prostate—A 20-year population-based analysis. Cancer 2022, 128, 3674–3680. [Google Scholar] [CrossRef] [PubMed]
- Busetto, G.M.; Giovannone, R.; Antonini, G.; Rossi, A.; Del Giudice, F.; Tricarico, S.; Ragonesi, G.; Gentile, V.; De Berardinis, E. Short-term pretreatment with a dual 5α-reductase inhibitor before bipolar transurethral resection of the prostate (B-TURP): Evaluation of prostate vascularity and decreased surgical blood loss in large prostates. BJU Int. 2014, 116, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.-H.; Chang, S.-W.; Tsai, L.-H.; Kan, H.-C.; Liu, J.-M.; Chuang, C.-K.; Pang, S.-T.; Yu, K.-J. Increasing incidence of prostate cancer in Taiwan: A study of related factors using a nationwide health and welfare database. Medicine 2020, 99, e22336. [Google Scholar] [CrossRef]
- Rabbani, F.; Stroumbakis, N.; Kava, B.R.; Cookson, M.S.; Fair, W.R. Incidence and clinical significance of false-negative sextant prostate biopsies. J. Urol. 1998, 159, 1247–1250. [Google Scholar] [CrossRef]
- King, C.R.; McNeal, J.E.; Gill, H.; Presti, J.C. Extended prostate biopsy scheme improves reliability of Gleason grading: Implications for radiotherapy patients. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 386–391. [Google Scholar] [CrossRef]
- Yang, C.H.; Lin, Y.S.; Weng, W.C.; Hsu, C.Y.; Tung, M.C.; Ou, Y.C. Incidental Prostate Cancer from Prostate with Benign Biopsies: A Predictive and Survival Analysis from Cohort Study. Int. J. Gen. Med. 2022, 15, 2807–2816. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Fütterer, J.J.; Briganti, A.; De Visschere, P.; Emberton, M.; Giannarini, G.; Kirkham, A.; Taneja, S.S.; Thoeny, H.; Villeirs, G.; Villers, A. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur. Urol. 2015, 68, 1045–1053. [Google Scholar] [CrossRef]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.C.; Lebastchi, A.H.; Hadaschik, B.A.; Emberton, M.; Moore, C.; Laguna, P.; Fütterer, J.J.; George, A.K. Role of MRI for the detection of prostate cancer. World J. Urol. 2021, 39, 637–649. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Eklund, M.; Jäderling, F.; Discacciati, A.; Bergman, M.; Annerstedt, M.; Aly, M.; Glaessgen, A.; Carlsson, S.; Grönberg, H.; Nordström, T. MRI-Targeted or Standard Biopsy in Prostate Cancer Screening. N. Engl. J. Med. 2021, 385, 908–920. [Google Scholar] [CrossRef]
- Mottet, N.; Cornford, P.; van den Bergh, R. EAU–ESTRO–ESUR–SIOG Guidelines on Prostate Cancer. EAU Guidelines Edn presented at the EAU Annual Congress 2022. In Proceedings of the EAU Annual Congress, Amsterdam. Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP_SIOG-Guidelines-on-Prostate-Cancer-2022_2022-04-25-063938_yfos.pdf (accessed on 21 February 2023).
- Bjurlin, M.A.; Carroll, P.R.; Eggener, S.; Fulgham, P.F.; Margolis, D.J.; Pinto, P.A.; Rosenkrantz, A.B.; Rubenstein, J.N.; Rukstalis, D.B.; Taneja, S.S.; et al. Update of the Standard Operating Procedure on the Use of Multiparametric Magnetic Resonance Imaging for the Diagnosis, Staging and Management of Prostate Cancer. J. Urol. 2020, 203, 706–712. [Google Scholar] [CrossRef]
- Dasgupta, P.; Davis, J.; Hughes, S. NICE guidelines on prostate cancer 2019. BJU Int. 2019, 124, 1. [Google Scholar] [CrossRef] [Green Version]
- National Comprehensive Cancer Network. Prostate Cancer Early Detection. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf (accessed on 24 January 2023).
- Wegelin, O.; van Melick, H.H.; Hooft, L.; Bosch, J.R.; Reitsma, H.B.; Barentsz, J.O.; Somford, D.M. Comparing Three Different Techniques for Magnetic Resonance Imaging-targeted Prostate Biopsies: A Systematic Review of In-bore versus Magnetic Resonance Imaging-transrectal Ultrasound fusion versus Cognitive Registration. Is There a Preferred Technique? Eur. Urol. 2017, 71, 517–531. [Google Scholar] [CrossRef]
- Noureldin, M.; Eldred-Evans, D.; Khoo, C.C.; Winkler, M.; Sokhi, H.; Tam, H.; Ahmed, H.U. Review article: MRI-targeted biopsies for prostate cancer diagnosis and management. World J. Urol. 2020, 39, 57–63. [Google Scholar] [CrossRef]
- Xiang, J.; Yan, H.; Li, J.; Wang, X.; Chen, H.; Zheng, X. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 17, 31. [Google Scholar] [CrossRef] [Green Version]
- Tu, X.; Liu, Z.; Chang, T.; Qiu, S.; Xu, H.; Bao, Y.; Yang, L.; Wei, Q. Transperineal Magnetic Resonance Imaging–Targeted Biopsy May Perform Better Than Transrectal Route in the Detection of Clinically Significant Prostate Cancer: Systematic Review and Meta-analysis. Clin. Genitourin. Cancer 2019, 17, e860–e870. [Google Scholar] [CrossRef]
- Hsieh, P.-F.; Chang, T.-Y.; Lin, W.-C.; Chang, H.; Chang, C.-H.; Huang, C.-P.; Yang, C.-R.; Chen, W.-C.; Chang, Y.-H.; Wang, Y.-D.; et al. A comparative study of transperineal software-assisted magnetic resonance/ultrasound fusion biopsy and transrectal cognitive fusion biopsy of the prostate. BMC Urol. 2022, 22, 72. [Google Scholar] [CrossRef]
- Zattoni, F.; Marra, G.; Kasivisvanathan, V.; Grummet, J.; Nandurkar, R.; Ploussard, G.; Olivier, J.; Chiu, P.K.; Valerio, M.; Gontero, P.; et al. The Detection of Prostate Cancer with Magnetic Resonance Imaging-Targeted Prostate Biopsies is Superior with the Transperineal vs the Transrectal Approach. A European Association of Urology-Young Academic Urologists Prostate Cancer Working Group Multi-Institutional Study. J. Urol. 2022, 208, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Halstuch, D.; Baniel, J.; Lifshitz, D.; Sela, S.; Ber, Y.; Margel, D. Characterizing the learning curve of MRI-US fusion prostate biopsies. Prostate Cancer Prostatic Dis. 2019, 22, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Gaziev, G.; Wadhwa, K.; Barrett, T.; Koo, B.C.; Gallagher, F.A.; Serrao, E.; Frey, J.; Seidenader, J.; Carmona, L.; Warren, A.; et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU Int. 2014, 117, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.-F.; Li, W.-J.; Lin, W.-C.; Chang, H.; Chang, C.-H.; Huang, C.-P.; Yang, C.-R.; Chen, W.-C.; Chang, Y.-H.; Wu, H.-C. Combining prostate health index and multiparametric magnetic resonance imaging in the diagnosis of clinically significant prostate cancer in an Asian population. World J. Urol. 2020, 38, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Kuru, T.H.; Wadhwa, K.; Chang, R.T.M.; Echeverria, L.M.C.; Roethke, M.; Polson, A.; Rottenberg, G.; Koo, B.; Lawrence, E.M.; Seidenader, J.; et al. Definitions of terms, processes and a minimum dataset for transperineal prostate biopsies: A standardization approach of the Ginsburg Study Group for Enhanced Prostate Diagnostics. BJU Int. 2013, 112, 568–577. [Google Scholar] [CrossRef]
- Szabo, R.J. “Free-Hand” Transperineal Prostate Biopsy Under Local Anesthesia: Review of the Literature. J. Endourol. 2021, 35, 525–543. [Google Scholar] [CrossRef]
- Urkmez, A.; Demirel, C.; Altok, M.; Bathala, T.K.; Shapiro, D.D.; Davis, J.W. Freehand versus Grid-Based Transperineal Prostate Biopsy: A Comparison of Anatomical Region Yield and Complications. J. Urol. 2021, 206, 894–902. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Hsieh, P.-F.; Li, T.-R.; Lin, W.-C.; Chang, H.; Huang, C.-P.; Chang, C.-H.; Yang, C.-R.; Yeh, C.-C.; Huang, W.-C.; Wu, H.-C. Combining prostate health index and multiparametric magnetic resonance imaging in estimating the histological diameter of prostate cancer. BMC Urol. 2021, 21, 161. [Google Scholar] [CrossRef]
- Tamada, T.; Kido, A.; Yamamoto, A.; Takeuchi, M.; Miyaji, Y.; Moriya, T.; Sone, T. Comparison of Biparametric and MultiparametricMRIfor Clinically Significant Prostate Cancer Detection WithPI-RADSVersion 2.1. J. Magn. Reson. Imaging 2021, 53, 283–291. [Google Scholar] [CrossRef]
- Cata, E.D.; Van Praet, C.; Andras, I.; Kadula, P.; Ognean, R.; Buzoianu, M.; Leucuta, D.; Caraiani, C.; Tamas-Szora, A.; Decaestecker, K.; et al. Analyzing the learning curves of a novice and an experienced urologist for transrectal magnetic resonance imaging-ultrasound fusion prostate biopsy. Transl. Androl. Urol. 2021, 10, 1956–1965. [Google Scholar] [CrossRef]
- Kasabwala, K.; Patel, N.; Cricco-Lizza, E.; Shimpi, A.A.; Weng, S.; Buchmann, R.M.; Motanagh, S.; Wu, Y.; Banerjee, S.; Khani, F.; et al. The Learning Curve for Magnetic Resonance Imaging/Ultrasound Fusion-guided Prostate Biopsy. Eur. Urol. Oncol. 2019, 2, 135–140. [Google Scholar] [CrossRef]
- Wysock, J.S.; Rosenkrantz, A.B.; Huang, W.C.; Stifelman, M.D.; Lepor, H.; Deng, F.-M.; Melamed, J.; Taneja, S.S. A Prospective, Blinded Comparison of Magnetic Resonance (MR) Imaging–Ultrasound Fusion and Visual Estimation in the Performance of MR-targeted Prostate Biopsy: The PROFUS Trial. Eur. Urol. 2013, 66, 343–351. [Google Scholar] [CrossRef]
- Checcucci, E.; Piramide, F.; Amparore, D.; De Cillis, S.; Granato, S.; Sica, M.; Verri, P.; Volpi, G.; Piana, A.; Garrou, D.; et al. Beyond the Learning Curve of Prostate MRI/TRUS Target Fusion Biopsy after More than 1000 Procedures. Urology 2021, 155, 39–45. [Google Scholar] [CrossRef]
- Hsieh, P.-F.; Chang, T.-Y.; Lin, W.-C.; Chang, H.; Chang, C.-H.; Huang, C.-P.; Yang, C.-R.; Chen, W.-C.; Chang, Y.-H.; Wang, Y.-D.; et al. Saturation target biopsy can overcome the learning curve of magnetic resonance imaging/ultrasound fusion biopsy of the prostate. J. Men’s Health 2022, 18, 127. [Google Scholar] [CrossRef]
- Muthigi, A.; George, A.K.; Sidana, A.; Kongnyuy, M.; Simon, R.; Moreno, V.; Merino, M.J.; Choyke, P.L.; Turkbey, B.; Wood, B.; et al. Missing the Mark: Prostate Cancer Upgrading by Systematic Biopsy over Magnetic Resonance Imaging/Transrectal Ultrasound Fusion Biopsy. J. Urol. 2017, 197, 327–334. [Google Scholar] [CrossRef]
- Van Houdt, P.J.; Ghobadi, G.; Schoots, I.G.; Heijmink, S.W.; Jong, J.; Poel, H.G.; Pos, F.J.; Rylander, S.; Bentzen, L.; Haustermans, K.; et al. Histopathological Features of MRI-Invisible Regions of Prostate Cancer Lesions. J. Magn. Reson. Imaging 2019, 51, 1235–1246. [Google Scholar] [CrossRef]
- Tay, K.J.; Scheltema, M.J.; Ahmed, H.U.; Barret, E.; Coleman, J.A.; Dominguez-Escrig, J.; Ghai, S.; Huang, J.; Jones, J.S.; Klotz, L.H.; et al. Patient selection for prostate focal therapy in the era of active surveillance: An International Delphi Consensus Project. Prostate Cancer Prostatic Dis. 2017, 20, 294–299. [Google Scholar] [CrossRef]
- Barzell, W.E.; Melamed, M.R. Appropriate Patient Selection in the Focal Treatment of Prostate Cancer: The Role of Transperineal 3-Dimensional Pathologic Mapping of the Prostate—A 4-Year Experience. Urology 2007, 70, 27–35. [Google Scholar] [CrossRef]
- Salciccia, S.; Capriotti, A.; Laganà, A.; Fais, S.; Logozzi, M.; De Berardinis, E.; Busetto, G.; Di Pierro, G.; Ricciuti, G.; Del Giudice, F.; et al. Biomarkers in Prostate Cancer Diagnosis: From Current Knowledge to the Role of Metabolomics and Exosomes. Int. J. Mol. Sci. 2021, 22, 4367. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Pan, P.-H.; Cheng, W.-M.; Wang, H.-K.; Shen, S.-H.; Liu, H.-T.; Cheng, H.-M.; Chen, W.-R.; Huang, T.-H.; Wei, T.-C.; et al. The Prostate Health Index aids multi-parametric MRI in diagnosing significant prostate cancer. Sci. Rep. 2021, 11, 1286. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-P.; Lin, T.-P.; Shen, S.-H.; Cheng, W.-M.; Huang, T.-H.; Huang, I.-S.; Fan, Y.-H.; Lin, C.-C.; Huang, E.Y.H.; Chung, H.-J.; et al. Combining prostate health index and multiparametric magnetic resonance imaging may better predict extraprostatic extension after radical prostatectomy. J. Chin. Med. Assoc. 2023, 86, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; Laguna, M.P.; Walz, J.; Pavlovich, C.P.; Bianco, F.; Gregg, J.; Lebastchi, A.H.; Lepor, H.; Macek, P.; Rais-Bahrami, S.; et al. Molecular biomarkers in the context of focal therapy for prostate cancer: Recommendations of a Delphi Consensus from the Focal Therapy Society. Minerva Urol. Nephrol. 2022, 74, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Calio, B.; Sidana, A.; Sugano, D.; Gaur, S.; Jain, A.; Maruf, M.; Xu, S.; Yan, P.; Kruecker, J.; Merino, M.; et al. Changes in prostate cancer detection rate of MRI-TRUS fusion vs systematic biopsy over time: Evidence of a learning curve. Prostate Cancer Prostatic Dis. 2017, 20, 436–441. [Google Scholar] [CrossRef]
- Lee, C.U.; Sung, S.H.; Jang, C.T.; Kang, M.; Sung, H.H.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Jeon, H.G. Cancer Location in Upgrading and Detection after Transperineal Template-Guided Mapping Biopsy for Patients in Active Surveillance and Negative Transrectal Ultrasonography-Guided Prostate Biopsy. Urol. Int. 2019, 103, 262–269. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chiu, H.-C.; Lin, W.-C.; Ho, T.-L.; Chang, H.; Chang, Y.-H.; Huang, C.-P.; Wu, H.-C.; Yang, C.-R.; Hsieh, P.-F. The Influence of Serum Prostate-Specific Antigen on the Accuracy of Magnetic Resonance Imaging Targeted Biopsy versus Saturation Biopsy in Patients with Previous Negative Biopsy. BioMed Res. Int. 2017, 2017, 7617148. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Lee, C.H.; Chia, D.; Lin, Z.; Huang, W.; Tan, C.H. Machine Learning in Prostate MRI for Prostate Cancer: Current Status and Future Opportunities. Diagnostics 2022, 12, 289. [Google Scholar] [CrossRef]
- Lai, C.-C.; Wang, H.-K.; Wang, F.-N.; Peng, Y.-C.; Lin, T.-P.; Peng, H.-H.; Shen, S.-H. Autosegmentation of Prostate Zones and Cancer Regions from Biparametric Magnetic Resonance Images by Using Deep-Learning-Based Neural Networks. Sensors 2021, 21, 2709. [Google Scholar] [CrossRef]


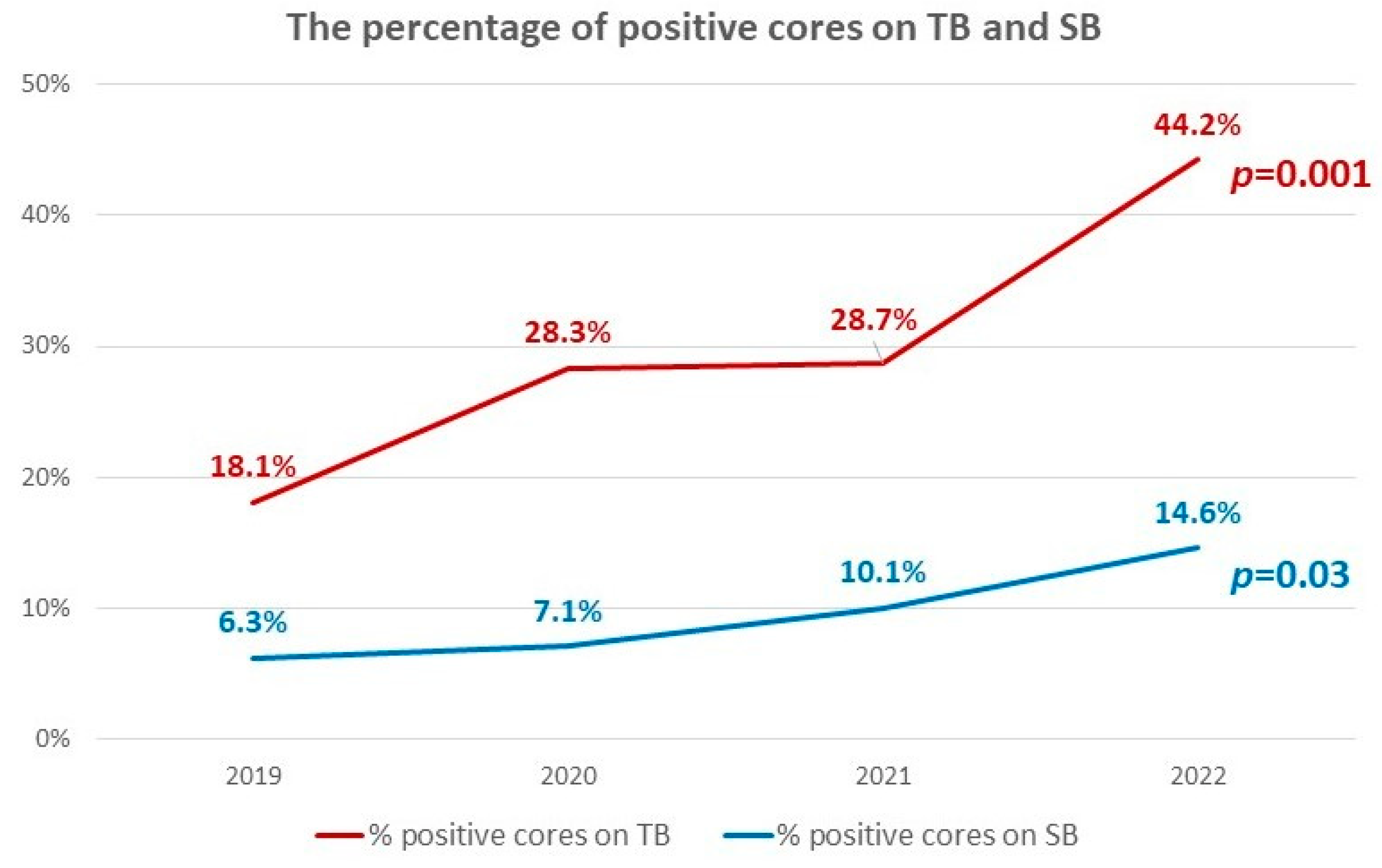
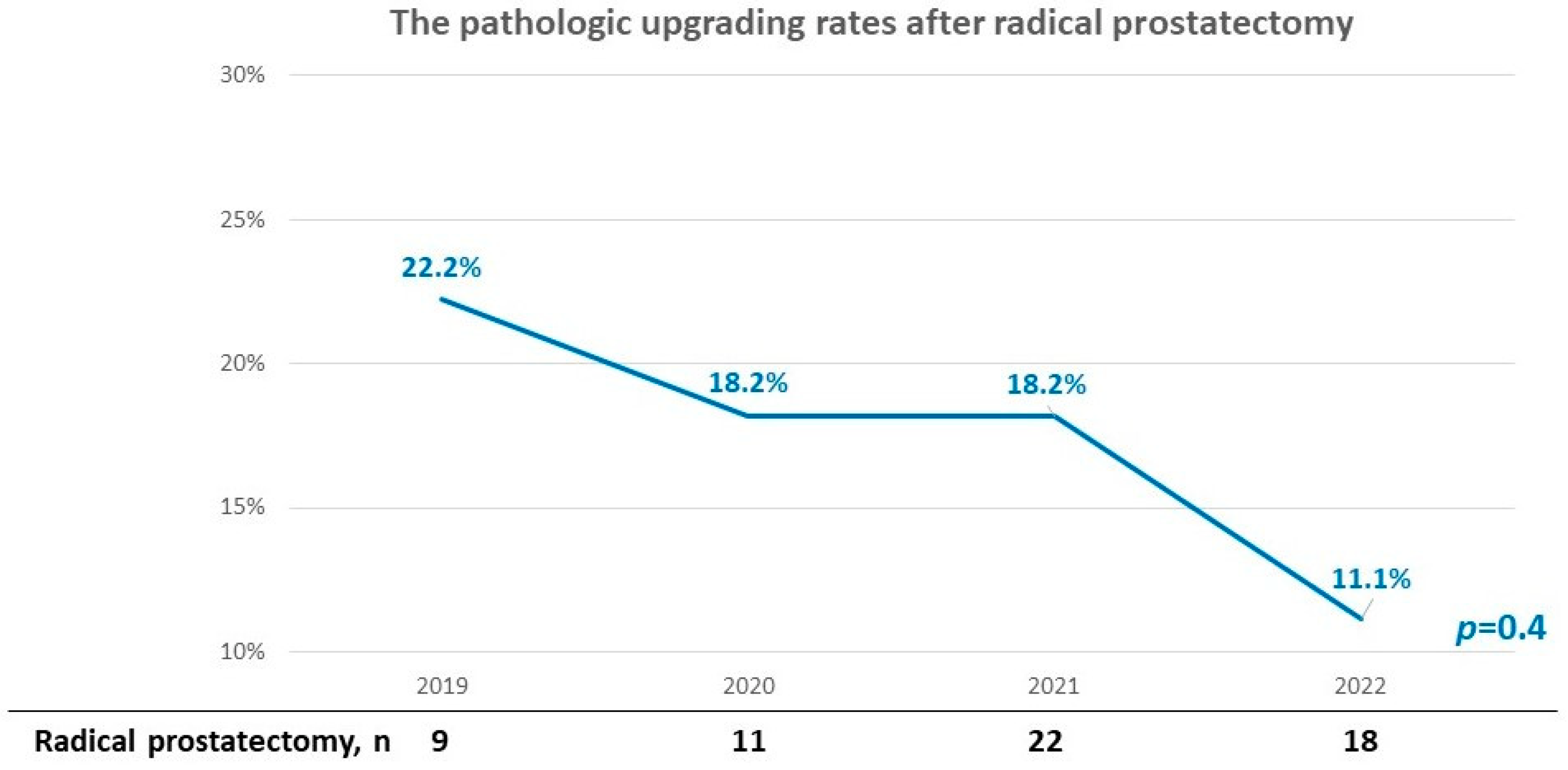
| Year | 2019 | 2020 | 2021 | 2022 | p Value |
|---|---|---|---|---|---|
| Case no. | 35 | 36 | 59 | 76 | |
| Age, mean ± SD | 67.1 ± 8.3 | 64.6 ± 9.0 | 67.8 ± 8.6 | 67.5 ± 8.0 | 0.17 |
| PSA (ng/mL), mean ± SD | 9.9 ± 6.5 | 11.9 ± 13.6 | 10.0 ± 11.5 | 11.6 ± 11.0 | 0.76 |
| Prostate volume (cm3), mean ± SD | 46.5 ± 34.6 | 48.5 ± 25.7 | 52.2 ± 24.5 | 52.8 ± 24.9 | 0.65 |
| Index lesion size (cm), mean ± SD | 14.3 ± 8.6 | 15.2 ± 8.2 | 12.5 ± 6.5 | 14.0 ± 9.2 | 0.4 |
| Biopsy cores per target (n), mean ± SD | 5.0 ± 2.1 | 7.1 ± 2.3 | 6.1 ± 2.3 | 5.3 ± 1.4 | 0.01 |
| Systematic biopsy cores (n), mean ± SD | 18.3 ± 3.7 | 19.9 ± 3.9 | 19.2 ± 3.1 | 18.1 ± 2.9 | 0.09 |
| PI-RADS score of index lesion | 0.32 | ||||
| 3 | 4 | 10 | 12 | 19 | |
| 4 | 17 | 13 | 31 | 37 | |
| 5 | 14 | 13 | 16 | 20 | |
| Negative biopsy within 5 years, n (%) | 9 (25.7%) | 11 (30.6%) | 23 (39.0%) | 11 (14.5%) | 0.02 |
| Abnormal DRE, n (%) | 11 (31.4%) | 8 (22.2%) | 19 (32.2%) | 18 (23.7%) | 0.67 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Year of biopsy | 1.10 | 0.86–1.42 | 0.4 | 1.51 | 1.03–2.20 | 0.03 |
| Age | 1.10 | 1.06–1.14 | <0.001 | 1.13 | 1.07–1.20 | <0.001 |
| PSA | 1.12 | 1.06–1.18 | <0.001 | 1.12 | 1.03–1.22 | 0.008 |
| Prostate volume | 0.96 | 0.95–0.98 | <0.001 | 0.95 | 0.93–0.97 | <0.001 |
| Size of Index lesion | 1.10 | 1.05–1.15 | <0.001 | 1.02 | 0.95–1.09 | 0.6 |
| Biopsy cores per target | 1.25 | 1.08–1.45 | 0.003 | 1.10 | 0.89–1.36 | 0.4 |
| PI-RADS score of index lesion | 3.82 | 2.39–6.12 | <0.001 | 2.38 | 1.16–4.89 | 0.02 |
| Negative biopsy within 5 years | 0.73 | 0.39–1.36 | 0.32 | 0.50 | 0.19–1.27 | 0.14 |
| Abnormal DRE | 1.99 | 1.05–3.75 | 0.03 | 1.05 | 0.43–2.57 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, P.-F.; Li, P.-I.; Lin, W.-C.; Chang, H.; Chang, C.-H.; Wu, H.-C.; Chang, Y.-H.; Wang, Y.-D.; Huang, W.-C.; Huang, C.-P. Learning Curve of Transperineal MRI/US Fusion Prostate Biopsy: 4-Year Experience. Life 2023, 13, 638. https://doi.org/10.3390/life13030638
Hsieh P-F, Li P-I, Lin W-C, Chang H, Chang C-H, Wu H-C, Chang Y-H, Wang Y-D, Huang W-C, Huang C-P. Learning Curve of Transperineal MRI/US Fusion Prostate Biopsy: 4-Year Experience. Life. 2023; 13(3):638. https://doi.org/10.3390/life13030638
Chicago/Turabian StyleHsieh, Po-Fan, Po-I Li, Wei-Ching Lin, Han Chang, Chao-Hsiang Chang, Hsi-Chin Wu, Yi-Huei Chang, Yu-De Wang, Wen-Chin Huang, and Chi-Ping Huang. 2023. "Learning Curve of Transperineal MRI/US Fusion Prostate Biopsy: 4-Year Experience" Life 13, no. 3: 638. https://doi.org/10.3390/life13030638
APA StyleHsieh, P.-F., Li, P.-I., Lin, W.-C., Chang, H., Chang, C.-H., Wu, H.-C., Chang, Y.-H., Wang, Y.-D., Huang, W.-C., & Huang, C.-P. (2023). Learning Curve of Transperineal MRI/US Fusion Prostate Biopsy: 4-Year Experience. Life, 13(3), 638. https://doi.org/10.3390/life13030638









