Role of the Interventional Radiologist in the Treatment of Desmoid Tumors
Abstract
1. Introduction
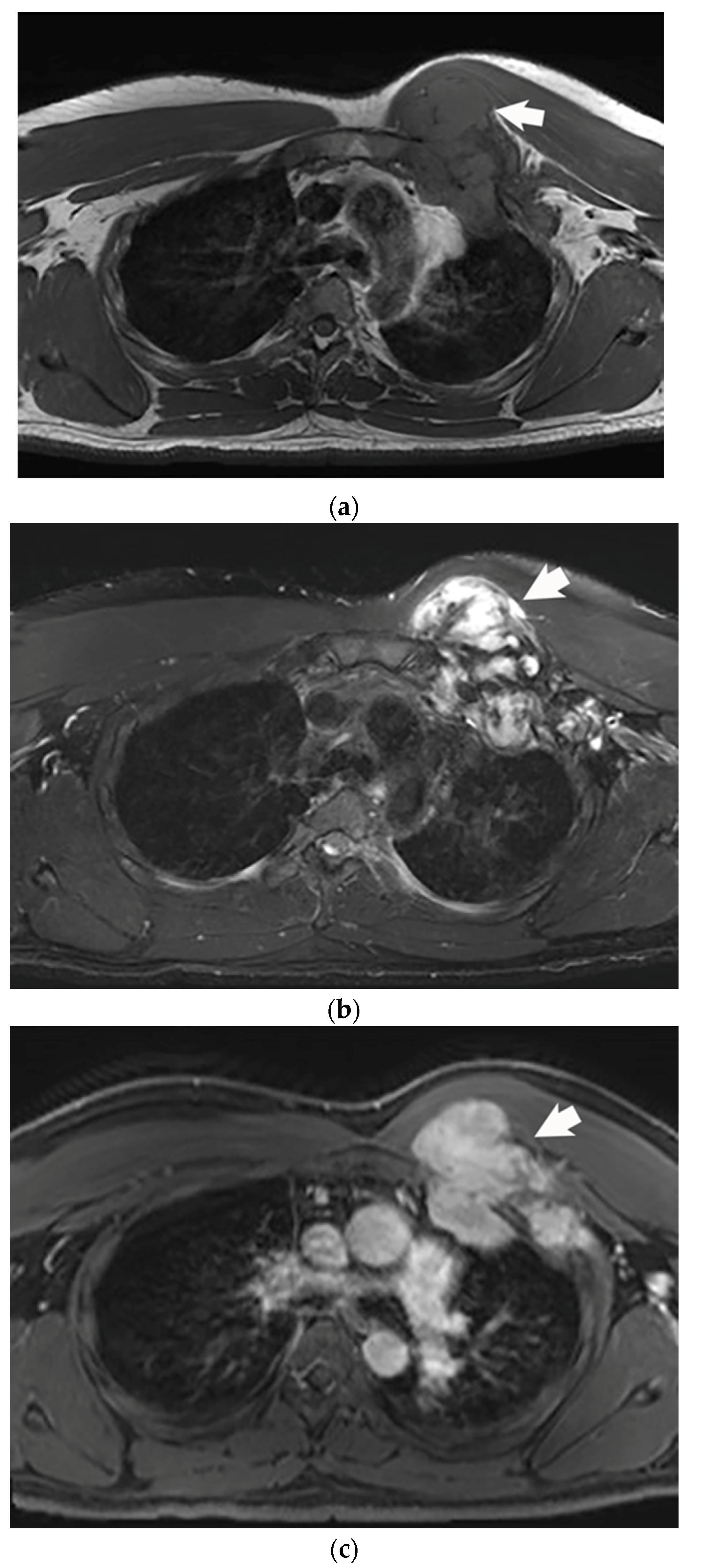
2. Imaging Characteristics
3. Management of Desmoid Tumors
4. Ablation
4.1. Chemical
4.2. Radiofrequency
4.3. Cryoablation
4.4. Microwave
4.5. High Intensity Focused Ultrasound
5. Transcatheter Embolization
6. Future Directions
7. Conclusions
Funding
Conflicts of Interest
References
- Kasper, B.; Ströbel, P.; Hohenberger, P. Desmoid Tumors: Clinical Features and Treatment Options for Advanced Disease. Oncologist 2011, 16, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, M.H.; Casparie, M.; Mathus-Vliegen, L.M.H.; Dekkers, O.M.; Hogendoorn, P.C.W.; Vasen, H.F.A. A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int. J. Cancer 2011, 129, 256–261. [Google Scholar] [CrossRef]
- Eastley, N.; McCulloch, T.; Esler, C.; Hennig, I.; Fairbairn, J.; Gronchi, A.; Ashford, R. Extra-abdominal desmoid fibromatosis: A review of management, current guidance and unanswered questions. Eur. J. Surg. Oncol. 2016, 42, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Crago, A.M.; Chmielecki, J.; Rosenberg, M.; O'Connor, R.; Byrne, C.; Wilder, F.G.; Thorn, K.; Agius, P.; Kuk, D.; Socci, N.D.; et al. Near universal detection of alterations in CTNNB1 and Wnt pathway regulators in desmoid-type fibromatosis by whole-exome sequencing and genomic analysis. Genes Chromosomes Cancer 2015, 54, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Le Guellec, S.; Soubeyran, I.; Rochaix, P.; Filleron, T.; Neuville, A.; Hostein, I.; Coindre, J.-M. CTNNB1 mutation analysis is a useful tool for the diagnosis of desmoid tumors: A study of 260 desmoid tumors and 191 potential morphologic mimics. Mod. Pathol. 2012, 25, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Braschi-Amirfarzan, M.; Keraliya, A.R.; Krajewski, K.M.; Tirumani, S.H.; Shinagare, A.B.; Hornick, J.; Baldini, E.H.; George, S.; Ramaiya, N.H.; Jagannathan, J.P. Role of imaging in management of desmoid-type fibromatosis: A primer for radiologists. Radiographics 2016, 36, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Thorner, P. Pediatric Radiology MRI of Fibromatosis: With Pathologic Correlation. Pediatr. Radiol. 1992, 22, 587–589. [Google Scholar] [CrossRef]
- Robbin, M.R.; Murphy, M.D.; Temple, H.T.; Kransdorf, M.; Choi, J. Imaging of Musculoskeletal Fibromatosis. Radiographics 2001, 21, 585–600. [Google Scholar] [CrossRef]
- Von Mehren, M.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; Gonzalez, R.J.; et al. NCCN Guidel. Version 2.2022 Soft Tissue Sarcoma. 2022. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1464 (accessed on 15 December 2022).
- Bonvalot, S.; Ternès, N.; Fiore, M.; Bitsakou, G.; Colombo, C.; Honoré, C.; Marrari, A.; Le Cesne, A.; Perrone, F.; Dunant, A.; et al. Spontaneous regression of primary abdominal wall desmoid tumors: More common than previously thought. Ann. Surg. Oncol. 2013, 20, 4096–4102. [Google Scholar] [CrossRef]
- Nuyttens, J.J.; Rust, P.F.; Thomas, C.R.; Turrisi, A.T. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: A comparative review of 22 articles. Cancer 2000, 88, 1517–1523. [Google Scholar] [CrossRef]
- Erdogan, O.; Parlakgumus, A.; Kulahci, O.; Irkorucu, O. Abdominal Wall Reconstruction for Desmoid Tumors Following Radical Resection from the Abdominoplasty Incision: Case Report. Niger. J. Clin. Pract. 2021, 24, 1100–1102. [Google Scholar] [PubMed]
- Constantinidou, A.; Jones, R.L.; Scurr, M.; Al-Muderis, O.; Judson, I. Pegylated liposomal doxorubicin, an effective, well-tolerated treatment for refractory aggressive fibromatosis. Eur. J. Cancer 2009, 45, 2930–2934. [Google Scholar] [CrossRef] [PubMed]
- Riedel, R.F.; Agulnik, M. Evolving strategies for management of desmoid tumor. Cancer 2022, 128, 3027–3040. [Google Scholar] [CrossRef] [PubMed]
- Alman, B.; Attia, S.; Baumgarten, C.; Benson, C.; Blay, J.-Y.; Bonvalot, S.; Breuing, J.; Cardona, K.; Casali, P.G.; van Coevorden, F.; et al. The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients. Eur. J. Cancer 2020, 127, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, R.L.; Gantzer, J.; de Marini, P.; Garnon, J.; Koch, G.; Buy, X.; Autrusseau, P.-A.; Auloge, P.; Dalili, D.; Kurtz, J.-E.; et al. Sporadic Desmoid Tumours: Systematic Review with Reflection on the Role of Cryoablation. Cardiovasc. Interv. Radiol. 2022, 45, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.W.I. Percutaneous Chemical Ablation of Desmoid Tumors. J. Vasc. Interv. Radiol. 2003, 14, 629–633. [Google Scholar] [CrossRef]
- Shiina, S.; Tagawa, K.; Unuma, T.; Takanashi, R.; Yoshiura, K.; Komatsu, Y.; Hata, Y.; Niwa, Y.; Shiratori, Y.; Terano, A.; et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991, 68, 1524–1530. [Google Scholar] [CrossRef]
- Ohnishi, K.; Yoshioka, H.; Ito, S.; Fujiwara, K. Treatment of nodular hepatocellular carcinoma larger than 3 cm with ultrasound-guided percutaneous acetic acid injection. Hepatology. 1996, 24, 1379–1385. [Google Scholar] [CrossRef]
- Hong, K.; Georgiades, C. Radiofrequency ablation: Mechanism of action and devices. J. Vasc. Interv. Radiol. 2010, 21 Suppl. 8. [Google Scholar] [CrossRef]
- Louis Hinshaw, J.; Lubner, M.G.; Ziemlewicz, T.J.; Lee, F.T.; Brace, C.L. Percutaneous tumor ablation tools: Microwave, radiofrequency, or cryoablation-what should you use and why? Radiographics 2014, 34, 1344–1362. [Google Scholar] [CrossRef]
- Tsz-Kan, T.; Man-Kwong, C.; Shu Shang-Jen, J.; Ying-Lee, L.; Wai Man-Wah, A.; Hon-Shing, F. Radiofrequency Ablation of Recurrent Fibromatosis. J. Vasc. Interv. Radiol. 2007, 18, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Ilaslan, H.; Schils, J.; Joyce, M.; Marks, K.; Sundaram, M. Radiofrequency ablation: Another treatment option for local control of desmoid tumors. Skeletal Radiol. 2010, 39, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, L.; Ravetta, V.; Viera, F.T.; Filisetti, C.; Siri, B.; Segalini, E.; Maestri, M.; Dominioni, T.; Alessiani, M.; Rossi, S.; et al. The challenge of extraabdominal desmoid tumour management in patients with Gardner’s syndrome: Radiofrequency ablation, a promising option. World J Surg Oncol. 2014, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Barrow, E.; Newton, K.; Rajashanker, B.; Lee, S.; Evans, D.G.; Hill, J. Successful radiofrequency ablation of an anterior abdominal wall desmoid in familial adenomatous polyposis. Color. Dis. 2013, 15, e160–e163. [Google Scholar] [CrossRef]
- Wang, L.; Xu, D.; Chen, L.; Huang, P. Percutaneous ultrasound-guided radiofrequency ablation for giant desmoid tumors of the intra-abdominal cavity in a patient with Gardner syndrome. J Cancer Res Ther. 2021, 17, 1286–1288. [Google Scholar] [CrossRef]
- Gage, A.A.; Baust, J. Mechanisms of Tissue Injury in Cryosurgery. Cryobiology. 1998, 37, 171–186. [Google Scholar] [CrossRef]
- Kujak, J.L.; Liu, P.T.; Johnson, G.B.; Callstrom, M.R. Early experience with percutaneous cryoablation of extra-abdominal desmoid tumors. Skelet. Radiol. 2010, 39, 175–182. [Google Scholar] [CrossRef]
- Havez, M.; Lippa, N.; Al-Ammari, S.; Kind, M.; Stoeckle, E.; Italiano, A.; Gangi, A.; Hauger, O.; Cornelis, F. Percutaneous Image-Guided Cryoablation in Inoperable Extra-abdominal Desmoid Tumors: A Study of Tolerability and Efficacy. Cardiovasc. Interv. Radiol. 2014, 37, 1500–1506. [Google Scholar] [CrossRef]
- Schmitz, J.J.; Schmit, G.D.; Atwell, T.D.; Callstrom, M.R.; Kurup, A.N.; Weisbrod, A.J.; Morris, J.M. Percutaneous cryoablation of extraabdominal desmoid tumors: A 10-year experience. Am. J. Roentgenol. 2016, 207, 190–195. [Google Scholar] [CrossRef]
- Redifer Tremblay, K.; Lea, W.B.; Neilson, J.C.; King, D.M.; Tutton, S.M. Percutaneous cryoablation for the treatment of extra-abdominal desmoid tumors. J. Surg. Oncol. 2019, 120, 366–375. [Google Scholar] [CrossRef]
- Saltiel, S.; Bize, P.E.; Goetti, P.; Gallusser, N.; Cherix, S.; Denys, A.; Becce, F.; Tsoumakidou, G. Cryoablation of extra-abdominal desmoid tumors: A single-center experience with literature review. Diagnostics 2020, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Bouhamama, A.; Lame, F.; Mastier, C.; Cuinet, M.; Thibaut, A.; Beji, H.; Ricoeur, A.; Blay, J.-Y.; Pilleul, F. Local Control and Analgesic Efficacy of Percutaneous Cryoablation for Desmoid Tumors. Cardiovasc. Interv. Radiol. 2020, 43, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Y.; Walsh, J.P.; Munk, P.L.; Mallinson, P.I.; Simmons, C.; Clarkson, P.W.; Jayaram, P.R.; Heran, M.K.; Ouellette, H.A. A Single-Center 10-Year Retrospective Analysis of Cryoablation for the Management of Desmoid Tumors. J. Vasc. Interv. Radiol. 2021, 32, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.; Alves, A.; Messiou, C.; Napolitano, A.; Strauss, D.; Hayes, A.; Smith, M.; Benson, C.; Jones, R.; Gennatas, S.; et al. Percutaneous cryoablation for desmoid fibromatosis: Initial experience at a UK centre. Clin Radiol. 2022, 77, 784–793. [Google Scholar] [CrossRef]
- Auloge, P.; Garnon, J.; Robinson, J.M.; Thenint, M.-A.; Koch, G.; Caudrelier, J.; Weiss, J.; Cazzato, R.L.; Kurtz, J.E.; Gangi, A. Percutaneous cryoablation for advanced and refractory extra-abdominal desmoid tumors. Int. J. Clin. Oncol. 2021, 26, 1147–1158. [Google Scholar] [CrossRef]
- Vora, B.M.K.; Munk, P.L.; Somasundaram, N.; Ouellette, H.A.; Mallinson, P.I.; Sheikh, A.; Kadir, H.A.; Tan, T.J.; Yan, Y.Y. Cryotherapy in extra-abdominal desmoid tumors: A systematic review and metaanalysis. PLoS ONE 2021, 16, e0261657. [Google Scholar] [CrossRef]
- Kurtz, J.-E.; Buy, X.; Deschamps, F.; Sauleau, E.; Bouhamama, A.; Toulmonde, M.; Honoré, C.; Bertucci, F.; Brahmi, M.; Chevreau, C.; et al. CRYODESMO-O1: A prospective, open phase II study of cryoablation in desmoid tumour patients progressing after medical treatment. Eur. J. Cancer 2021, 143, 78–87. [Google Scholar] [CrossRef]
- Wright, A.S.; Sampson, L.A.; Warner, T.F.; Mahvi, D.M.; Lee, F.T. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005, 236, 132–139. [Google Scholar] [CrossRef]
- Brace, C.L. Radiofrequency and Microwave Ablation of the Liver, Lung, Kidney, and Bone: What Are the Differences? Curr. Probl. Diagn. Radiol. 2009, 38, 135–143. [Google Scholar] [CrossRef]
- Martínez-Martínez, A.; García-Espinosa, J.; Ramos-Bossini, A.J.L.; Santiago, F.R. Percutaneous microwave ablation of desmoid fibromatosis. Korean J. Radiol. 2021, 22, 944–950. [Google Scholar] [CrossRef]
- Griffin, M.O.; Kulkarni, N.M.; O’Connor, S.D.; Sudakoff, G.S.; Lea, W.B.; Tutton, S.M. Magnetic Resonance-Guided Focused Ultrasound: A Brief Review with Emphasis on the Treatment of Extra-abdominal Desmoid Tumors. Ultrasound Q. 2019, 35, 346–354. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Tang, J. Ultrasound-guided high intensity focused ultrasound treatment for extra-abdominal desmoid tumours: Preliminary results. Int. J. Hyperth. 2011, 27, 648–653. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, Y.; Zhou, M.; Ying, X.; Hu, X. High-intensity focused ultrasound treatment for intra-abdominal desmoid tumors: A report of four cases. J. Med. Ultrason. 2016, 43, 279–284. [Google Scholar] [CrossRef]
- Zhong, X.; Hu, X.; Zhao, P.; Wang, Y.; Fang, X.F.; Shen, J.; Shen, H.; Yuan, Y. The efficacy of low-power cumulative high-intensity focused ultrasound treatment for recurrent desmoid tumor. Cancer Med. 2022, 11, 2079–2084. [Google Scholar] [CrossRef]
- Mo, S.; Chen, J.; Zhang, R.; Yang, C.; Wang, T.; Chen, L.; Chen, W. High-Intensity Focused Ultrasound Ablation for Postoperative Recurrent Desmoid Tumors: Preliminary Results. Ultrasound Med Biol. 2022, 48, 638–645. [Google Scholar] [CrossRef]
- Avedian, R.S.; Bitton, R.; Gold, G.; Butts-Pauly, K.; Ghanouni, P. Is MR-guided High-intensity Focused Ultrasound a Feasible Treatment Modality for Desmoid Tumors? Clin. Orthop. Relat. Res. 2016, 474, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Fuchs, B.; Binkert, C.A. Mid-term results of MR-guided high-intensity focused ultrasound treatment for relapsing superficial desmoids. Int. J. Hyperth. 2019, 36, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Bucknor, M.D.; Rieke, V. MRgFUS for desmoid tumors within the thigh: Early clinical experiences. J. Ther. Ultrasound 2017, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ghanouni, P.; Dobrotwir, A.; Bazzocchi, A.; Bucknor, M.; Bitton, R.; Rosenberg, J.; Telischak, K.; Busacca, M.; Ferrari, S.; Albisinni, U.; et al. Magnetic resonance-guided focused ultrasound treatment of extra-abdominal desmoid tumors: A retrospective multicenter study. Eur. Radiol. 2017, 27, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Elnekave, E.; Atar, E.; Amar, S.; Bruckheimer, E.; Knizhnik, M.; Yaniv, I.; Dujovny, T.; Feinmesser, M.; Ash, S. Doxorubicin-Eluting Intra-Arterial Therapy for Pediatric Extra-Abdominal Desmoid Fibromatoses: A Promising Approach for a Perplexing Disease. J. Vasc. Interv. Radiol. 2018, 29, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Evans, H.L.; Benjamin, R.S. Combination chemotherapy in adult desmoid tumors. Cancer 1993, 72, 3244–3247. [Google Scholar] [CrossRef] [PubMed]
- Garbay, D.; Le Cesne, A.; Penel, N.; Chevreau, C.; Marec-Berard, P.; Blay, J.-Y.; Debled, M.; Isambert, N.; Thyss, A.; Bompas, E.; et al. Chemotherapy in patients with desmoid tumors: A study from the French Sarcoma Group (FSG). Ann. Oncol. 2012, 23, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Keohan, M.L.; Gounder, M.M.; Crago, A.M.; Erinjeri, J.P. Transarterial Chemoembolization with Doxorubicin Eluting Beads for Extra-Abdominal Desmoid Tumors: Initial Experience. Cardiovasc. Interv. Radiol. 2022, 45, 1141–1151. [Google Scholar] [CrossRef] [PubMed]

| Imaging Modality | Advantages | Disadvantages |
|---|---|---|
| Ultrasound |
|
|
| CT |
|
|
| MRI |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldberg, D.; Woodhead, G.; Hannallah, J.; Young, S. Role of the Interventional Radiologist in the Treatment of Desmoid Tumors. Life 2023, 13, 645. https://doi.org/10.3390/life13030645
Goldberg D, Woodhead G, Hannallah J, Young S. Role of the Interventional Radiologist in the Treatment of Desmoid Tumors. Life. 2023; 13(3):645. https://doi.org/10.3390/life13030645
Chicago/Turabian StyleGoldberg, Daniel, Gregory Woodhead, Jack Hannallah, and Shamar Young. 2023. "Role of the Interventional Radiologist in the Treatment of Desmoid Tumors" Life 13, no. 3: 645. https://doi.org/10.3390/life13030645
APA StyleGoldberg, D., Woodhead, G., Hannallah, J., & Young, S. (2023). Role of the Interventional Radiologist in the Treatment of Desmoid Tumors. Life, 13(3), 645. https://doi.org/10.3390/life13030645







