Evaluation of Serum Iron Parameters among Men Performing Regular Physical Activity—A Preliminary Study
Abstract
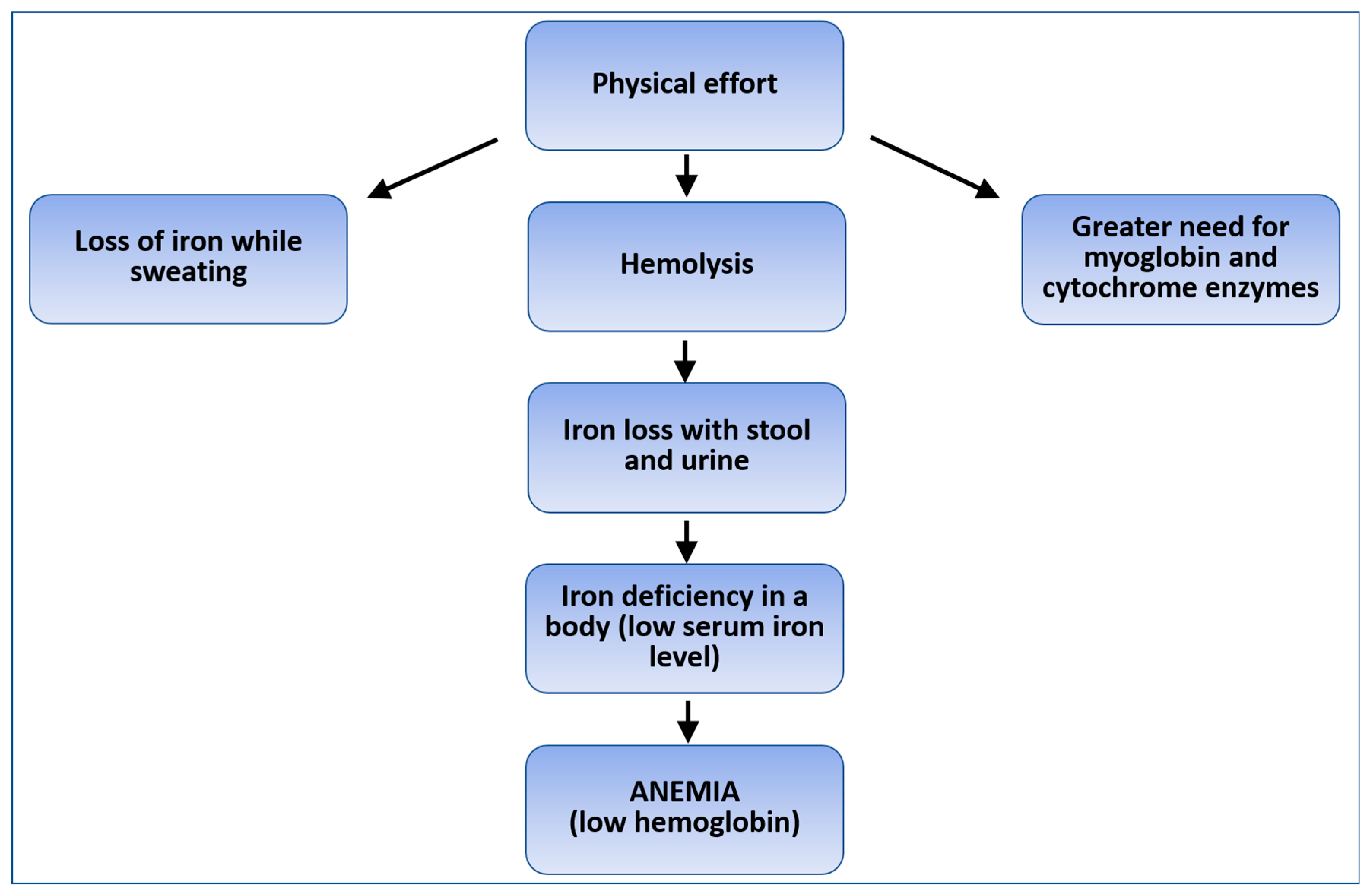
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Changes in Serum Ferritin Levels
4.2. Changes in Red Blood Cell Parameters
4.3. Changes in the WBC Count
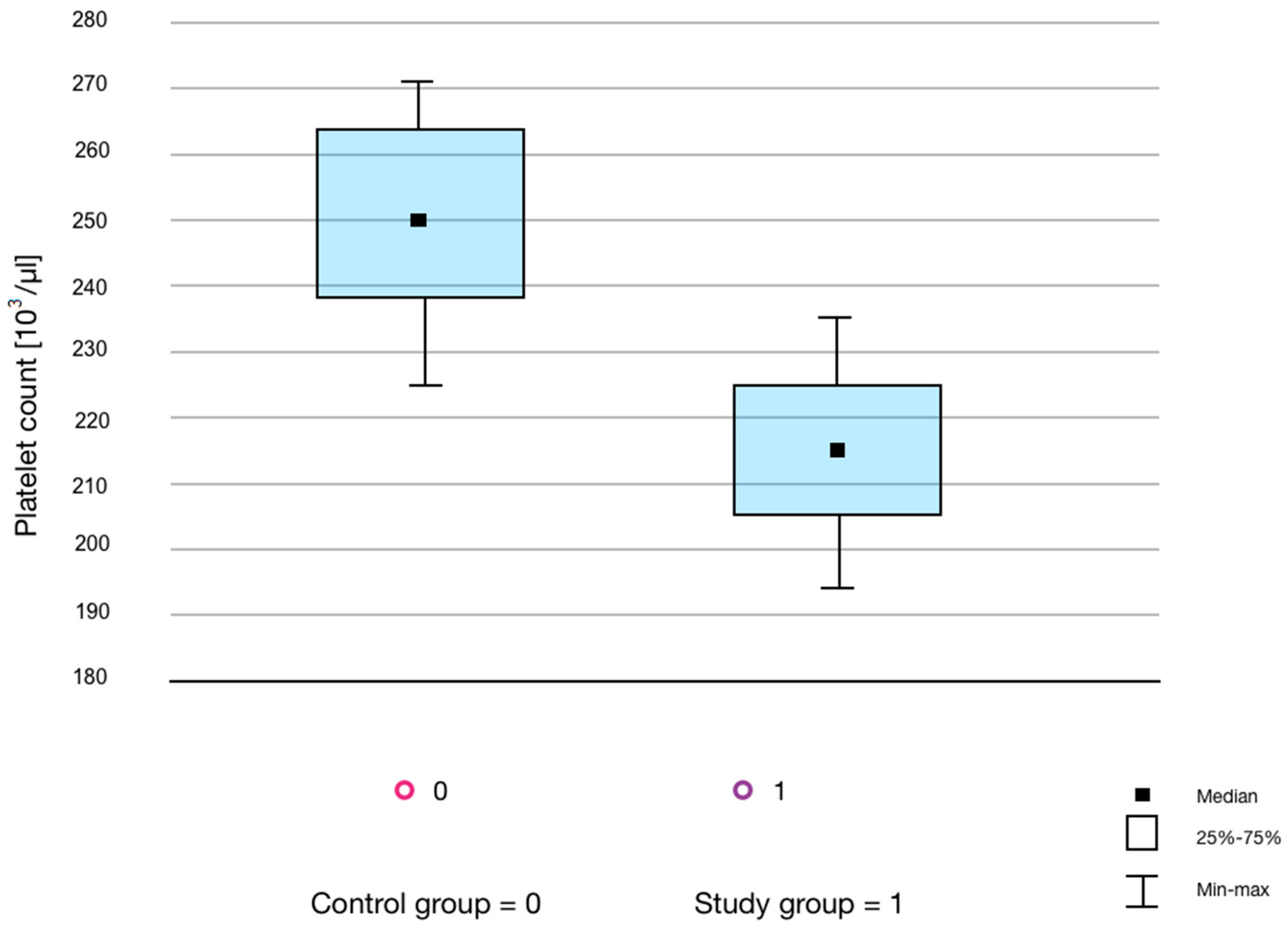
4.4. Changes in the PLT Count
4.5. Goals and Weaknesses of the Experiment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gattermann, N.; Muckenthaler, M.U.; Kulozik, A.E.; Metzgeroth, G.; Hastka, J. The Evaluation of Iron Deficiency and Iron Overload. Dtsch. Arztebl. Int. 2021, 118, 847–856. [Google Scholar] [CrossRef]
- Weyh, C.; Krüger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel-Abramowski, S.; Waeber, G.; Gassner, C.; Buser, A.; Frey, B.M.; Favrat, B.; Tissot, J.-D. Physiology of Iron Metabolism. Transfus. Med. Hemother. 2014, 41, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Maniňo, M.M.; Grijota, F.J.; Bartołomé, I.; Siguier-Coll, J.; Román, V.T.; Muňoz, D. Influence of physical training on erythrocyte concentrations of iron, phosphorus and magnesium. J. Int. Soc. Sport. Nutr. 2020, 17, 8. [Google Scholar]
- McKay, A.; Sim, M.; Moretti, D.; Hall, R.; Stellingwerff, T.; Burden, R.; Peeling, P. Methodological Considerations for Investigating Iron Status and Regulation in Exercise and Sport Science Studies. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 359–370. [Google Scholar] [CrossRef]
- Plays, M.; Müller, S.; Rodriguez, R. Chemistry and Biology of Ferritin. Metallomics 2021, 13, mfab021. [Google Scholar] [CrossRef]
- Roy, R.; Kück, M.; Radziwolek, L.; Kerling, A. Iron Deficiency in Adolescent and Young Adult German Athletes-A Retrospective Study. Nutrients 2022, 14, 4511. [Google Scholar] [CrossRef]
- DePalma, R.G.; Hayes, V.W.; O’Leary, T.J. Optimal serum ferritin level range: Iron status measure and inflammatory biomarker. Metallomics 2021, 13, mfab030. [Google Scholar] [CrossRef]
- Bauri, S.; Martin, J. Investigation of iron deficiency anaemia. Clin. Med. 2018, 18, 242–244. [Google Scholar] [CrossRef]
- Lee, N.K.; Cho, S.; Kim, I.-S. Ferritin—A multifaceted protein scaffold for biotherapeutics. Exp. Mol. Med. 2022, 54, 1652–1657. [Google Scholar] [CrossRef]
- Muhoberac, B.B.; Vida, R. Iron, Ferritin, Hereditary Ferritinopathy, and Neurodegeneration. Front. Neurosci. 2019, 13, 1195. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G. Iron metabolism in the anemia of chronic disease. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 682–693. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; van Assen, S. Exercise-induced anaemia: A forgotten cause of iron deficiency anaemia in young adults. Br. J. Gen. Pract. 2015, 65, 268–269. [Google Scholar] [CrossRef]
- Cohen, D.C.; Winstanley, A.; Engledow, A.; Windsor, A.; Skipworth, J.R. Marathon-induced ischemic colitis: Why running is not always good for you. Am. J. Emerg. Med. 2009, 27, e5–e255. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Trinder, D. Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 2008, 103, 381–391. [Google Scholar] [CrossRef]
- Grijota, F.J.; Román, V.T.; Siguier-Coll, J.; Robles-Gil, M.C.; Muňoz, D.; Maynar-Mariňo, M. Total Iron Concentrations in Different Biological Matrices-Influence of Physical Training. Nutrients 2022, 14, 3549. [Google Scholar] [CrossRef]
- Schmidt, M.; Ohlendorf, D.; Gronberg, D.A.; Wanke, E.M. Fit to Teach? Cardiorespiratory Capacity, Vitamin D3, and Ferritin in Physical Education Teachers with Specialization in Dance. J. Strength Cond. Res. 2021, 35, 1156–1164. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P.; Hatch-McChesney, A.; Allen, J.T.; Wilson, M.A.; Carrigan, C.T.; Murphy, N.E.; Teien, H.K.; Martini, S.; Gwin, J.A.; et al. Energy deficit increases hepcidin and exacerbates declines in dietary iron absorption following strenuous physical activity: A randomized-controlled cross-over trial. Am. J. Clin. Nutr. 2021, 113, 359–369. [Google Scholar] [CrossRef]
- DeLoughery, T.G. Microcytic anemia. N. Engl. J. Med. 2014, 371, 1324–1331. [Google Scholar] [CrossRef]
- Braga, F.; Pasqualetti, S.; Frusciante, E.; Borrillo, F.; Chibireva, M.; Panteghini, M. Harmonization Status of Serum Ferritin Measurements and Implications for Use as Marker of Iron-Related Disorders. Clin. Chem. 2022, 68, 1202–1210. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef]
- Nabhana, D.; Bielko, S.; Sinex, J.A.; Surhoff, K.; Moreaud, W.J.; Schumacher, Y.O.; Bahr, R.; Chapman, R.F. Serum ferritin distribution in elite athletes. J. Sci. Med. Sport 2020, 23, 554–558. [Google Scholar] [CrossRef]
- Mörwald, K.; Aigner, E.; Bergsten, P.; Brunner, S.M.; Forslund, A.; Kullberg, J.; Ahlström, H.; Manell, H.; Roomp, K.; Schütz, S.; et al. Serum Ferritin Correlates with Liver Fat in Male Adolescents with Obesity. Front. Endocrinol. 2020, 11, 340. [Google Scholar] [CrossRef]
- Shattnawi, K.K.; Alomari, M.A.; Al-Sheyab, N.; Salameh, A.B. The relationship between plasma ferritin levels and body mass index among adolescents. Sci. Rep. 2018, 8, 15307. [Google Scholar] [CrossRef]
- Shakaroun, D.; Lazar, M.H.; Horowitz, J.C.; Jennings, J.H. Serum Ferritin as a Predictor of Outcomes in Hospitalized Patients with COVID-19 Pneumonia. J. Intensive Care Med. 2023, 38, 21–26. [Google Scholar] [CrossRef]
- Clénin, G.; Cordes, M.; Huber, A.; Schumacher, Y.O.; Noack, P.; Scales, J.; Kriemler, S. Iron deficiency in sports—Definition, influence on performance and therapy. Swiss Med. Wkly. 2015, 145, w14196. [Google Scholar] [CrossRef]
- Morgado, J.P.; Monteiro, C.P.; Teles, J.; Reis, J.F.; Matias, C.; Seixas, M.T.; Alvim, M.G.; Bourbon, M.; Laires, M.J.; Alves, F. Immune cell changes in response to a swimming training session during a 24-h recovery period. Appl. Physiol. Nutr. Metab. 2016, 41, 476–483. [Google Scholar] [CrossRef]
- Bachero-Mena, B.; Pareja-Blanco, F.; González-Badillo, J.J. Enhanced Strength and Sprint Levels, and Changes in Blood Parameters during a Complete Athletics Season in 800 m High-Level Athletes. Front. Physiol. 2017, 8, 637. [Google Scholar] [CrossRef]
- Lai, S.-W.; Tsai, K.-Z.; Lin, Y.-P.; Liu, P.-Y.; Lin, Y.-K.; Chang, P.-Y.; Dai, M.-S.; Chao, T.-Y.; Han, C.-L.; Lin, G.-M. Association of red blood cell size and physical fitness in a military male cohort: The CHIEF study. Scand. J. Med. Sci. Sport. 2021, 31, 295–302. [Google Scholar] [CrossRef]
- Schmidt, W.; Prommer, N. Effects of various training modalities on blood volume. Scand. J. Med. Sci. Sport. 2008, 18, 57–69. [Google Scholar] [CrossRef]
- Horn, P.L.; Pyne, D.B.; Hopkins, W.G.; Barnes, C.J. Lower white blood cell counts in elite athletes training for highly aerobic sports. Eur. J. Appl. Physiol. 2010, 110, 925–932. [Google Scholar] [CrossRef]
- Malczewska-Lenczowska, J.; Sitkowski, D.; Orysiak, J.; Pokrywka, A.; Szygula, Z. Total haemoglobin mass, blood volume and morphological indices among athletes from different sport disciplines. Arch. Med. Sci. 2013, 9, 780–787. [Google Scholar] [CrossRef]
- Heber, S.; Volf, I. Effects of Physical (In)activity on Platelet Function. Biomed. Res. Int. 2015, 2015, 165078. [Google Scholar] [CrossRef]
- Davis, R.B.; Boyd, D.G.; McKinney, M.E.; Jones, C.C. Effects of exercise and exercise conditioning on blood platelet function. Med. Sci. Sport. Exerc. 1990, 22, 49–53. [Google Scholar] [CrossRef]

| Control | Sportsmen | p | |
|---|---|---|---|
| Age (years) mean (minimum–maximum) | 25 (21–40) | 27 (22–38) | 0.132 |
| Physical activity (minutes/week) mean (minimum–maximum) | 35 (20–50) | 225 (180–360) | 0.00089 |
| Body mass index (kg/m2) | 18.5–24.9 | ||
| Iron supplementation | no | ||
| Diet | normal | ||
| Smoking | no | ||
| Acute/Chronic diseases | no | ||
| Medicaments | no | ||
| Stress | no | ||
| Well-condition | yes | ||
| Parameter | Control Group (n = 20) | Study Group (n = 20) | ||||
|---|---|---|---|---|---|---|
| Median | Lower-Upper Quartile | Median | Lower-Upper Quartile | p | r-Pearson | |
| RBC [106/µL] | 5.08 | 4.91–5.45 | 5.17 | 4.87–5.31 | 0.79 | −0.36 |
| HGB [g/dL] | 15.90 | 15.40–16.55 | 15.55 | 15.00–15.85 | 0.18 | −0.35558 |
| HCT [%] | 47.00 | 44.90–49.85 | 46.75 | 44.55–47.60 | 0.41 | −0.3342 |
| MCV [µm3] | 91.00 | 88.20–94.00 | 90.55 | 89.00–92.10 | 0.46 | 0.2891 |
| MCH [pg] | 30.90 | 29.50–31.50 | 30.10 | 29.80–30.55 | 0.27 | −0.1639 |
| MCHC [g/dL] | 33.60 | 32.85–34.30 | 33.40 | 32.90–33.75 | 0.33 | 0.1433 |
| RDW-CV [%] | 13.10 | 12.80–13.65 | 13.60 | 12.95–13.95 | 0.09 | - |
| RDW-SD [µm3] | 40.30 | 39.50–43.70 | 41.20 | 40.30–44.10 | 0.59 | - |
| PLT [103/µL] | 245.50 | 212.00–283.50 | 217 | 195.50–250.50 | 0.04 | 0.0403 |
| WBC [103/µL] | 5.63 | 5.20–7.56 | 5.70 | 4.87–6.51 | 0.31 | −0.1515 |
| Ferritin—measurement 1 [ng/mL] | 21.53 | 16.40–39.53 | 14.87 | 9.19–22.24 | - | |
| Ferritin—measurement 2 [ng/mL] | 25.00 | 17.65–52.78 | 16.74 | 9.36–22.18 | - | |
| Ferritin—average [ng/mL] | 23.12 | 17.44–46.16 | 15.81 | 9.26–22.69 | 0.01 | −0.0856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamelska, K.; Rzepka, M.; Olszewska-Słonina, D.; Woźniak, A.; Szewczyk-Golec, K.; Hołyńska-Iwan, I. Evaluation of Serum Iron Parameters among Men Performing Regular Physical Activity—A Preliminary Study. Life 2023, 13, 670. https://doi.org/10.3390/life13030670
Zamelska K, Rzepka M, Olszewska-Słonina D, Woźniak A, Szewczyk-Golec K, Hołyńska-Iwan I. Evaluation of Serum Iron Parameters among Men Performing Regular Physical Activity—A Preliminary Study. Life. 2023; 13(3):670. https://doi.org/10.3390/life13030670
Chicago/Turabian StyleZamelska, Klaudia, Mateusz Rzepka, Dorota Olszewska-Słonina, Alina Woźniak, Karolina Szewczyk-Golec, and Iga Hołyńska-Iwan. 2023. "Evaluation of Serum Iron Parameters among Men Performing Regular Physical Activity—A Preliminary Study" Life 13, no. 3: 670. https://doi.org/10.3390/life13030670
APA StyleZamelska, K., Rzepka, M., Olszewska-Słonina, D., Woźniak, A., Szewczyk-Golec, K., & Hołyńska-Iwan, I. (2023). Evaluation of Serum Iron Parameters among Men Performing Regular Physical Activity—A Preliminary Study. Life, 13(3), 670. https://doi.org/10.3390/life13030670








