Efficacy and Safety of Gel Immersion Endoscopic Mucosal Resection for Non-Pedunculated Colorectal Polyps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Statement of Ethics
2.2. Outcome Measures
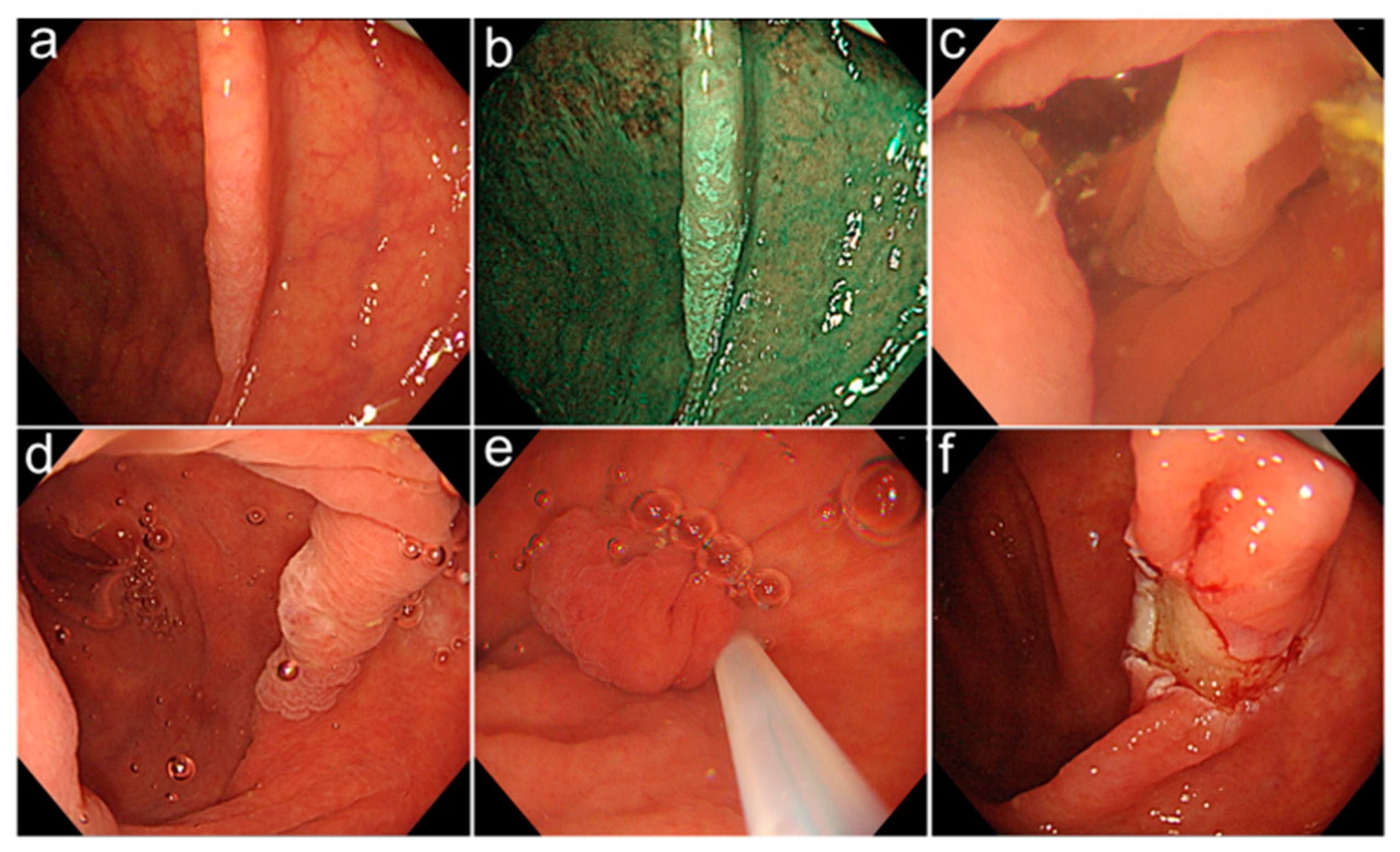
2.3. Endoscopic Procedures and Equipment
3. Results
3.1. Patients and Polyp Characteristics
3.2. Outcomes
3.3. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; Van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic Polypectomy and Long-Term Prevention of Colorectal-Cancer Deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef]
- East, J.E.; Atkin, W.S.; Bateman, A.C.; Clark, S.K.; Dolwani, S.; Ket, S.N.; Leedham, S.J.; Phull, P.S.; Rutter, M.D.; Shepherd, N.A.; et al. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 2017, 66, 1181–1196. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, T.; Takeuchi, Y.; Asai, S.; Yokota, I.; Akamine, E.; Kato, M.; Akamatsu, T.; Tada, K.; Komeda, Y.; Iwatate, M.; et al. A comparison of the resection rate for cold and hot snare polypectomy for 4–9 mm colorectal polyps: A multicentre randomised controlled trial (CRESCENT study). Gut 2018, 67, 1950–1957. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, A.; Nakayama, Y.; Kajiyama, M.; Tanaka, N.; Sano, K.; Graham, D.Y. Removal of small colorectal polyps in anticoagulated patients: A prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest. Endosc. 2014, 79, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Ferlitsch, M.; Moss, A.; Hassan, C.; Bhandari, P.; Dumonceau, J.-M.; Paspatis, G.; Jover, R.; Langner, C.; Bronzwaer, M.; Nalankilli, K.; et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017, 49, 270–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaltenbach, T.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Gupta, S.; Lieberman, D.; Robertson, D.J.; Shaukat, A.; Syngal, S.; Rex, D.K. Endoscopic Removal of Colorectal Lesions: Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2020, 115, 435–464. [Google Scholar] [CrossRef] [Green Version]
- Oka, S.; Tanaka, S.; Saito, Y.; Iishi, H.; Kudo, S.E.; Ikematsu, H.; Igarashi, M.; Saitoh, Y.; Inoue, Y.; Kobayashi, K.; et al. Local recurrence after endoscopic resection for large colorectal neoplasia: A multicenter prospective study in Japan. Am. J. Gastroenterol. 2015, 110, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H.; Srivastava, A.; Bensen, S.P.; Anderson, P.; Rothstein, R.I.; Gordon, S.R.; Levy, L.C.; Toor, A.; Mackenzie, T.A.; Rosch, T.; et al. Incomplete polyp resection during colonoscopy—Results of the complete adenoma resection (CARE) study. Gastroenterology 2013, 144, 74–80.e71. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Garimall, S.; Scott, F.I.; Ahmad, N.A.; Kochman, M.L.; Ginsberg, G.G.; Chandrasekhara, V. En bloc endoscopic mucosal resection is equally effective for sessile serrated polyps and conventional adenomas. Surg. Endosc. 2018, 32, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Fujii, T.; Saito, Y.; Matsuda, T. Local recurrence after endoscopic resection of colorectal tumors. Int. J. Color. Dis. 2009, 24, 225–230. [Google Scholar] [CrossRef]
- Binmoeller, K.F.; Weilert, F.; Shah, J.; Bhat, Y.; Kane, S. “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest. Endosc. 2012, 75, 1086–1091. [Google Scholar] [CrossRef]
- Kawamura, T.; Sakai, H.; Ogawa, T.; Sakiyama, N.; Ueda, Y.; Shirakawa, A.; Okada, Y.; Sanada, K.; Nakase, K.; Mandai, K.; et al. Feasibility of Underwater Endoscopic Mucosal Resection for Colorectal Lesions: A Single Center Study in Japan. Gastroenterol. Res. 2018, 11, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Siau, K.; Ishaq, S.; Cadoni, S.; Kuwai, T.; Yusuf, A.; Suzuki, N. Feasibility and outcomes of underwater endoscopic mucosal resection for ≥ 10° mm colorectal polyps. Surg. Endosc. 2018, 32, 2656–2663. [Google Scholar] [CrossRef]
- Yen, A.W.; Leung, J.W.; Wilson, M.D.; Leung, F.W. Underwater versus conventional endoscopic resection of nondiminutive nonpedunculated colorectal lesions: A prospective randomized controlled trial (with video). Gastrointest. Endosc. 2020, 91, 643–654.e642. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, T.; Uedo, N.; Akasaka, T.; Iwatsubo, T.; Nakatani, Y.; Akamatsu, T.; Kawamura, T.; Takeuchi, Y.; Fujii, S.; Kusaka, T.; et al. Comparison of Underwater vs Conventional Endoscopic Mucosal Resection of Intermediate-Size Colorectal Polyps. Gastroenterology 2019, 157, 451–461.e452. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Takezawa, T.; Hashimoto, K.; Ohmori, A.; Shinozaki, S.; Nagayama, M.; Sakamoto, H.; Miura, Y.; Hayashi, Y.; Sunada, K.; et al. Gel immersion endoscopy: Innovation in securing the visual field—Clinical experience with 265 consecutive procedures. Endosc. Int. Open 2021, 09, E1123–E1127. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Yano, T.; Takezawa, T.; Sakamoto, H.; Osawa, H.; Lefor, A.; Yamamoto, H. Gel immersion endoscopy simplifies hemostasis during endoscopic submucosal dissection using the pocket-creation method. Endoscopy 2018, 50, E294–E295. [Google Scholar] [CrossRef] [Green Version]
- Yano, T.; Nemoto, D.; Ono, K.; Miyata, Y.; Numao, N.; Iwashita, C.; Nagayama, M.; Takahashi, H.; Lefor, A.K.; Yamamoto, H. Gel immersion endoscopy: A novel method to secure the visual field during endoscopy in bleeding patients (with videos). Gastrointest. Endosc. 2016, 83, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Sudo, G.; Saito, M.; Onuma, K.; Takada, Y.; Yawata, A.; Nakase, H. Gel immersion endoscopic mucosal resection for early gastric cancer near the pyloric ring. Endoscopy 2022, 54, E644–E645. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, A.; Kuwai, T.; Miyauchi, T.; Shimura, H.; Shimura, K. Gel immersion endoscopy-facilitated endoscopic mucosal resection of a superficial nonampullary duodenal epithelial tumor: A novel approach. VideoGIE 2021, 6, 422–426. [Google Scholar] [CrossRef]
- Kuwabara, H.; Chiba, H.; Tachikawa, J.; Okada, N.; Arimoto, J.; Nakaoka, M. Efficacy of under-gel endoscopic mucosal resection method for colonic lesion extending into the diverticulum. Endoscopy 2022, 54, E292–E293. [Google Scholar] [CrossRef]
- Takada, K.; Hotta, K.; Imai, K. Gel immersion endoscopic mucosal resection with acetic acid spray for sessile serrated lesion extending close to the appendiceal orifice. Dig. Endosc. 2022, 34, e115–e116. [Google Scholar] [CrossRef] [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest. Endosc. 2003, 58, S3–S43. [CrossRef] [PubMed]
- Sano, Y.; Tanaka, S.; Kudo, S.-E.; Saito, S.; Matsuda, T.; Wada, Y.; Fujii, T.; Ikematsu, H.; Uraoka, T.; Kobayashi, N.; et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig. Endosc. 2016, 28, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Keklikkiran, C.; Ozdogan, O.C. Thermal ablation of mucosal defect margins reduces adenoma recurrence after colonic endoscopic mucosal resection. Turk. J. Gastroenterol. 2019, 30, 580–581. [Google Scholar] [CrossRef]
- Watabe, H.; Yamaji, Y.; Okamoto, M.; Kondo, S.; Ohta, M.; Ikenoue, T.; Kato, J.; Togo, G.; Matsumura, M.; Yoshida, H.; et al. Risk assessment for delayed hemorrhagic complication of colonic polypectomy: Polyp-related factors and patient-related factors. Gastrointest. Endosc. 2006, 64, 73–78. [Google Scholar] [CrossRef]
- Wada, Y.; Kudo, S.-E.; Tanaka, S.; Saito, Y.; Iishii, H.; Ikematsu, H.; Igarashi, M.; Saitoh, Y.; Inoue, Y.; Kobayashi, K.; et al. Predictive factors for complications in endoscopic resection of large colorectal lesions: A multicenter prospective study. Surg. Endosc. 2015, 29, 1216–1222. [Google Scholar] [CrossRef]
- Nakajima, T.; Saito, Y.; Tanaka, S.; Iishi, H.; Kudo, S.-E.; Ikematsu, H.; Igarashi, M.; Saitoh, Y.; Inoue, Y.; Kobayashi, K.; et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg. Endosc. 2013, 27, 3262–3270. [Google Scholar] [CrossRef]
- Kobayashi, N.; Yoshitake, N.; Hirahara, Y.; Konishi, J.; Saito, Y.; Matsuda, T.; Ishikawa, T.; Sekiguchi, R.; Fujimori, T. Matched case-control study comparing endoscopic submucosal dissection and endoscopic mucosal resection for colorectal tumors. J. Gastroenterol. Hepatol. 2012, 27, 728–733. [Google Scholar] [CrossRef]
- Miyakawa, A.; Kuwai, T.; Sakuma, Y.; Kubota, M.; Nakamura, A.; Itobayashi, E.; Shimura, H.; Suzuki, Y.; Shimura, K. A feasibility study comparing gel immersion endoscopic resection and underwater endoscopic mucosal resection for a superficial nonampullary duodenal epithelial tumor. Endoscopy 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Yamashina, T.; Shimatani, M.; Takahashi, Y.; Takeo, M.; Saito, N.; Matsumoto, H.; Kasai, T.; Kano, M.; Sumimoto, K.; Mitsuyama, T.; et al. Gel Immersion Endoscopic Mucosal Resection (EMR) for Superficial Nonampullary Duodenal Epithelial Tumors May Reduce Procedure Time Compared with Underwater EMR (with Video). Gastroenterol. Res. Pract. 2022, 2022, 2040792. [Google Scholar] [CrossRef] [PubMed]
| Scope | PCF-H290ZI/CF-EZ1500DI (Olympus Co., Tokyo, Japan) |
| Gel | Viscoclear® (Otsuka Pharmaceutical Factory, Tokushima, Japan) |
| Attachment | Elastic touch® (Top, Tokyo, Japan) Distal hood® (Olympus, Tokyo, Japan) |
| Channel device | BioShield Irrigator® (Fujifilm Medical, Tokyo, Japan) |
| Electrosurgical unit | VIO 300D ENDOCUT Q EFFECT 3/DURATION 2/INTERVAL 2 |
| Snare | CAPTIVATORII® (15/20 mm) (Boston scientific, Tokyo, Japan) SnareMater Plus® (15 mm) (Olympus, Tokyo, Japan) |
| No. of patients, n | n = 20 |
| Gender, male | 14 |
| Median age, years (range) | 70 (53–90) |
| Antithrombotic drugs | 2 |
| No. of lesions, n | n = 25 |
| Location, n (%) | |
| Cecum | 6 (24) |
| Ascending colon | 8 (32) |
| Transverse colon | 4 (16) |
| Descending colon | 3 (12) |
| Sigmoid colon | 4 (16) |
| Rectum | 0 (0) |
| Morphology, n (%) | |
| 0-Is/Isp | 3 (12) |
| 0-IIa | 22 (88) |
| NBI magnifying findings, n (%) | |
| JNET Type 1 | 9 (36) |
| JNET Type 2A | 14 (56) |
| JNET Type 2B | 2 (8) |
| Median tumor size, mm (IQR) | 15 (10–18) |
| n = 25 | |
|---|---|
| En bloc resection, n (%) | 20 (80) |
| R0 resection, n (%) | 18 (72) |
| R1 resection, n (%) | 5 (20) |
| RX resection, n (%) | 2 (8) |
| Including submucosal tissue, % | 100 |
| Median resection time, sec (IQR) | 195 (156–290) |
| Histological diagnosis, n (%) | |
| Adenoma | 12 (48) |
| Tis | 4 (16) |
| SSL | 9 (36) |
| Complication, n (%) | |
| Perforation | 0 |
| Delayed bleeding | 0 |
| Post polypectomy coagulation syndrome | 0 |
| En Bloc Resection | R0 Resection | |
|---|---|---|
| Tumor size, mm | ||
| 6–9 | 4/4 (100) | 4/4 (100) |
| 10–19 | 12/16 (75) | 11/16 (69) |
| 20–26 | 4/5 (80) | 3/5 (60) |
| Histological type | ||
| Adenoma | 10/12 (83) | 9/12 (75) |
| Tis | 4/4 (100) | 3/4 (75) |
| SSL | 6/9 (67) | 6/9 (67) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashizawa, H.; Hotta, K.; Imai, K.; Ito, S.; Kishida, Y.; Takada, K.; Okumura, T.; Kawata, N.; Yoshida, M.; Maeda, Y.; et al. Efficacy and Safety of Gel Immersion Endoscopic Mucosal Resection for Non-Pedunculated Colorectal Polyps. Life 2023, 13, 711. https://doi.org/10.3390/life13030711
Ashizawa H, Hotta K, Imai K, Ito S, Kishida Y, Takada K, Okumura T, Kawata N, Yoshida M, Maeda Y, et al. Efficacy and Safety of Gel Immersion Endoscopic Mucosal Resection for Non-Pedunculated Colorectal Polyps. Life. 2023; 13(3):711. https://doi.org/10.3390/life13030711
Chicago/Turabian StyleAshizawa, Hiroshi, Kinichi Hotta, Kenichiro Imai, Sayo Ito, Yoshihiro Kishida, Kazunori Takada, Taishi Okumura, Noboru Kawata, Masao Yoshida, Yuki Maeda, and et al. 2023. "Efficacy and Safety of Gel Immersion Endoscopic Mucosal Resection for Non-Pedunculated Colorectal Polyps" Life 13, no. 3: 711. https://doi.org/10.3390/life13030711
APA StyleAshizawa, H., Hotta, K., Imai, K., Ito, S., Kishida, Y., Takada, K., Okumura, T., Kawata, N., Yoshida, M., Maeda, Y., Yamamoto, Y., Minamide, T., Sato, J., Ishiwatari, H., Matsubayashi, H., & Ono, H. (2023). Efficacy and Safety of Gel Immersion Endoscopic Mucosal Resection for Non-Pedunculated Colorectal Polyps. Life, 13(3), 711. https://doi.org/10.3390/life13030711







