Demystifying the Value of Minimal Clinically Important Difference in the Cardiothoracic Surgery Context
Abstract
1. Introduction
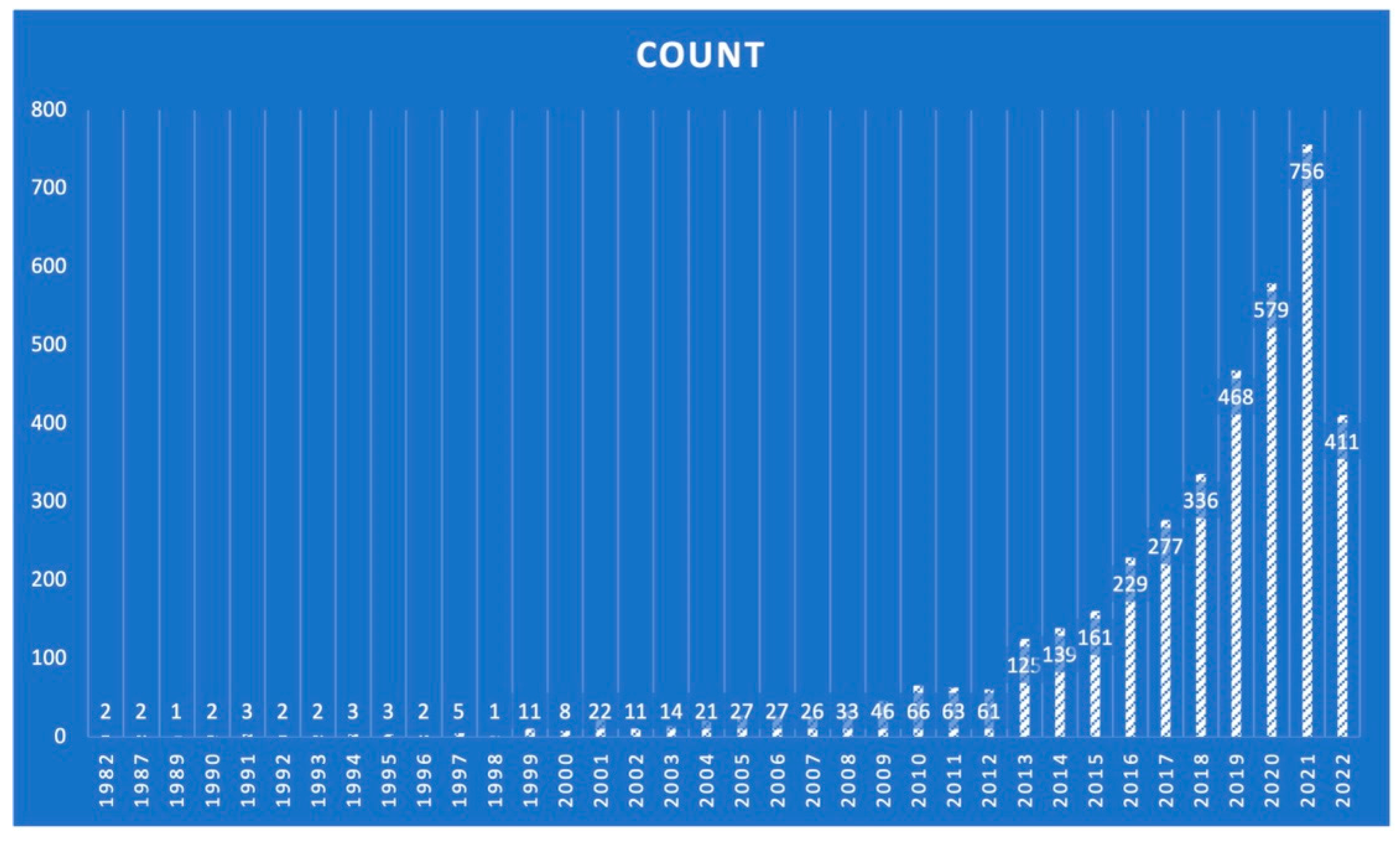
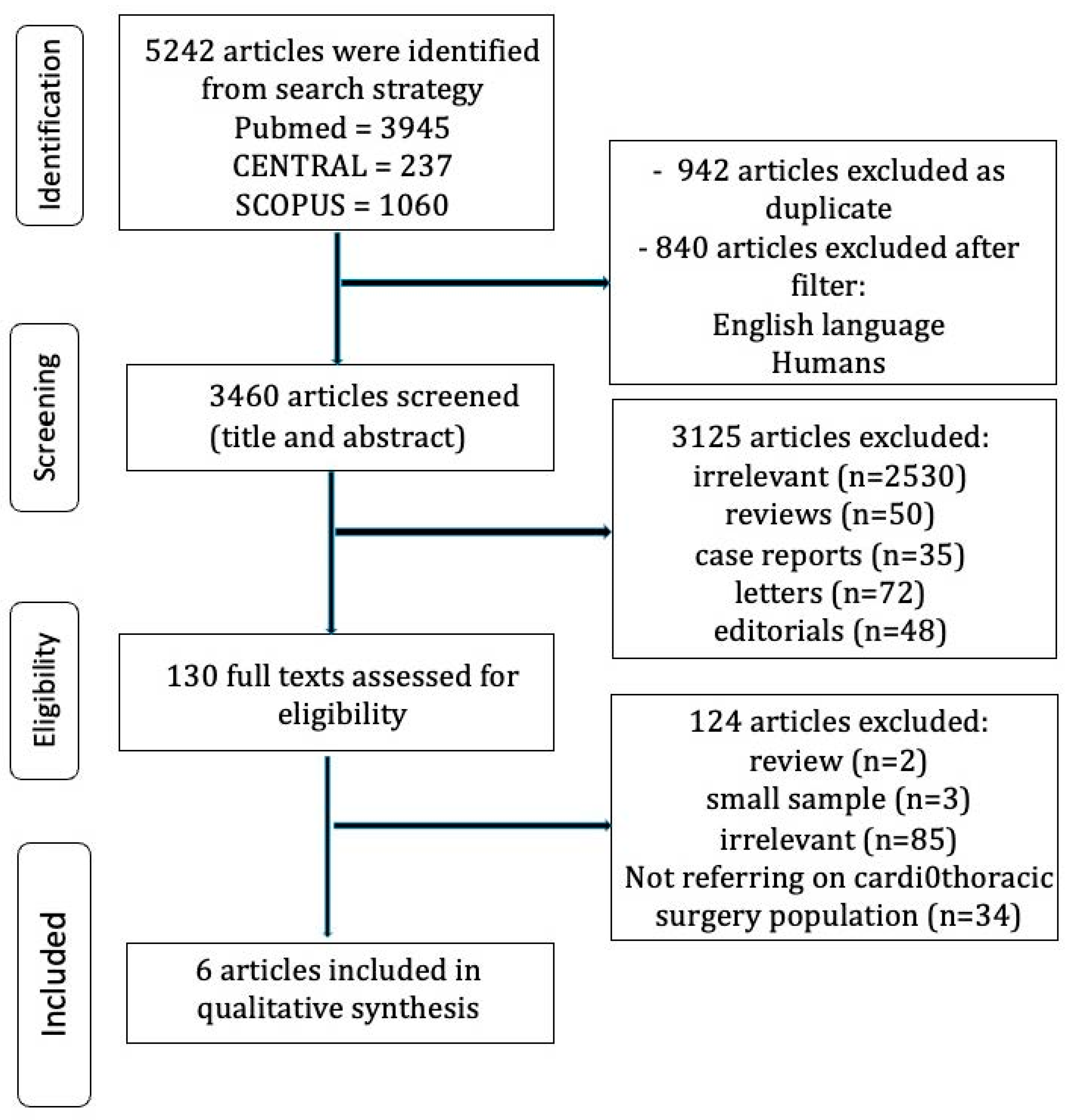
2. Materials and Methods
3. Results
3.1. The Rationale behind MCID Implementation
3.2. Different Methodological Approaches for MCID
- An important difference that represents a real alteration as perceived at the population or individual level.
- A difference reflecting cost-effectiveness associated with healthcare institutions, perceived as systems.
- A significant difference as perceived by patients in those cases where the interpretation of measures is not clear.
- An important difference associated with a prognostic factor, to reach a reduction regarding an event of interest within a certain population.
- A minimum of important differences that are perceived by individuals.
3.3. Distribution Methods Approach
3.4. Anchor-Based Approach
3.5. Consensus or Delphi Approach
3.6. Potential Pitfalls When Implementing MCIDs
4. Discussion
4.1. MCID Implementation in Cardiothoracic Surgery
4.2. MCID Implementation in the Context of Heart Failure and Transcatheter Aortic Valve Replacement (TAVR)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of health status: Ascertaining the minimal clinically important difference. Control. Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.E.; Singh, S.J.; Sewell, L.; Guyatt, G.H.; Morgan, M.D.L. Development of a self-reported Chronic Respiratory Questionnaire (CRQ-SR). Thorax 2001, 56, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Falcoz, P.E.; Chocron, S.; Stoica, L.; Kalili, D.; Puyraveau, M.; Mercier, M.; Etievent, J.-P. Open heart surgery: One-year self-assessment of quality of life and functional outcome. Ann. Thorac. Surg. 2003, 76, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Rumsfeld, J.S.; Mawhinney, S.; Mccarthy, M.; Shroyer, L.A.W.; Villanueva, C.B.; O’brien, M.; Moritz, T.E.; Henderson, W.G.; Grover, F.L.; Sethi, G.K. Health-related quality of life as a predictor of mortality following coronary artery bypass graft surgery. Participants of the department of veterans affairs cooperative study group on processes, structures, and outcomes of care in cardiac surgery. JAMA 1999, 281, 1298–1303. [Google Scholar] [CrossRef]
- Rumsfeld, J.S.; Magid, D.J.; O’Brien, M.; McCarthy, M.; MaWhinney, S.; Shroyer, A.L.; Moritz, T.E.; Henderson, W.G.; Sethi, G.K.; Grover, F.L.; et al. Changes in health-related quality of life following coronary artery bypass graft surgery. Ann. Thorac. Surg. 2001, 72, 2026–2032. [Google Scholar] [CrossRef]
- Turner, D.; Schunemann, H.J.; Griffith, L.E.; Beaton, D.E.; Griffiths, A.M.; Critch, J.N.; Guyatt, G.H. The minimal detectable change cannot reliably replace the minimal important difference. J. Clin. Epidemiol. 2010, 63, 28–36. [Google Scholar] [CrossRef]
- Livingston, E.H.; Elliot, A.; Hynan, L.; Cao, J. Effect size estimation: A necessary component of statistical analysis. Arch. Surg. 2009, 144, 706–712. [Google Scholar] [CrossRef]
- Troosters, T. How important is a minimal difference? Eur. Respir. J. 2011, 37, 755–756. [Google Scholar] [CrossRef]
- McGlothlin, A.E.; Lewis, R.J. Minimal clinically important difference: Defining what really matters to patients. JAMA 2014, 312, 1342–1343. [Google Scholar] [CrossRef]
- Redelmeier, D.A.; Lorig, K. Assessing the clinical importance of symptomatic improvements. An illustration in rheumatology. Arch. Intern. Med. 1993, 153, 1337–1342. [Google Scholar] [CrossRef]
- Hinman, R.S.; McCrory, P.; Pirotta, M.; Relf, I.; Forbes, A.; Crossley, K.; Williamson, E.; Kyriakides, M.; Novy, K.; Metcalf, B.; et al. Acupuncture for chronic knee pain: A randomized clinical trial. JAMA 2014, 312, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Grand, N.; Bouchet, J.B.; Zufferey, P.; Beraud, A.M.; Awad, S.; Sandri, F.; Campisi, S.; Fuzellier, J.F.; Molliex, S.; Vola, M.; et al. Quality of Life After Cardiac Surgery Based on the Minimal Clinically Important Difference Concept. Ann. Thorac. Surg. 2018, 106, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Schunemann, H.J.; Guyatt, G.H. Commentary—Goodbye M(C)ID! Hello MID, where do you come from? Health Serv. Res. 2005, 40, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Carette, S.; Ford, P.M.; Kean, W.F.; le Riche, N.G.; Wells, L.A.; Campbell, J. Osteoarthritis antirheumatic drug trials: III, setting the delta for clinical trials: Results of a consensus development (Delphi) exercise. J. Rheumatol. 1992, 19, 451–457. [Google Scholar]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W., Jr.; Schuler, T.C. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Redelmeier, D.A.; Bayoumi, A.M.; Goldstein, R.S.; Guyatt, G.H. Interpreting small differences in functional status: The Six Minute Walk test in chronic lung disease patients. Am. J. Respir. Crit. Care Med. 1997, 155, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Ikramuddin, S.; Blackstone, R.P.; Brancatisano, A.; Toouli, J.; Shah, S.; Wolfe, B.; Fujioka, K.; Maher, J.; Swain, J.; Que, F.; et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: The ReCharge randomized clinical trial. JAMA 2014, 312, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Houchen-Wolloff, L.; Evans, R.A. Unravelling the mystery of the ‘minimum important difference’ using practical outcome measures in chronic respiratory disease. Chron. Respir. Dis. 2019, 16, 1479973118816491. [Google Scholar] [CrossRef]
- Blokzijl, F.; Houterman, S.; van Straten, B.H.; Daeter, E.; Bruinsma, G.J.B.B.; Dieperink, W.; Reneman, M.F.; Keus, F.; van der Horst, I.C.; Mariani, M.A. The impact of surgical aortic valve replacement on quality of life-a multicenter study. J. Thorac. Cardiovasc. Surg. 2019, 161, 1204–1210.e7. [Google Scholar] [CrossRef]
- Auensen, A.; Hussain, A.I.; Garratt, A.M.; Gullestad, L.L.; Pettersen, K.I. Patient-reported outcomes after referral for possible valve replacement in patients with severe aortic stenosis. Eur. J. Cardiothorac. Surg. 2018, 53, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, L.; Caligari, M.; Acquati, C.; Nicolazzi, S.; Paracchini, G.; Sardano, D.; Giordano, A.; Marcassa, C.; Corrà, U. Functional capacity assessment and Minimal Clinically Important Difference in post-acute cardiac patients: The role of Short Physical Performance Battery. Eur. J. Prev. Cardiol. 2021, 29, 1008–1014. [Google Scholar] [CrossRef]
- Meenaghan, S.M.; Nugent, G.M.; Dee, E.C.; Smith, H.A.; McMahon, C.J.; Nolke, L. Health-Related Quality of Life in Pediatric Cardiac Patients After Extracorporeal Life Support. Pediatr. Cardiol. 2021, 42, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, G.J.; Maldonado, F.; Chen, H.; Rickman, O.; Roller, L.; Walston, C.; Katsis, J.; Lentz, R. Minimal clinically important difference for chest discomfort in patients undergoing pleural interventions. BMJ Open Respir. Res. 2020, 7, e000667. [Google Scholar] [CrossRef]
- Nicholls, S.G.; Quach, P.; Von Elm, E.; Guttmann, A.; Moher, D.; Petersen, I.; Sørensen, H.T.; Smeeth, L.; Langan, S.M.; Benchimol, E.I. The Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PloS Med. 2015, 12, e1001885. [Google Scholar]
- Raj, A.A.; Pavord, D.I.; Birring, S.S. Clinical cough IV: What is the minimal important difference for the Leicester Cough Questionnaire? Handb. Exp. Pharmacol. 2009, 187, 311–320. [Google Scholar]
- Joshi, A.; Kale, S.; Chandel, S.; Pal, D.K. Likert scale: Explored and explained. Br. J. Appl. Sci. Technol. 2015, 7, 396–403. [Google Scholar] [CrossRef]
- Varni, J.W.; Burwinkle, T.M.; Seid, M.; Skarr, D. The PedsQL 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambul. Pediatr. 2003, 3, 329–341. [Google Scholar] [CrossRef]
- Jain, S.S.; Cohen, D.J.; Zhang, Z.; Uriel, N.; Sayer, G.; Lindenfeld, J.; Abraham, W.T.; Mack, M.J.; Stone, G.W.; Arnold, S.V. Defining a Clinically Important Change in 6-Minute Walk Distance in Patients with Heart Failure and Mitral Valve Disease. Circ. Heart. Fail. 2021, 14, e007564. [Google Scholar] [CrossRef]
- Butler, J.; Khan, M.S.; Lindenfeld, J.; Abraham, W.T.; Savarese, G.; Salsali, A.; Zeller, C.; Peil, B.; Filippatos, G.; Ponikowski, P.; et al. Minimally Clinically Important Difference in Health Status Scores in Patients With HFrEF vs HFpEF. ACC Hear. Fail. 2022, 10, 651–661. [Google Scholar] [CrossRef]
- Khan, M.S.; Anker, S.D.; Friede, T.; Jankowska, E.A.; Metra, M.; Piña, I.L.; Coats, A.J.; Rosano, G.; Roubert, B.; Goehring, U.-M.; et al. Minimal Clinically Important Difference for Six-minute Walk Test in Patients with HFrEF and Iron Deficiency. J. Card. Fail. 2022, in press. [CrossRef]
- Surman, T.L.; Abrahams, J.M.; Kim, J.; Surman, H.E.; Roberts-Thomson, R.; Montarello, J.M.; Edwards, J.; Worthington, M.; Beltrame, J. Quality of life and frailty outcomes following surgical and transcatheter aortic valve replacement. J. Cardiothorac. Surg. 2022, 17, 113. [Google Scholar] [CrossRef]
- Stenman, M.; Sartipy, U. Depression screening in cardiac surgery patients. Heart Lung Circ. 2018, 28, 953–958. [Google Scholar] [CrossRef]
- Gondi, K.T.; Tam, M.C.; Chetcuti, S.J.; Pagani, F.D.; Grossman, P.M.; Deeb, G.M.; Menees, D.P.; Haft, J.W.; Patel, H.J.; Aaronson, K.D.; et al. Transcatheter Aortic Valve Replacement for Left Ventricular Assist Device–Related Aortic Regurgitation: The Michigan Medicine Experience. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 2, 100530. [Google Scholar] [CrossRef]
| Study ID | Country of Origin | Study Population | MCID Methodology | Variables | Outcomes |
|---|---|---|---|---|---|
| Grand 2018 [12] | France | 326 | Anchor-based | Short-form survey SF-36 questionnaire | Post-cardiac surgery quality of life (QoL) improvement |
| Blokzijl 2021 [20] | Multinational | 899 | Anchor-based | SF-36 questionnaire | Post-AVR (aortic valve replacement) QoL improvement |
| Auensen 2018 [21] | Norway | 442 | Distribution- and anchor-based | EQ-5D and SF-36 questionnaires | Post-AVR QoL enhancement |
| Rinaldo 2022 [22] | Italy | 392 | Anchor-based | SPPB test; patient global impression of change was used as an anchor | Post-cardiac surgery rehabilitation QoL improvement |
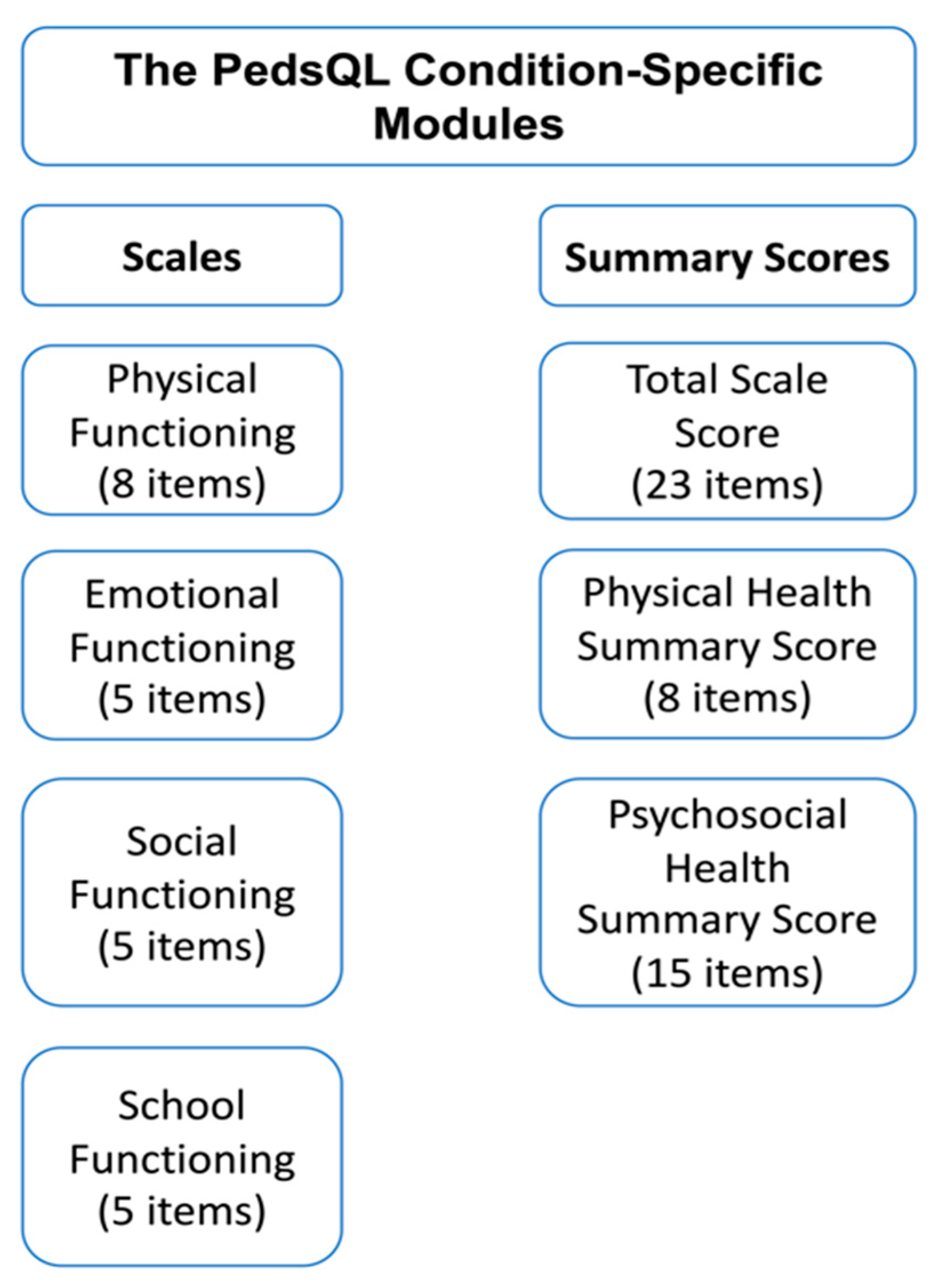
| Meenaghan 2021 [23] | Ireland | 41 | Anchor-based | PedsQL questionnaire | QoL improvement in pediatric cardiac patients after extracorporeal life support |
| Dahlberg 2020 [24] | USA | 262 | Anchor-based | VAS | Chest discomfort in patients undergoing therapeutic thoracentesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magouliotis, D.E.; Bareka, M.; Rad, A.A.; Christodoulidis, G.; Athanasiou, T. Demystifying the Value of Minimal Clinically Important Difference in the Cardiothoracic Surgery Context. Life 2023, 13, 716. https://doi.org/10.3390/life13030716
Magouliotis DE, Bareka M, Rad AA, Christodoulidis G, Athanasiou T. Demystifying the Value of Minimal Clinically Important Difference in the Cardiothoracic Surgery Context. Life. 2023; 13(3):716. https://doi.org/10.3390/life13030716
Chicago/Turabian StyleMagouliotis, Dimitrios E., Metaxia Bareka, Arian Arjomandi Rad, Grigorios Christodoulidis, and Thanos Athanasiou. 2023. "Demystifying the Value of Minimal Clinically Important Difference in the Cardiothoracic Surgery Context" Life 13, no. 3: 716. https://doi.org/10.3390/life13030716
APA StyleMagouliotis, D. E., Bareka, M., Rad, A. A., Christodoulidis, G., & Athanasiou, T. (2023). Demystifying the Value of Minimal Clinically Important Difference in the Cardiothoracic Surgery Context. Life, 13(3), 716. https://doi.org/10.3390/life13030716










