Prebiotic Synthesis of ATP: A Terrestrial Volcanism-Dependent Pathway
Abstract
:1. Introduction
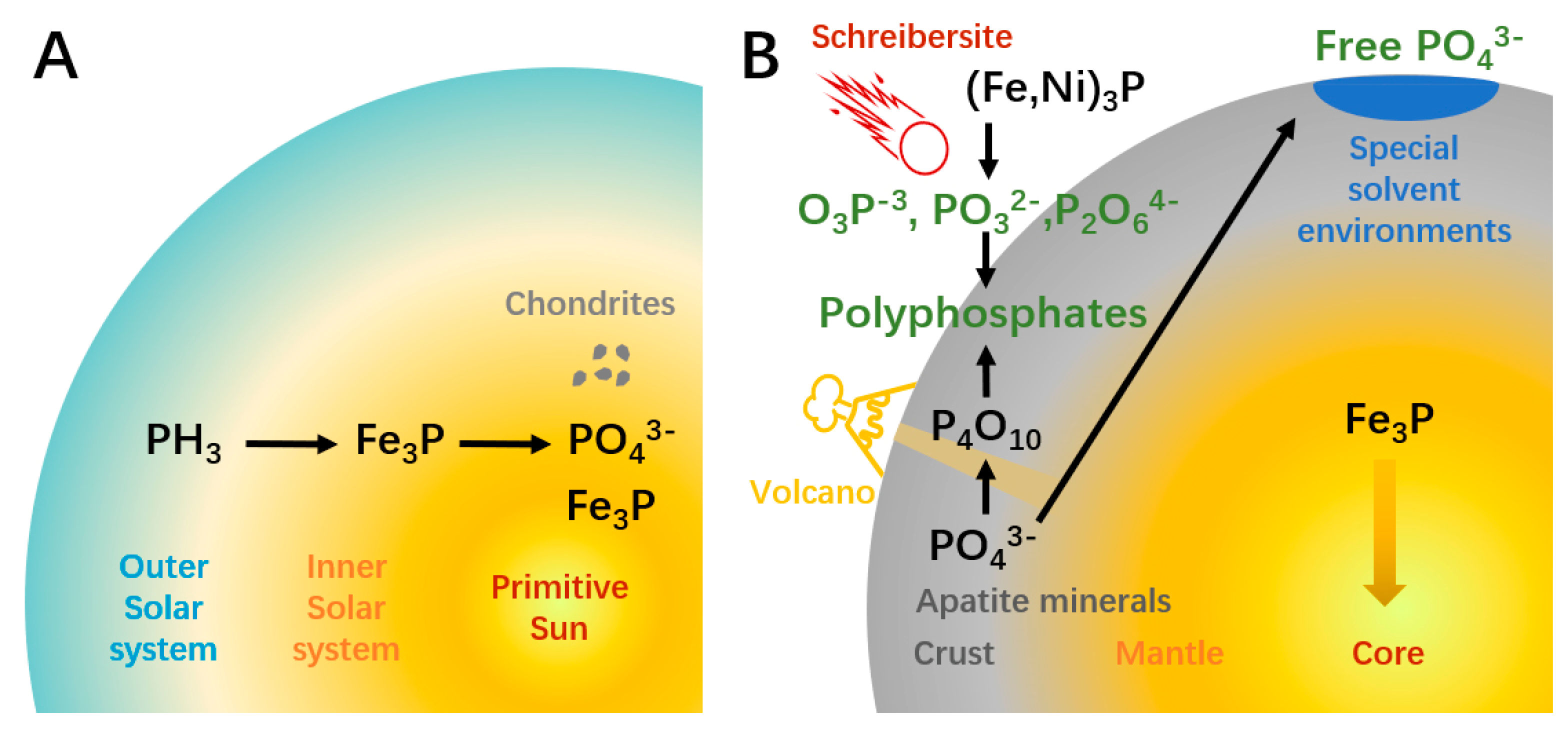
2. Source of Materials Required for ATP Synthesis
3. Plausible Pathways to ATP
3.1. Adenine Synthesis
3.2. Ribose Synthesis
3.3. Adenosine Synthesis and Phosphorylation
3.4. A Terrestrial Volcanism-Dependent Pathway to ATP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, H.-F.; Kong, D.-X.; Shen, L.; Chen, L.-L.; Ma, B.-G.; Zhang, H.-Y. Distribution patterns of small-molecule ligands in the protein universe and implications for origin of life and drug discovery. Genome Biol. 2007, 8, R176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, X.-Y.; Zhang, H.-Y. Cofactors as molecular fossils to trace the origin and evolution of proteins. ChemBioChem 2020, 21, 3161–3168. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Malinovska, L.; Saha, S.; Wang, J.; Alberti, S.; Krishnan, Y.; Hyman, A.A. ATP as a biological hydrotrope. Science 2017, 356, 753–756. [Google Scholar] [CrossRef]
- Chu, X.-Y.; Xu, Y.-Y.; Tong, X.-Y.; Wang, G.; Zhang, H.-Y. The legend of ATP: From origin of life to precision medicine. Metabolites 2022, 12, 461. [Google Scholar] [CrossRef]
- Goldford, J.E.; Hartman, H.; Smith, T.F.; Segrè, D. Remnants of an ancient metabolism without phosphate. Cell 2017, 168, 1126–1134.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldford, J.E.; Hartman, H.; Marsland, R.; Segrè, D. Environmental boundary conditions for the origin of life converge to an organo-sulfur metabolism. Nat. Ecol. Evol. 2019, 3, 1715–1724. [Google Scholar] [CrossRef]
- Raanan, H.; Poudel, S.; Pike, D.H.; Nanda, V.; Falkowski, P.G. Small protein folds at the root of an ancient metabolic network. Proc. Natl. Acad. Sci. USA 2020, 117, 7193–7199. [Google Scholar] [CrossRef]
- Pasek, M.A. Thermodynamics of prebiotic phosphorylation. Chem. Rev. 2020, 120, 4690–4706. [Google Scholar] [CrossRef]
- Fialho, D.M.; Karunakaran, S.C.; Greeson, K.W.; Martínez, I.; Schuster, G.B.; Krishnamurthy, R.; Hud, N.V. Depsipeptide nucleic acids: Prebiotic formation, oligomerization, and self-assembly of a new proto-nucleic acid candidate. J. Am. Chem. Soc. 2021, 143, 13525–13537. [Google Scholar] [CrossRef]
- Palme, H.; Lodders, K.; Jones, A. Solar system abundances of the elements. In Treatise on Geochemistry, 2nd ed.; Davis, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 15–36. [Google Scholar]
- Sossi, P.A.; Burnham, A.D.; Badro, J.; Lanzirotti, A.; Newville, M.; O’Neill, H.S.C. Redox state of Earth’s magma ocean and its venus-like early atmosphere. Sci. Adv. 2020, 6, eabd1387. [Google Scholar] [CrossRef]
- Villanueva, G.L.; Mumma, M.J.; Novak, R.E.; Käufl, H.U.; Hartogh, P.; Encrenaz, T.; Tokunaga, A.; Khayat, A.; Smith, M.D. Strong water isotopic anomalies in the Martian atmosphere: Probing current and ancient reservoirs. Science 2015, 348, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Vu, T.H.; Hodyss, R.; Choukroun, M.; Johnson, P.V. Chemistry of frozen sodium–magnesium–sulfate–chloride brines: Implications for surface expression of Europa’s ocean composition. Astrophys. J. Lett. 2016, 816, L26. [Google Scholar] [CrossRef]
- Keane, T.C. Mechanism for the coupled photochemistry of ammonia and acetylene: Implications for giant planets, comets and interstellar organic synthesis. Orig. Life Evol. Biosph. 2017, 47, 223–248. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.M.; Kamp, L.W.; Matson, D.L.; Irwin, P.G.J.; Baines, K.H.; Boryta, M.D.; Leader, F.E.; Jaumann, R.; Smythe, W.D.; Sotin, C.; et al. Saturn’s Titan: Surface change, ammonia, and implications for atmospheric and tectonic activity. Icarus 2009, 199, 429–441. [Google Scholar] [CrossRef]
- Gibb, B.C. A Chemist’s guide to the Solar system. Nat. Chem. 2015, 7, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Dalle Ore, C.M.; Cruikshank, D.P.; Protopapa, S.; Scipioni, F.; McKinnon, W.B.; Cook, J.C.; Grundy, W.M.; Schmitt, B.; Stern, S.A.; Moore, J.M.; et al. Detection of ammonia on Pluto’s surface in a region of geologically recent tectonism. Sci. Adv. 2019, 5, eaav5731. [Google Scholar] [CrossRef] [Green Version]
- Pasek, M.A. Phosphorus volatility in the early Solar nebula. Icarus 2019, 317, 59–65. [Google Scholar] [CrossRef]
- Walton, C.R.; Shorttle, O.; Jenner, F.E.; Williams, H.M.; Golden, J.; Morrison, S.M.; Downs, R.T.; Zerkle, A.; Hazen, R.M.; Pasek, M. Phosphorus mineral evolution and prebiotic chemistry: From minerals to microbes. Earth Sci. Rev. 2021, 221, 103806. [Google Scholar] [CrossRef]
- Pasek, M.A.; Gull, M.; Herschy, B. Phosphorylation on the early Earth. Chem. Geol. 2017, 475, 149–170. [Google Scholar] [CrossRef]
- Schreiber, U.; Locker-Grütjen, O.; Mayer, C. Hypothesis: Origin of life in deep-reaching tectonic faults. Orig. Life Evol. Biosph. 2012, 42, 47–54. [Google Scholar] [CrossRef]
- Gull, M.; Zhou, M.; Fernández, F.M.; Pasek, M.A. Prebiotic phosphate ester syntheses in a deep eutectic solvent. J. Mol. Evol. 2014, 78, 109–117. [Google Scholar] [CrossRef]
- Schwartz, A.W. Prebiotic phosphorylation-nucleotide synthesis with apatite. Biochim. Biophys. Acta 1972, 281, 477–480. [Google Scholar] [CrossRef]
- Toner, J.D.; Catling, D.C. A Carbonate-rich lake solution to the phosphate problem of the origin of life. Proc. Natl. Acad. Sci. USA 2020, 117, 883–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gull, M. Prebiotic phosphorylation reactions on the early Earth. Challenges 2014, 5, 193–212. [Google Scholar] [CrossRef] [Green Version]
- Gan, D.; Ying, J.; Zhao, Y. Prebiotic chemistry: The role of trimetaphosphate in prebiotic chemical evolution. Front. Chem. 2022, 10, 941228. [Google Scholar] [CrossRef]
- Yamagata, Y.; Watanabe, H.; Saitoh, M.; Namba, T. Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature 1991, 352, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Miller, S.L. Are polyphosphates or phosphate esters prebiotic reagents? J. Mol. Evol. 1995, 41, 639–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBeath, T.M.; Lombi, E.; McLaughlin, M.J.; Bünemann, E.K. Polyphosphate-fertilizer solution stability with time, temperature, and PH. Z. Nutr. Soil. Sc. 2007, 170, 387–391. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Usuzan. Available online: https://www.data.jma.go.jp/svd/vois/data/tokyo/STOCK/souran_eng/volcanoes/015_usuzan.pdf (accessed on 15 June 2022).
- Pasek, M.A.; Dworkin, J.P.; Lauretta, D.S. A Radical pathway for organic phosphorylation during schreibersite corrosion with implications for the origin of life. Geochim. Cosmochim. Acta 2007, 71, 1721–1736. [Google Scholar] [CrossRef]
- Gull, M.; Mojica, M.A.; Fernández, F.M.; Gaul, D.A.; Orlando, T.M.; Liotta, C.L.; Pasek, M.A. Nucleoside phosphorylation by the mineral schreibersite. Sci. Rep. 2015, 5, 17198. [Google Scholar] [CrossRef] [Green Version]
- Sydow, C.; Seiband, C.; Siegle, A.F.; Trapp, O. Phosphorylation in liquid sulfur dioxide under prebiotically plausible conditions. Commun. Chem. 2022, 5, 143. [Google Scholar] [CrossRef]
- Pasek, M.A. Rethinking early earth phosphorus geochemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 853–858. [Google Scholar] [CrossRef] [Green Version]
- Pasek, M.A.; Harnmeijer, J.P.; Buick, R.; Gull, M.; Atlas, Z. Evidence for reactive reduced phosphorus species in the early Archean ocean. Proc. Natl. Acad. Sci. USA 2013, 110, 10089–10094. [Google Scholar] [CrossRef] [Green Version]
- Kitadai, N.; Maruyama, S. Origins of building blocks of life: A review. Geosci. Front. 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, R.; Krishnamurthy, R. Chemistry of abiotic nucleotide synthesis. Chem. Rev. 2020, 120, 4766–4805. [Google Scholar] [CrossRef]
- Green, N.J.; Xu, J.; Sutherland, J.D. Illuminating life’s origins: UV photochemistry in abiotic synthesis of biomolecules. J. Am. Chem. Soc. 2021, 143, 7219–7236. [Google Scholar] [CrossRef] [PubMed]
- Oró, J. Synthesis of adenine from ammonium cyanide. Biochem. Biophys. Res. Commun. 1960, 2, 407–412. [Google Scholar] [CrossRef]
- Shapiro, R. The Prebiotic role of adenine: A critical analysis. Orig. Life Evol. Biosph. 1995, 25, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Sasselov, D.D.; Grotzinger, J.P.; Sutherland, J.D. The origin of life as a planetary phenomenon. Sci. Adv. 2020, 6, eaax3419. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, R.; Ferris, J.; Orgel, L.E. Conditions for purine synthesis: Did prebiotic synthesis occur at low temperatures? Science 1966, 153, 72–73. [Google Scholar] [CrossRef]
- Levy, M. Prebiotic synthesis of adenine and amino acids under Europa-like conditions. Icarus 2000, 145, 609–613. [Google Scholar] [CrossRef]
- Miyakawa, S.; James Cleaves, H.; Miller, S.L. The cold origin of life: A. implications based on the hydrolytic stabilities of hydrogen cyanide and formamide. Orig. Life Evol. Biosph. 2002, 32, 195–208. [Google Scholar] [CrossRef]
- Miyakawa, S.; Cleaves, H.J.; Miller, S.L. The cold origin of life: B. implications based on pyrimidines and purines produced from frozen ammonium cyanide solutions. Orig. Life Evol. Biosph. 2002, 32, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Villafañe-Barajas, S.A.; Ruiz-Bermejo, M.; Rayo-Pizarroso, P.; Gálvez-Martínez, S.; Mateo-Martí, E.; Colín-García, M. A Lizardite–hcn interaction leading the increasing of molecular complexity in an alkaline hydrothermal scenario: Implications for origin of life studies. Life 2021, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Villafañe-Barajas, S.A.; Ruiz-Bermejo, M.; Rayo-Pizarroso, P.; Colín-García, M. Characterization of HCN-derived thermal polymer: Implications for chemical evolution. Processes 2020, 8, 968. [Google Scholar] [CrossRef]
- LaRowe, D.E.; Regnier, P. Thermodynamic potential for the abiotic synthesis of adenine, cytosine, guanine, thymine, uracil, ribose, and deoxyribose in hydrothermal systems. Orig. Life Evol. Biosph. 2008, 38, 383–397. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Hirobe, M.; Higashiyama, K.; Takahashi, H.; Suzuki, K.T. Reaction mechanism for purine ring formation as studied by 13C-15N coupling. Tetrahedron Lett. 1978, 19, 4039–4042. [Google Scholar] [CrossRef]
- Saladino, R. A possible prebiotic synthesis of purine, adenine, cytosine, and 4(3H)-pyrimidinone from formamide implications for the origin of life. Bioorg. Med. Chem. 2001, 9, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Neri, V.; Crestini, C.; Costanzo, G.; Graciotti, M.; Di Mauro, E. Synthesis and degradation of nucleic acid components by formamide and iron sulfur minerals. J. Am. Chem. Soc. 2008, 130, 15512–15518. [Google Scholar] [CrossRef]
- Barks, H.L.; Buckley, R.; Grieves, G.A.; Di Mauro, E.; Hud, N.V.; Orlando, T.M. Guanine, adenine, and hypoxanthine production in UV-irradiated formamide solutions: Relaxation of the requirements for prebiotic purine nucleobase formation. Chem. Eur. J. Chem. Biol. 2010, 11, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L.; Chalmers, J.H.; Cleaves, H.J. Is Formamide a geochemically plausible prebiotic solvent? Phys. Chem. Chem. Phys. 2016, 18, 20085–20090. [Google Scholar] [CrossRef] [PubMed]
- Niether, D.; Afanasenkau, D.; Dhont, J.K.G.; Wiegand, S. Accumulation of formamide in hydrothermal pores to form prebiotic nucleobases. Proc. Natl. Acad. Sci. USA 2016, 113, 4272–4277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferus, M.; Pietrucci, F.; Saitta, A.M.; Knížek, A.; Kubelík, P.; Ivanek, O.; Shestivska, V.; Civiš, S. Formation of nucleobases in a Miller–Urey reducing atmosphere. Proc. Natl. Acad. Sci. USA 2017, 114, 4306–4311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, M.; Miller, S.L. The Stability of the RNA bases: Implications for the origin of life. Proc. Natl. Acad. Sci. USA 1998, 95, 7933–7938. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, E. Purine and pyrimidines in sediments from the experimental Mohole. Science 1964, 146, 1680–1681. [Google Scholar] [CrossRef]
- Butlerow, A. Bildung einer zuckerartigen substanz durch synthese. Ann. Chem. Pharm. 1861, 120, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Cody, G.D.; Heying, E.; Alexander, C.M.O.; Nittler, L.R.; Kilcoyne, A.L.D.; Sandford, S.A.; Stroud, R.M. Establishing a molecular relationship between chondritic and cometary organic solids. Proc. Natl. Acad. Sci. USA 2011, 108, 19171–19176. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.P.; Gladstone, G.R.; Yung, Y.L. Photochemical production of formaldehyde in Earth’s primitive atmosphere. Science 1980, 210, 183–185. [Google Scholar] [CrossRef]
- Inaba, S. Primary formation path of formaldehyde in hydrothermal vents. Orig. Life Evol. Biosph. 2018, 48, 1–22. [Google Scholar] [CrossRef]
- Omran, A. Plausibility of the formose reaction in alkaline hydrothermal vent environments. Orig. Life Evol. Biosph. 2020. [Google Scholar] [CrossRef]
- Ricardo, A.; Carrigan, M.A.; Olcott, A.N.; Benner, S.A. Borate minerals stabilize ribose. Science 2004, 303, 196. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Mayagoitia, Á.; Horton, S.R.; Sumpter, B.G.; Šponer, J.; Šponer, J.E.; Fuentes-Cabrera, M. On the stabilization of ribose by silicate minerals. Astrobiology 2011, 11, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Usami, K.; Okamoto, A. Hydroxyapatite: Catalyst for a one-pot pentose formation. Org. Biomol. Chem. 2017, 15, 8888–8893. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Pitsch, S.; Kittaka, A.; Wagner, E.; Wintner, C.E.; Eschenmoser, A.; Ohlofjgewidmet, G. Chemistry of a-aminonitriles. Aldomerization of glycolaldehyde phosphate to rac-hexose 2, 4, 6-triphosphates and (in presence of formaldehyde) rac-pentose 2, 4-diphosphates: Rac-allose 2, 4, 6-triphosphate and rac-ribose 2, 4-diphosphate are the main reaction products. Helv. Chim. Acta 1990, 73, 1410–1468. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.-R.; Wang, X. A Plausible prebiotic selection of ribose for RNA—Formation, dynamic isolation, and nucleotide synthesis based on metal-doped-clays. Chem 2021, 7, 3292–3308. [Google Scholar] [CrossRef]
- Georgelin, T.; Jaber, M.; Fournier, F.; Laurent, G.; Costa-Torro, F.; Maurel, M.-C.; Lambert, J.-F. Stabilization of ribofuranose by a mineral surface. Carbohydr. Res. 2015, 402, 241–244. [Google Scholar] [CrossRef]
- Sagi, V.N.; Punna, V.; Hu, F.; Meher, G.; Krishnamurthy, R. Exploratory experiments on the chemistry of the “glyoxylate scenario”: Formation of ketosugars from dihydroxyfumarate. J. Am. Chem. Soc. 2012, 134, 3577–3589. [Google Scholar] [CrossRef]
- Larralde, R.; Robertson, M.P.; Miller, S.L. Rates of decomposition of ribose and other sugars: Implications for chemical evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 8158–8160. [Google Scholar] [CrossRef] [Green Version]
- Fuller, W.D.; Sanchez, R.A.; Orgel, L.E. Studies in prebiotic synthesis: VI. Synthesis of purine nucleosides. J. Mol. Biol. 1972, 67, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Benner, S.A. Prebiotic stereoselective synthesis of purine and noncanonical pyrimidine nucleotide from nucleobases and phosphorylated carbohydrates. Proc. Natl. Acad. Sci. USA 2017, 114, 11315–11320. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Kim, J. A Prebiotic synthesis of canonical pyrimidine and purine ribonucleotides. Astrobiology 2019, 19, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.A.; Orgel, L.E. Studies in prebiotic synthesis: V. Synthesis and photoanomerization of pyrimidine nucleosides. J. Mol. Biol. 1970, 47, 531–543. [Google Scholar] [CrossRef]
- Powner, M.W.; Gerland, B.; Sutherland, J.D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Stairs, S.; Nikmal, A.; Bučar, D.-K.; Zheng, S.-L.; Szostak, J.W.; Powner, M.W. Divergent prebiotic synthesis of pyrimidine and 8-oxo-purine ribonucleotides. Nat. Commun. 2017, 8, 15270. [Google Scholar] [CrossRef] [Green Version]
- Ponnamperuma, C.; Sagan, C.; Mariner, R. Synthesis of adenosine triphosphate under possible primitive earth conditions. Nature 1963, 199, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Etaix, E.; Orgel, L.E. Phosphorylation of nucleosides in aqueous solution using trimetaphosphate: Formation of nucleoside triphosphates. J. Carbohydr. Nucleosides Nucleotides 1978, 5, 91–110. [Google Scholar]
- Cheng, C.; Fan, C.; Wan, R.; Tong, C.; Miao, Z.; Chen, J.; Zhao, Y. Phosphorylation of adenosine with trimetaphosphate under simulated prebiotic conditions. Orig. Life Evol. Biosph. 2002, 32, 219–224. [Google Scholar] [CrossRef]
- Stockbridge, R.B.; Wolfenden, R. The Intrinsic reactivity of ATP and the catalytic proficiencies of kinases acting on glucose, N-acetylgalactosamine, and homoserine. J. Biol. Chem. 2009, 284, 22747–22757. [Google Scholar] [CrossRef] [Green Version]
- Tokuriki, N.; Tawfik, D.S. Protein dynamism and evolvability. Science 2009, 324, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Noor, E.; Flamholz, A.I.; Jayaraman, V.; Ross, B.L.; Cohen, Y.; Patrick, W.M.; Gruic-Sovulj, I.; Tawfik, D.S. Uniform binding and negative catalysis at the origin of enzymes. Protein Sci. 2022, 31, e4381. [Google Scholar] [CrossRef]
- Caetano-Anollés, G.; Kim, K.M.; Caetano-Anollés, D. The Phylogenomic roots of modern biochemistry: Origins of proteins, cofactors and protein biosynthesis. J. Mol. Evol. 2012, 74, 1–34. [Google Scholar] [CrossRef]
- Kang, S.-K.; Chen, B.-X.; Tian, T.; Jia, X.-S.; Chu, X.-Y.; Liu, R.; Dong, P.-F.; Yang, Q.-Y.; Zhang, H.-Y. ATP selection in a random peptide library consisting of prebiotic amino acids. Biochem. Biophys. Res. Commun. 2015, 466, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Seligmann, H. First arrived, first served: Competition between codons for codon-amino acid stereochemical interactions determined early genetic code assignments. Sci. Nat. 2020, 107, 20. [Google Scholar] [CrossRef]
- Zhou, L.; O’Flaherty, D.K.; Szostak, J.W. Template-directed copying of RNA by non-enzymatic ligation. Angew. Chem. Int. Ed. 2020, 59, 15682–15687. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, W. The Origin of biological homochirality along with the origin of life. PLoS Comput. Biol. 2020, 16, e1007592. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.-Y.; Zhang, H.-Y. Protein homochirality may be derived from primitive peptide synthesis by RNA. Astrobiology 2021, 21, 628–635. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Ying, J.; Liu, Y.; Zhang, G.; Zhao, Y. Selection of amino acid chirality induced by cyclic dipeptide synthesis in plausible prebiotic conditions. Front. Astron. Space Sci. 2022, 9, 794932. [Google Scholar] [CrossRef]
- Pinna, S.; Kunz, C.; Halpern, A.; Harrison, S.A.; Jordan, S.F.; Ward, J.; Werner, F.; Lane, N. A Prebiotic basis for ATP as the universal energy currency. PLoS Biol. 2022, 20, e3001437. [Google Scholar] [CrossRef]
- Chu, X.-Y.; Wang, G.; Zhang, H.-Y. ATP as an anti-aging agent: Beyond the energy reservoir. Drug Discov. Today 2021, 26, 2783–2785. [Google Scholar] [CrossRef]
- Tong, X.-Y.; Liao, X.; Gao, M.; Lv, B.-M.; Chen, X.-H.; Chu, X.-Y.; Zhang, Q.-Y.; Zhang, H.-Y. Identification of NUDT5 inhibitors from approved drugs. Front. Mol. Biosci. 2020, 7, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, Y.; Chikaraishi, Y.; Ohkouchi, N.; Ogawa, N.O.; Glavin, D.P.; Dworkin, J.P.; Abe, C.; Nakamura, T. Extraterrestrial ribose and other sugars in primitive meteorites. Proc. Natl. Acad. Sci. USA 2019, 116, 24440–24445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oba, Y.; Takano, Y.; Furukawa, Y.; Koga, T.; Glavin, D.P.; Dworkin, J.P.; Naraoka, H. Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites. Nat. Commun. 2022, 13, 2008. [Google Scholar] [CrossRef]
- Oba, Y.; Takano, Y.; Naraoka, H.; Furukawa, Y.; Glavin, D.P.; Dworkin, J.P.; Tachibana, S. Extraterrestrial hexamethylenetetramine in meteorites—A precursor of prebiotic chemistry in the inner Solar system. Nat. Commun. 2020, 11, 6243. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Jiménez-Serra, I.; Puente-Sánchez, F.; Aguirre, J. The emergence of interstellar molecular complexity explained by interacting networks. Proc. Natl. Acad. Sci. USA 2022, 119, e2119734119. [Google Scholar] [CrossRef]
- NASA Confirms Thousands of Massive, Ancient Volcanic Eruptions on Mars. Available online: https://www.nasa.gov/feature/goddard/2021/nasa-confirms-thousands-of-massive-ancient-volcanic-eruptions-on-mars (accessed on 16 September 2022).
- Segura, A. Nitrogen fixation on early mars by volcanic lightning and other sources. Geophys. Res. Lett. 2005, 32, L05203. [Google Scholar] [CrossRef]
- Peplow, M. Formaldehyde claim inflames martian debate. Nature 2005, news050221-15. [Google Scholar] [CrossRef]
- Adcock, C.T.; Hausrath, E.M.; Forster, P.M. Readily available phosphate from minerals in early aqueous environments on mars. Nat. Geosci. 2013, 6, 824–827. [Google Scholar] [CrossRef]

| Difficulties | Solutions |
|---|---|
| Synthesis of adenine requires a high concentration of HCN, but it is too active and unstable to accumulate on primitive Earth. | Store HCN in less reactive salts in mineral-rich water. Convert HCN to polymer under freezing or hydrothermal environments. Formamide generated during HCN hydrolysis can be concentrated under a volcanic environment. HCN independent pathways. |
| Synthesis of ribose with formose reaction requires a relatively high concentration of formaldehyde, but it is easily converted to formate and methanol in solution. | The continuously generated formaldehyde by the volcano-facilitated photochemical and electrochemical reactions. The continuously generated formaldehyde by hydrothermal vents. |
| The product of the formose reaction is complex, while ribose is not a major one. | Borate, silicate, and phosphate minerals can enhance ribose production. Clays can select ribose from complex reaction products. The glyoxylate scenario. |
| Ribose is unstable. | Preserve ribose by solid phase or low temperature. Using ribose as soon as possible. |
| The efficiency of condensing ribose and nucleobase is low. | Phosphorylated ribose is a better substrate. Construct nucleobase on phosphorylated ribose. |
| The phosphorylation of adenosine is inefficient in solution. | Trimetaphosphate could be a more effective phosphorylation reagent. Wet-dry cycles and metal ions promote the phosphorylation reaction. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, X.-Y.; Zhang, H.-Y. Prebiotic Synthesis of ATP: A Terrestrial Volcanism-Dependent Pathway. Life 2023, 13, 731. https://doi.org/10.3390/life13030731
Chu X-Y, Zhang H-Y. Prebiotic Synthesis of ATP: A Terrestrial Volcanism-Dependent Pathway. Life. 2023; 13(3):731. https://doi.org/10.3390/life13030731
Chicago/Turabian StyleChu, Xin-Yi, and Hong-Yu Zhang. 2023. "Prebiotic Synthesis of ATP: A Terrestrial Volcanism-Dependent Pathway" Life 13, no. 3: 731. https://doi.org/10.3390/life13030731
APA StyleChu, X.-Y., & Zhang, H.-Y. (2023). Prebiotic Synthesis of ATP: A Terrestrial Volcanism-Dependent Pathway. Life, 13(3), 731. https://doi.org/10.3390/life13030731







