EDTA and IAA Ameliorates Phytoextraction Potential and Growth of Sunflower by Mitigating Cu-Induced Morphological and Biochemical Injuries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Soil
2.2. Experimental Design
2.3. Morphological Parameters
2.4. Estimation of Chlorophyll Contents
2.5. Estimation of Phytohormones
2.6. Metabolite Determination
2.7. Determination of Antioxidant Response
2.8. Estimation of the Copper in Plant Biomass
2.9. Data Analysis
3. Results
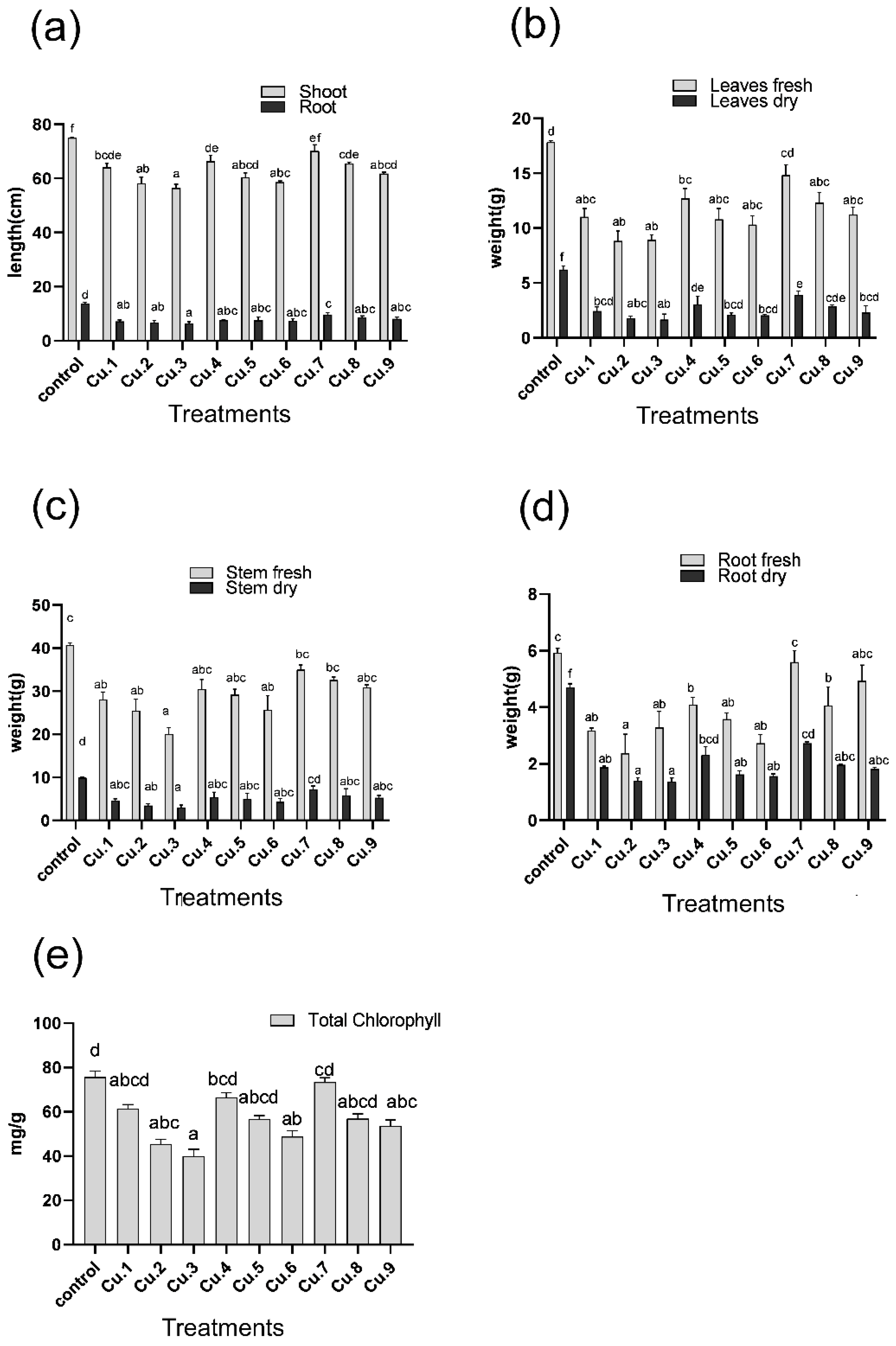
3.1. Effect of Copper on the Growth of Sunflower
3.2. Effect of Copper on Total Chlorophyll Contents
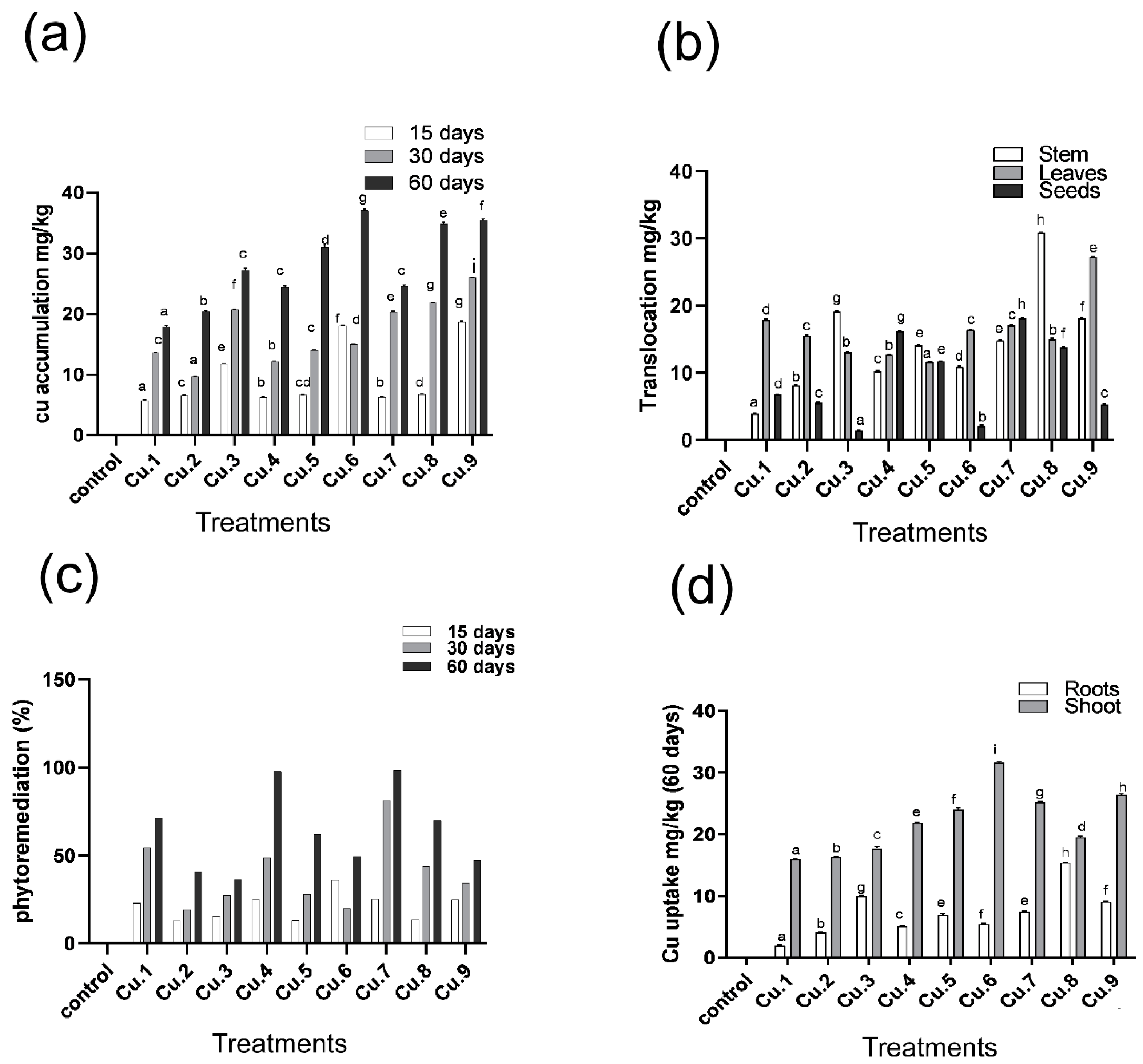
3.3. Metal Accumulation and Translocation to Aerial Parts of the Host
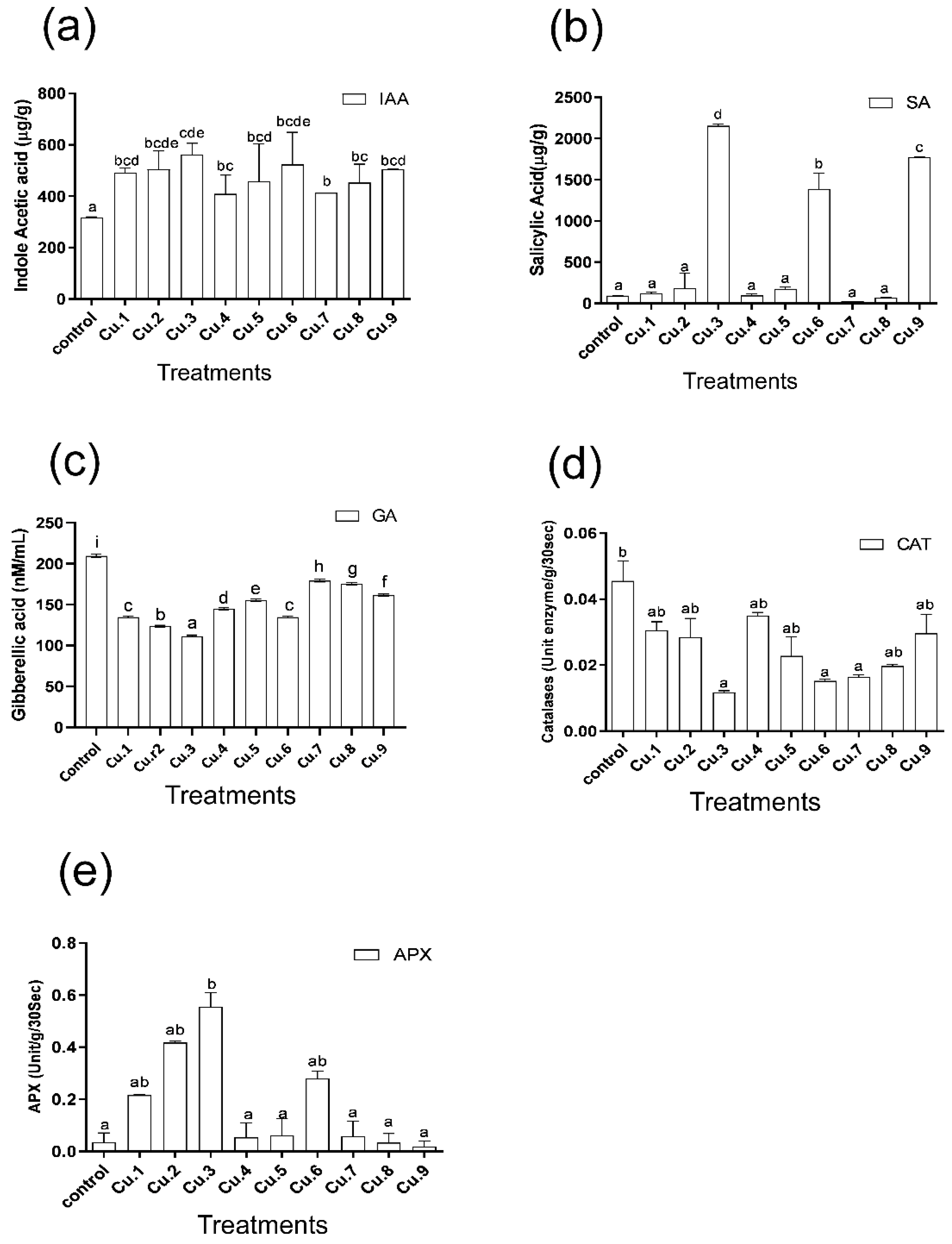
3.4. Production of Phytohormones
3.5. Response of Antioxidants
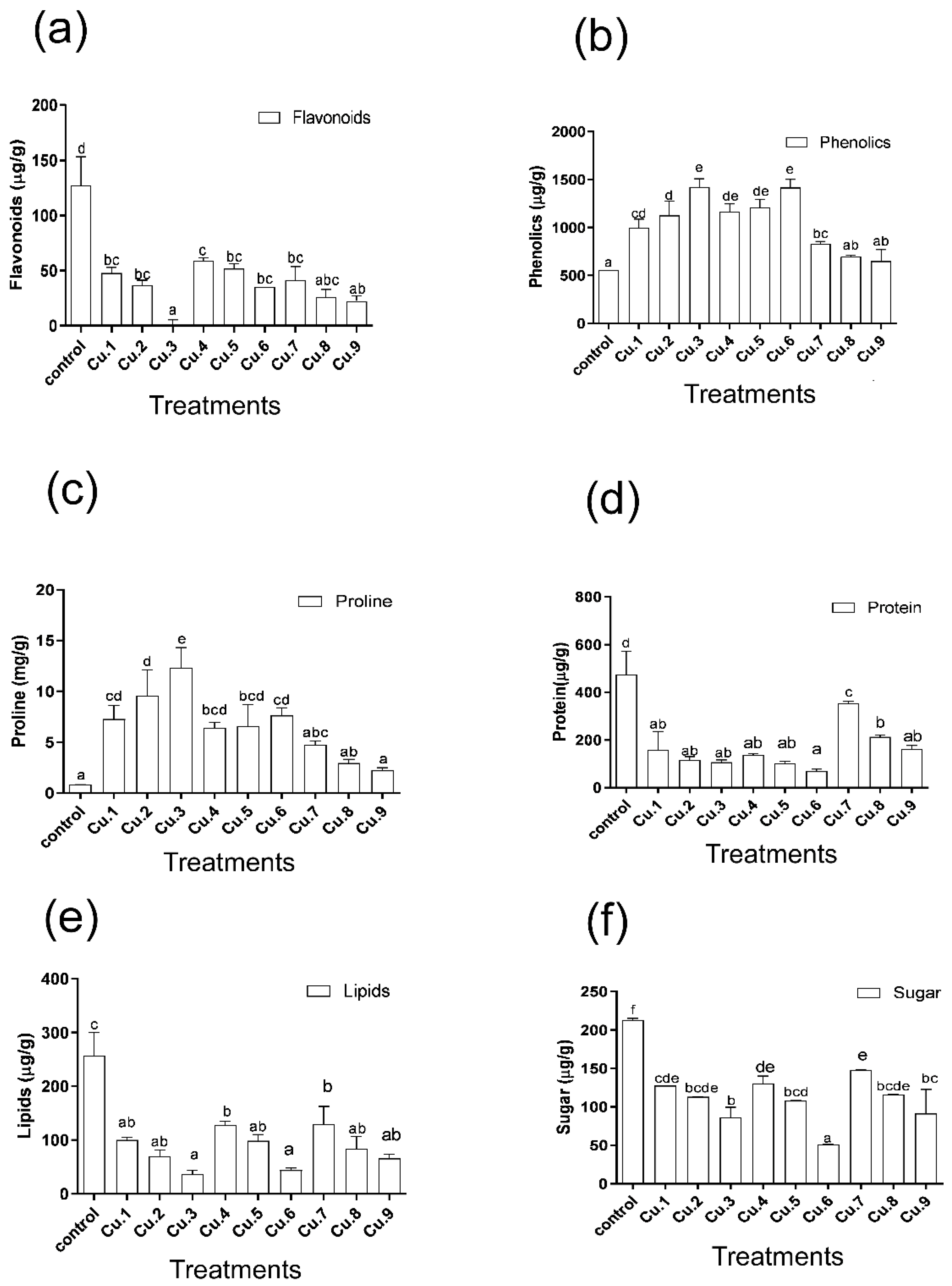
3.6. Production of Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amouei, A.; Cherati, A.; Naghipour, D. Heavy metals contamination and risk assessment of surface soils of Babol in northern Iran. Health Scope 2018, 7, e62423. [Google Scholar] [CrossRef]
- Roy, R.N.; Finck, A.; Blair, G.; Tandon, H.L.S.; AGL; FAO; Land and Water Development Division. Plant nutrition for food security. FAO Fertil. Plant Nutr. Bull. 2006, 16, 368. [Google Scholar]
- Khator, K.; Shekhawat, G.J.A.P.P. Cd-and Cu-induced phytotoxicity on 2–3 leaf stage of Cyamopsis tetragonoloba and its regulation by nitrate reductase and ROS quenching enzyme. Acta Physiol. Plant. 2020, 42, 120. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A.J.E.S.; Research, P. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, M.; Irshad, M.; Rahman, H.; Qasim, M.; Afridi, S.G.; Qadir, M.; Hussain, A. Alleviation of heavy metal toxicity and phytostimulation of Brassica campestris L. by endophytic Mucor sp. MHR-7. Ecotoxicol. Environ. Saf. 2017, 142, 139–149. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Husna; Murad, W. Phytohormones producing rhizobacterium alleviates chromium toxicity in Helianthus annuus L. by reducing chromate uptake and strengthening antioxidant system. Chemosphere 2020, 258, 127386. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Kogbara, R.B. A review of the mechanical and leaching performance of stabilized/solidified contaminated soils. Environ. Rev. 2014, 22, 66–86. [Google Scholar] [CrossRef]
- Fabbricino, M.; Ferraro, A.; Luongo, V.; Pontoni, L.; Race, M. Soil washing optimization, recycling of the solution, and ecotoxicity assessment for the remediation of Pb-contaminated sites using EDDS. Sustainability 2018, 10, 636. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Cui, C. Remediation of heavy metal-contaminated soils by electrokinetic technology: Mechanisms and applicability. Chemosphere 2021, 265, 129071. [Google Scholar] [CrossRef]
- Raj, D.; Kumar, A.; Maiti, S.K. Brassica juncea (L.) Czern. (Indian mustard): A putative plant species to facilitate the phytoremediation of mercury contaminated soils. Int. J. Phytoremediation 2020, 22, 733–744. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Hasanuzzaman, M.; Rizwan, M.; Irshad, S.; Shafiq, F.; Iqbal, M.; Alharbi, B.M.; Alnusaire, T.S.J.P. Jute: A potential candidate for phytoremediation of metals—A review. Plants 2020, 9, 258. [Google Scholar] [CrossRef]
- Shahid, M.; Austruy, A.; Echevarria, G.; Arshad, M.; Sanaullah, M.; Aslam, M.; Nadeem, M.; Nasim, W.; Dumat, C.J.S.; Journal, S.C.A.I. EDTA-enhanced phytoremediation of heavy metals: A review. Soil Sediment Contam. Int. J. 2014, 23, 389–416. [Google Scholar] [CrossRef]
- De Oliveira, L.S.; Brondani, G.E.; Molinari, L.V.; Dias, R.Z.; Teixeira, G.L.; Gonçalves, A.N.; de Almeida, M. Optimal cytokinin/auxin balance for indirect shoot organogenesis of Eucalyptus cloeziana and production of ex vitro rooted micro-cuttings. J. For. Res. 2022, 33, 1573–1584. [Google Scholar] [CrossRef]
- Ben Massoud, M.; Karmous, I.; El Ferjani, E.; Chaoui, A.J.J.o.P.I. Alleviation of copper toxicity in germinating pea seeds by IAA, GA3, Ca and citric acid. J. Plant Interactions 2018, 13, 21–29. [Google Scholar] [CrossRef]
- Arnon, D. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Hussain, A.; Hasnain, S.J. Interactions of bacterial cytokinins and IAA in the rhizosphere may alter phytostimulatory efficiency of rhizobacteria. World J. Microbiol. Biotechnol. 2011, 27, 2645–2654. [Google Scholar] [CrossRef]
- Warrier, R.; Paul, M.; Vineetha, M.J.G. Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Environ. Sci. Biol. 2013, 3, 90–97. [Google Scholar]
- Ismail, I.; Hamayun, M.; Sayyed, A.; Din, I.; Gul, H.; Hussain, A. Gibberellin and indole acetic acid production capacity of endophytic fungi isolated from Zea mays L. Int. J. Biosci. 2016, 8, 35–43. [Google Scholar]
- El Far, M.M.; Taie, H. Antioxidant activities, total anthocyanins, phenolics and flavonoids contents of some sweetpotato genotypes under stress of different concentrations of sucrose and sorbitol. Aust. J. Basic Appl. Sci. 2009, 3, 3609–3616. [Google Scholar]
- Bates, C.J. Proline and hydroxyproline excretion and vitamin C status in elderly human subjects. J. Clin. Sci. Mol. Med. 1977, 52, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Lee, I.-J. Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, and jasmonic acid signals in soybean. J. Plant Growth Regul. 2013, 32, 22–30. [Google Scholar] [CrossRef]
- Asada, K. Ascorbate peroxidase–a hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant. 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pandey, B.; Suthar, S.; Singh, V. Accumulation and health risk of heavy metals in sugarcane irrigated with industrial effluent in some rural areas of Uttarakhand, India. Process. Saf. Environ. Prot. 2016, 102, 655–666. [Google Scholar] [CrossRef]
- Jamal, A.; Sarim, M. Heavy metals distribution in different soil series of district Swabi, Khyber Pakhunkhawa, Pakistan. World Sci. News 2018, 105, 1–13. [Google Scholar]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Feil, S.B.; Pii, Y.; Valentinuzzi, F.; Tiziani, R.; Mimmo, T.; Cesco, S. Copper toxicity affects phosphorus uptake mechanisms at molecular and physiological levels in Cucumis sativus plants. Plant Physiol. Biochem. 2020, 157, 138–147. [Google Scholar] [CrossRef]
- Mohamed, H.; Gomaa, E. Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescens on growth and pigment composition of radish plants (Raphanus sativus) under NaCl stress. Photosynthetica 2012, 50, 263–272. [Google Scholar] [CrossRef]
- Ismail, A.H.; Mehmood, A.; Qadir, M.; Husna, A.I.; Hamayun, M.; Khan, N. Thermal stress alleviating potential of endophytic fungus rhizopus oryzae inoculated to sunflower (Helianthus annuus L.) and soybean (Glycine max L.). Pak. J. Bot. 2020, 52, 1857–1865. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, N.; Khan, M.N.; Qadir, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Rehman, K.U.; Lee, I.-J. Antimicrobial and plant growth-promoting activities of bacterial endophytes isolated from Calotropis procera (Ait.) WT Aiton. Biocell 2021, 45, 363. [Google Scholar] [CrossRef]
- Borker, A.R.; David, K.; Singhal, N. Analysis of time varying response on uptake patterns of Cu and Zn ions under application of ethylene diamine disuccinic acid and gibberellic acid in Lolium perenne. Chemosphere 2020, 260, 127541. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Irshad, M.; Ahmad, A.; Lodhi, M.A.; Lee, I.-J. Phytohormones Producing Acinetobacter bouvetii P1 Mitigates Chromate Stress in Sunflower by Provoking Host Antioxidant Response. Antioxidants 2021, 10, 1868. [Google Scholar] [CrossRef]
- Husna; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Murad, W.; Irshad, M.; Qadir, M.; Kim, H.-Y. Pseudocitrobacter anthropi reduces heavy metal uptake and improves phytohormones and antioxidant system in Glycine max L. World J. Microbiol. Biotechnol. 2021, 37, 195. [Google Scholar] [CrossRef]
- Husna; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Qadir, M.; Alataway, A.; Dewidar, A.Z.; Elansary, H.O.; Lee, I.-J. Phytohormones producing rhizobacteria alleviate heavy metals stress in soybean through multilayered response. Microbiol. Res. 2023, 266, 127237. [Google Scholar] [CrossRef]
- Husna, H.; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Qadir, M.; Asim, S.; Lee, I.-J. Stemphylium lycopersici and Stemphylium solani improved antioxidant system of soybean under chromate stress. Front. Microbiol. 2022, 13, 4314. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Bałabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef]
- Hayat, K.; Menhas, S.; Bundschuh, J.; Zhou, P.; Niazi, N.K.; Amna; Hussain, A.; Hayat, S.; Ali, H.; Wang, J. Plant growth promotion and enhanced uptake of Cd by combinatorial application of Bacillus pumilus and EDTA on Zea mays L. Int. J. Phytoremediation 2020, 22, 1372–1384. [Google Scholar] [CrossRef]
- Jumali, S.S.; Said, I.M.; Ismail, I.; Zainal, Z. Genes induced by high concentration of salicylic acid in ‘Mitragyna speciosa’. Aust. J. Crop Sci. 2011, 5, 296–303. [Google Scholar]
- Raigond, P.; Buckseth, T.; Singh, B.; Kaundal, B.; Singh, R.K.; Singh, B.P. Influence of Photoperiod and EDTA Salts on Endogenous Gibberellic Acid Concentration of Tissue Culture Grown Potato Microplants. Agric. Res. 2019, 8, 176–183. [Google Scholar] [CrossRef]
- Ismaila, A.H.; Qadira, M.; Husnaa, M.I.; Ahmadb, A.; Hamayuna, M. Endophytic fungi isolated from Citrullus colocynthesl. Leaves and Their potential for secretion of indole acetic acid and gibberellin. Appl. Environ. Biol. Sci. 2018, 8, 80–84. [Google Scholar]
- Rai, K.K.; Pandey, N.; Rai, S.P. Salicylic acid and nitric oxide signaling in plant heat stress. Physiol. Plant. 2020, 168, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Říha, M.; Karlíčková, J.; Filipský, T.; Macáková, K.; Rocha, L.; Bovicelli, P.; Silvestri, I.P.; Saso, L.; Jahodář, L.; Hrdina, R. In vitro evaluation of copper-chelating properties of flavonoids. RSC Adv. 2014, 4, 32628–32638. [Google Scholar] [CrossRef]
- Contreras, R.A.; Pizarro, M.; Köhler, H.; Sáez, C.A.; Zúñiga, G.E. Copper stress induces antioxidant responses and accumulation of sugars and phytochelatins in Antarctic Colobanthus quitensis (Kunth) Bartl. Biol. Res. 2018, 51, 48. [Google Scholar] [CrossRef] [PubMed]
- Kısa, D.; Elmastaş, M.; Öztürk, L.; Kayır, Ö. Responses of the phenolic compounds of Zea mays under heavy metal stress. Appl. Biol. Chem. 2016, 59, 813–820. [Google Scholar] [CrossRef]
- Rico, M.; López, A.; Santana-Casiano, J.M.; Gonzàlez, A.G.; Gonzàlez-Dàvila, M. Variability of the phenolic profile in the diatom Phaeodactylum tricornutum growing under copper and iron stress. Limnol. Oceanogr. 2013, 58, 144–152. [Google Scholar] [CrossRef]
- Farid, H.T. The effect of the marine cyanobacterium (Trichodesmium erythraeum) on iron speciation in seawater. Ph.D. Dissertation, Southern Cross University, East Lismore, Australia, 2016. [Google Scholar]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Surówka, E.; Hura, T. Osmoprotectants and nonenzymatic antioxidants in halophytes. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–31. [Google Scholar]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rana, M.S.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Soliman, M.H.; Elkelish, A. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef]
- Husna; Hussain, A.; Shah, M.; Hamayun, M.; Qadir, M.; Iqbal, A. Heavy metal tolerant endophytic fungi Aspergillus welwitschiaeimproves growth, ceasing metal uptake and strengthening antioxidant system inGlycine maxL. Environ. Sci. Pollut. Res. 2022, 29, 15501–15515. [Google Scholar] [CrossRef]
- Nonogaki, K. New insights into sympathetic regulation of glucose and fat metabolism. Dibetologia 2000, 43, 533–549. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Shah, M.; Lee, I.J.; Iqbal, A.; Irshad, M.; Ismail; Sayyed, A.; Husna; Ahmad, A.; et al. Comparative assessment of chromate bioremediation potential of Pantoea conspicua and Aspergillus niger. J. Hazard. Mater. 2022, 424, 127314. [Google Scholar] [CrossRef]
- Chandra, J.; Keshavkant, S. Mechanisms underlying the phytotoxicity and genotoxicity of aluminum and their alleviation strategies: A review. Chemosphere 2021, 278, 130384. [Google Scholar] [CrossRef]
- Wang, S.-H.; Yang, Z.-M.; Yang, H.; Lu, B.; Li, S.-Q.; Lu, Y.-P. Copper-induced stress and antioxidative responses in roots of Brassica juncea L. Bot. Bull. Acad. Sin. 2004, 45, 203–212. [Google Scholar]
- Ouzounidou, G.; Čiamporová, M.; Moustakas, M.; Karataglis, S. Responses of maize (Zea mays L.) plants to copper stress—I. Growth, mineral content and ultrastructure of roots. Environ. Exp. Bot. 1995, 35, 167–176. [Google Scholar] [CrossRef]
- Shah, N.; Qadir, M.; Irshad, M.; Hussain, A.; Hamayun, M.; Murad, W.; Khan, A.; Al-Harrasi, A. Enhancement of Cadmium Phytoremediation Potential of Helianthus annuus L. with Application of EDTA and IAA. Metabolites 2022, 12, 1049. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Nadia. Enhancement of chromate phytoremediation and soil reclamation potential of Brassica campestris L. by Aspergillus niger. Environ. Sci. Pollut. Res. 2022, 30, 9471–9482. [Google Scholar] [CrossRef]
- Pratap, H.; Mamboya, F.; Mtolera, M.; Björk, M. The effect of copper on the daily growth rate and photosynthetic efficiency of the brown macroalga Padina boergesenii. In Proceedings of the Conference on Advances on Marine Sciences in Tanzania, Zanzibar, Tanzania, 28 June–1 July 1999. [Google Scholar]
- Wu, X.; Yang, Y.; Liu, H.; Yue, Z.; Gao, X.; Yang, F.; Xing, X. Effects of dietary copper supplementation on nutrient digestibility, serum biochemical indices, and growth rate of young female mink (Neovison vison). Czech J. Anim. Sci. 2014, 59, 529–537. [Google Scholar] [CrossRef]
- Jiang, B.; Ma, Y.; Zhu, G.; Li, J. Prediction of soil copper phytotoxicity to barley root elongation by an EDTA extraction method. J. Hazard. Mater. 2020, 389, 121869. [Google Scholar] [CrossRef]
- Mir, A.R.; Alam, P.; Hayat, S. Auxin regulates growth, photosynthetic efficiency and mitigates copper induced toxicity via modulation of nutrient status, sugar metabolism and antioxidant potential in Brassica juncea. Plant Physiol. Biochem. 2022, 185, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.C.; Rahman, M.M.; Boyce, A.N.; Abas, M.R. Heavy metals phyto-assessment in commonly grown vegetables: Water spinach (I. aquatica) and okra (A. esculentus). Springerplus 2016, 5, 469. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Gill, S.S.; Tuteja, R. Plant responses to abiotic stresses: Shedding light on salt, drought, cold and heavy metal stress. In Omics and Plant Abiotic Stress Tolrance; Bentham Science Publisher Ltd.: Potomac, MD, USA, 2011; Volume 1, pp. 39–64. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, N.; Irshad, M.; Hussain, A.; Qadir, M.; Murad, W.; Khan, A.; Awais, M.; Alrefaei, A.F.; Ali, S. EDTA and IAA Ameliorates Phytoextraction Potential and Growth of Sunflower by Mitigating Cu-Induced Morphological and Biochemical Injuries. Life 2023, 13, 759. https://doi.org/10.3390/life13030759
Shah N, Irshad M, Hussain A, Qadir M, Murad W, Khan A, Awais M, Alrefaei AF, Ali S. EDTA and IAA Ameliorates Phytoextraction Potential and Growth of Sunflower by Mitigating Cu-Induced Morphological and Biochemical Injuries. Life. 2023; 13(3):759. https://doi.org/10.3390/life13030759
Chicago/Turabian StyleShah, Naila, Muhammad Irshad, Anwar Hussain, Muhammad Qadir, Waheed Murad, Asif Khan, Muhammad Awais, Abdulwahed Fahad Alrefaei, and Sajid Ali. 2023. "EDTA and IAA Ameliorates Phytoextraction Potential and Growth of Sunflower by Mitigating Cu-Induced Morphological and Biochemical Injuries" Life 13, no. 3: 759. https://doi.org/10.3390/life13030759
APA StyleShah, N., Irshad, M., Hussain, A., Qadir, M., Murad, W., Khan, A., Awais, M., Alrefaei, A. F., & Ali, S. (2023). EDTA and IAA Ameliorates Phytoextraction Potential and Growth of Sunflower by Mitigating Cu-Induced Morphological and Biochemical Injuries. Life, 13(3), 759. https://doi.org/10.3390/life13030759











