Nanoparticles Induced Oxidative Damage in Reproductive System and Role of Antioxidants on the Induced Toxicity
Abstract
1. Introduction
2. Nanoparticles and Reactive Oxygen Species (ROS)
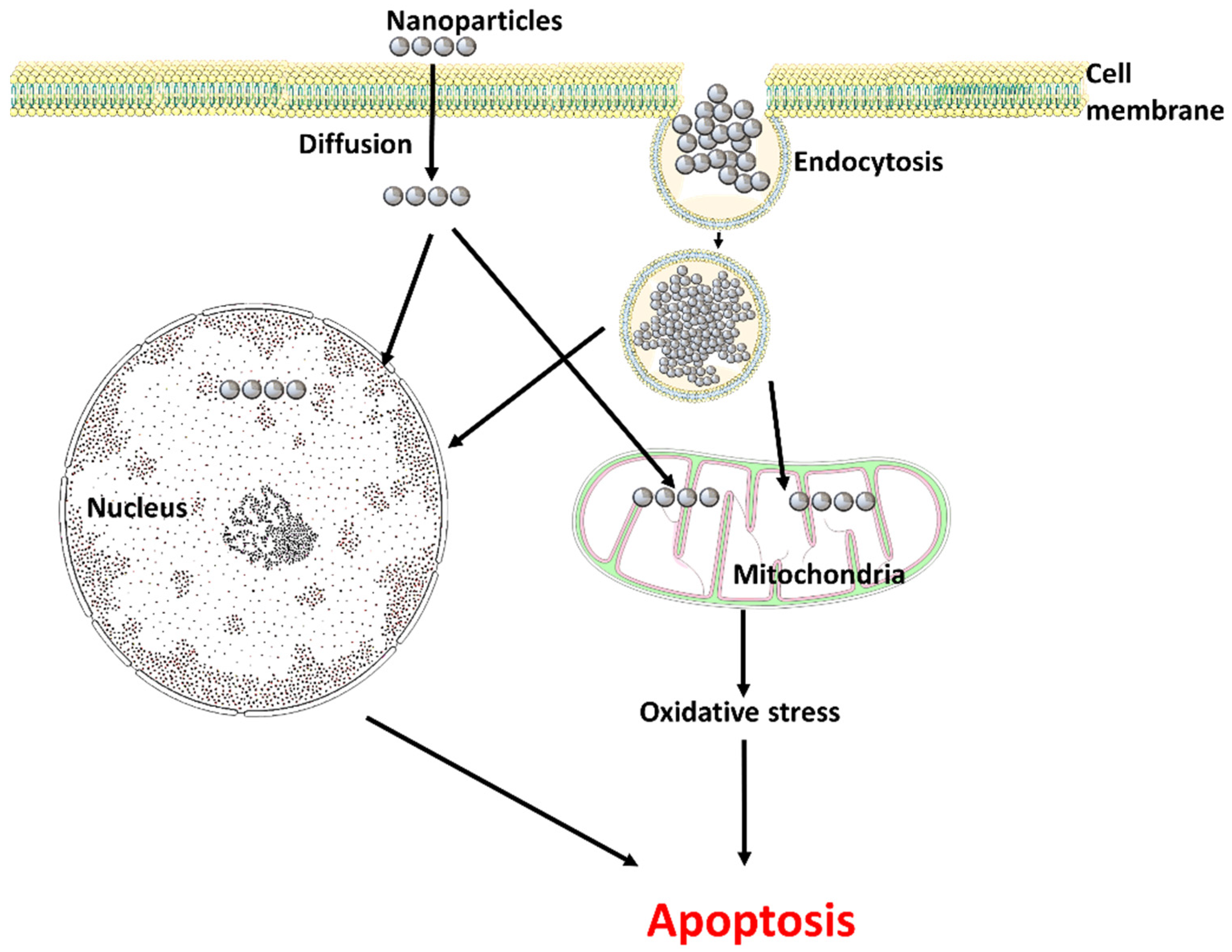
3. Entry of Nanoparticles into the Reproductive System
4. Impact on the Male Reproductive System
| S. No | Nanoparticles | Animal Model | Toxicity Effect | References |
|---|---|---|---|---|
| 1. | Silica nanoparticles | Male Albino Rats | Higher levels of micronucleus frequencies and malondialdehyde levels, and lesser catalase and glutathione activity in testicular tissues. | [44] |
| 2. | ZnO nanoparticles | TM-4 Sertoli cell line and GC2-spd spermatocyte cell line of mouse | Breakdown of the cell membrane and outer membrane of mitochondria in Sertoli cells; down-regulating the production of gap junction proteins; disruption of BTB disruption. | [42] |
| Mouse testis Leydig cells | Decreased antioxidant enzyme gene expression (SOD) and increased steroidogenesis-related gene expression. | [45] | ||
| 3. | Titanium oxide nanoparticles | Pregnant mouse model | Apoptosis of the olfactory bulb occurs with decreased sperm production and motility; disordered and disrupted seminiferous tubules. | [46] |
| Mouse, rat, and porcine Leydig cells | 17–α hydroxylase/C17-20 lyase and cholesterol side-chain cleavage enzyme gene and protein expression are affected by TNF- α to decrease testosterone synthesis. | [49] | ||
| 4. | Cerium oxide nanoparticles | Pregnant mouse model | Involvement in the prepubertal spermatogenesis and germ cell; reduction of germ cells, deformation of Sertoli cells; impairment steroidogenesis. | [53] |
Spermatogenesis and Toxicity
5. Impact on the Female Reproductive System
| S. No. | Nanoparticles | Animal Model | Toxicity Effect | References |
|---|---|---|---|---|
| 1. | ZnO nanoparticles | Female zebrafish | Autophagy and apoptosis occurring in a caspase-dependent manner; increased oxidative stress by inducing mutant ovarian p53 protein; necroptosis; follicular developmental retardation; deformation of oocyte ovulation, and decreased female zebrafish fertility. | [69] |
| 2. | Cadmium oxide nanoparticles | Pregnant mouse model | Weight gain, increased uterus weight, and decreased weight of placenta; decreased quantity of estrogen receptors. | [70] |
| 3. | Titanium oxide nanoparticles | Female mice | Up-regulation of Cyp17a1 resulted in enhanced estradiol production; up-regulation of bmf genes; apoptotic genes were down-regulated. | [72] |
| 4. | Cerium oxide nanoparticles | Mouse oocytes | Accumulation in the zona pellucida (ZP) of oocytes; DNA damage due to follicular cell endocytosis and zona pellucida trapping. | [73] |
| 5. | Silver nanoparticles | Ovaries of female albino rats | Inhibition of the ovulation; activation of oxidative stress factors in ovarian cells resulting in apoptosis. | [75] |
Nanotoxicity on the Steroidogenic Pathway
6. Nanotoxicity Quantification Tests
6.1. Assay for the Determination of ROS Production Due to Oxidative Stress
6.1.1. Superoxide Dismutase (SOD) Assay
6.1.2. Catalase Assay
6.1.3. Glutathione Peroxidase Assay
6.2. Other Methods
7. Role of Antioxidants in Nanoparticle-Induced Stress
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aziz, Z.A.A.; Mohd-Nasir, H.; Ahmad, A.; Setapar, S.H.M.; Peng, W.L.; Chuo, S.C.; Khatoon, A.; Umar, K.; Yaqoob, A.A.; Ibrahim, M.N.M. Role of nanotechnology for design and development of cosmeceutical: Application in makeup and skin care. Front. Chem. 2019, 7, 739. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Rajakumar, G.; Chung, I.M. Nanotechnology: Current uses and future applications in the food industry. 3 Biotech 2018, 8, 74. [Google Scholar] [CrossRef]
- Abiodun-Solanke, I.; Ajayi, D.; Arigbede, A. Nanotechnology and its application in dentistry. Ann. Med. Health Sci. Res. 2014, 4, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.; Joy, J.; George, S.C. Potential applications of nanotechnology in transportation: A review. J. King Saud Univ. Sci. 2019, 31, 586–594. [Google Scholar] [CrossRef]
- Samrot, A.V.; Angalene, J.L.A.; Roshini, S.M.; Stefi, S.M.; Preethi, R.; Raji, P. Purification, characterization and exploitation of Azadirachta indica gum for the production of drug loaded nanocarrier. Mater. Res. Express 2020, 7, 055007. [Google Scholar] [CrossRef]
- Samrot, A.V.; Angalene, J.L.A.; Roshini, S.M.; Stefi, S.M.; Preethi, R.; Raji, P.; Kumar, A.M.; Paulraj, P.; Kumar, S.S. Purification, characterization and utilization of polysaccharide of Araucaria heterophylla gum for the synthesis of curcumin loaded nanocarrier. Int. J. Biol. Macromol. 2019, 1, 93–400. [Google Scholar] [CrossRef] [PubMed]
- Akçan, R.; Aydogan, H.C.; Yildirim, M.Ş.; Taştekin, B.; Sağlam, N. Nanotoxicity: A challenge for future medicine. Turk. J. Med. Sci. 2020, 50, 1180–1196. [Google Scholar] [CrossRef]
- Habas, K.; Demir, E.; Guo, C.; Brinkworth, M.H.; Anderson, D. Toxicity mechanisms of nanoparticles in the male reproductive system. Drug Metab. Rev. 2014, 53, 604–617. [Google Scholar] [CrossRef]
- Shalviri, A.; Cai, P.; Rauth, A.M.; Henderson, J.T.; Wu, X.Y. Evaluation of new bi-functional terpolymeric nanoparticles for simultaneous in vivo optical imaging and chemotherapy of breast cancer. Drug Deliv. Transl. Res. 2012, 2, 437–453. [Google Scholar] [CrossRef]
- Cypriyana, P.J.J.; Saigeetha, S.; Samrot, A.V.; Ponniah, P.; Chakravarthi, S. Overview on toxicity of nanoparticles, it’s mechanism, models used in toxicity studies and disposal methods–A review. Biocatal. Agric. Biotechnol. 2021, 36, 102117. [Google Scholar] [CrossRef]
- Samrot, A.V.; Singh, S.P.R.; Deenadhayalan, R.; Rajesh, V.V.; Padmanaban, S.; Radhakrishnan, K. Nanoparticles, a Double-Edged Sword with Oxidant as Well as Antioxidant Properties—A Review. Oxygen 2022, 2, 591–604. [Google Scholar] [CrossRef]
- Ge, X.; Cao, Z.; Chu, L. The Antioxidant Effect of the Metal and Metal-Oxide Nanoparticles. Antioxidants 2022, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidant activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Song, B.; Wu, J.; Zhang, Y.; Chen, A.; Shao, L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018, 13, 8487–8506. [Google Scholar] [CrossRef]
- Working, P.K. Male reproductive toxicology: Comparison of the human to animal models. Environ. Health Perspect. 1988, 77, 37–44. [Google Scholar] [CrossRef]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Reactive oxygen species in male reproduction: A boon or a bane? Andrologia 2021, 53, e13577. [Google Scholar] [CrossRef]
- Derakhshi, M.; Daemi, S.; Shahini, P.; Habibzadeh, A.; Mostafavi, E.; Ashkarran, A.A. Two-dimensional nanomaterials beyond graphene for biomedical applications. J. Funct. Biomater. 2022, 13, 27. [Google Scholar] [CrossRef]
- Mihailovic, V.; Stankovic, J.S.K.; Selakovic, D.; Rosic, G. An overview of the beneficial role of antioxidants in the treatment of nanoparticle-induced toxicities. Oxidative Med. Cell. Longev. 2021, 2021, 7244677. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Singh, A.K.; Kumar, R.; Gupta, A.; Pandey, A.K.; Pandey, A.K. Nanoparticles as modulators of oxidative stress. Nanotechnol. Mod. Anim. Biotechnol. 2019, 29–35. [Google Scholar] [CrossRef]
- Larson, J.K.; Carvan, M.J., III; Hutz, R.J. Engineered nanomaterials: An emerging class of novel endocrine disruptors. Biol. Reprod. 2014, 91, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Sies, H. (Ed.) Oxidative Stress: Introduction. In Oxidative Stress Oxidants and Antioxidants; Academic Press: London, UK, 1991; Volume 15e22. [Google Scholar]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef]
- Mao, J.; Hu, Y.; Ruan, L.; Ji, Y.; Lou, Z. Role of endoplasmic reticulum stress in depression (Review). Mol. Med. Rep. 2019, 20, 4774–4780. [Google Scholar] [CrossRef]
- Bettigole, S.E.; Glimcher, L.H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015, 33, 107–138. [Google Scholar] [CrossRef]
- Carrara, M.; Prischi, F.; Nowak, P.R.; Kopp, M.C.; Ali, M.M. Noncanonical binding of BiP ATPase domain to Ire1 and Perk is dissociated by unfolded protein CH1 to initiate ER stress signaling. Elife 2015, 4, e03522. [Google Scholar] [CrossRef]
- Čapek, J.; Roušar, T. Detection of Oxidative Stress Induced by Nanomaterials in Cells-The Roles of Reactive Oxygen Species and Glutathione. Molecules 2021, 26, 4710. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, C.; Aronstam, R. Toxicity of transition metal oxide nanoparticles: Recent insights from in vitro Studies. Materials 2010, 3, 4842–4859. [Google Scholar] [CrossRef]
- Brohi, R.D.; Wang, L.; Talpur, H.S.; Wu, D.; Khan, F.A.; Bhattarai, D.; Rehman, Z.U.; Farmanullah, F.; Huo, L.J. Toxicity of nanoparticles on the reproductive system in animal models: A review. Front. Pharmacol. 2017, 8, 606. [Google Scholar] [CrossRef]
- Marziyeh, A.; Keyhanfar, F.; Moosavi, M.A.; Shabani, R.; Mehdizadeh, M.; Varma, R.S. Potential toxicity of nanoparticles on the reproductive system animal models: A review. J. Reprod. Immunol. 2021, 148, 103384. [Google Scholar]
- Dianová, L.; Tirpák, F.; Halo, M.; Slanina, T.; Massányi, M.; Stawarz, R.; Formicki, G.; Madeddu, R.; Massányi, P. Effects of Selected Metal Nanoparticles (Ag, ZnO, TiO2) on the Structure and Function of Reproductive Organs. Toxics 2022, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, D.; Toader, A.; Hoteiuc, O. Oxidative Stress Produced by Urban Atmospheric Nanoparticles. In Nanomaterials—Toxicity, Human Health and Environment; Clichici, S., Filip, A., do Nascimento, G.M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Zhou, Q.; Yue, Z.; Li, Q.; Zhou, R.; Liu, L. Exposure to PbSe nanoparticles and male reproductive damage in a rat model. Environ. Sci. Technol. 2019, 53, 13408–13416. [Google Scholar] [CrossRef] [PubMed]
- Jamnongjit, M.; Gill, A.; Hammes, S.R. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc. Natl. Acad. Sci. USA 2005, 102, 16257–16262. [Google Scholar] [CrossRef]
- Hou, C.C.; Zhu, J.Q. Nanoparticles and female reproductive system: How do nanoparticles affect oogenesis and embryonic development. Oncotarget 2017, 8, 109799–109817. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Iftikhar, M.; Noureen, A.; Uzair, M.; Jabeen, F.; Daim, M.A.; Cappello, T. Perspectives of Nanoparticles in Male Infertility: Evidence for Induced Abnormalities in Sperm Production. Int. J. Environ. Res. Public Health 2021, 18, 1758. [Google Scholar] [CrossRef]
- Vassal, M.; Rebelo, S.; de Lourdes Pereira, M. Metal Oxide Nanoparticles: Evidence of Adverse Effects on the Male Reproductive System. Int. J. Mol. Sci. 2021, 22, 8061. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, C.; Ji, G.; Liu, H.; Mo, Y.; Tollerud, D.J.; Gu, A.; Zhang, Q. Sublethal effects of ZnOnanoparticles on male reproductive cells. Toxicol. In Vitro 2016, 35, 131–138. [Google Scholar] [CrossRef]
- Mathias, F.T.; Romano, R.M.; Kizys, M.M.; Kasamatsu, T.; Giannocco, G. Daily exposure to silver nanoparticles during prepubertal development decreases adult sperm and reproductive parameters. Nanotoxicology 2015, 9, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Azouz, R.A.; Korany, R.M.S.; Noshy, A. Silica Nanoparticle–Induced Reproductive Toxicity in Male Albino Rats via Testicular Apoptosis and Oxidative Stress. Biol. Trace Elem. Res. 2022, 201, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Bara, N.; Kaul, G. Enhanced steroidogenic and altered antioxidant response by ZnO nanoparticles in mouse testis Leydig cells. Toxicol. Ind. Health 2018, 34, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Suzuki, K.; Ishihara, A.; Kubo-Irie, M.; Fujimoto, R.; Tabata, M.; Oshio, S.; Nihei, Y.; Ihara, T.; Sugamata, M. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J. Health Sci. 2009, 55, 95–102. [Google Scholar] [CrossRef]
- Meena, R.; Kajal, K.; Paulraj, R. Cytotoxic and genotoxic effects of titanium dioxide nanoparticles in testicular cells of male Wistar rat. Appl. Biochem. Biotechnol. 2015, 175, 825–840. [Google Scholar] [CrossRef]
- Shahin, N.N.; Mohamed, M.M. Nano-sized titanium dioxide toxicity in rat prostate and testis: Possible ameliorative effect of morin. Toxicol. Appl. Pharmacol. 2017, 334, 129–141. [Google Scholar] [CrossRef]
- Xiong, Y.; Hales, D.B. The role of tumor necrosis factor-alpha in the regulation of mouse Leydig cell steroidogenesis. Endocrinology 1993, 132, 2438–2444. [Google Scholar] [CrossRef]
- Lauvås, A.J.; Skovmand, A.; Poulsen, M.S.; Kyjovska, Z.O.; Roursgaard, M.; Goericke-Pesch, S.; Vogel, U.; Hougaard, K.S. Airway exposure to TiO2 nanoparticles and quartz and effects on sperm counts and testosterone levels in male mice. Reprod. Toxicol. 2019, 90, 134–140. [Google Scholar] [CrossRef]
- Ogunsuyi, O.M.; Ogunsuyi, O.I.; Akanni, O.; Alabi, O.A.; Alimba, C.G.; Adaramoye, O.A.; Cambier, S.; Eswara, S.; Gutleb, A.C.; Bakare, A.A. Alteration of sperm parameters and reproductive hormones in Swiss mice via oxidative stress after co-exposure to titanium dioxide and ZnOnanoparticles. Andrologia 2020, 52, e13758. [Google Scholar] [CrossRef]
- Miura, N.; Ohtani, K.; Hasegawa, T.; Yoshioka, H.; Hwang, G.W. High sensitivity of testicular function to titanium nanoparticles. J. Toxicol. Sci. 2017, 42, 359–366. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, H.J. Toxicity of Cerium Oxide Nanoparticles on Neonatal Testicular Development in Mouse Organ Culture. Reprod. Toxicol. 2022, 111, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Bhavya, K.S.; Sahithya, C.S.; Sowmya, N. Evaluation of toxicity of chemically synthesised gold nanoparticles against Eudrilus eugeniae. J. Clust. Sci. 2018, 29, 1217–1225. [Google Scholar] [CrossRef]
- Samrot, A.V.; Saipriya, C.; Agnes, L.A.; Roshini, S.M.; Cypriyana, J.; Saigeetha, S.; Raji, P.; Kumar, S. Evaluation of nanotoxicity of Araucaria heterophylla gum derived green synthesized silver nanoparticles on Eudrilus eugeniae and Danio rerio. J. Clust. Sci. 2019, 30, 1017–1024. [Google Scholar] [CrossRef]
- Samrot, A.V.; Ujjala, B.; Padmanaban, S.; Yamini, P.; Rabel, A.M. A study on toxicity of chemically synthesised silver nanoparticle on Eudriluseugeniae. Toxicol. Environ. Health Sci. 2018, 10, 162–167. [Google Scholar] [CrossRef]
- Shobana, N.; Prakash, P.; Samrot, A.V.; Saigeetha, S.; Sathiyasree, M.; Thirugnanasambandam, R.; Sridevi, V.; Kumar, M.B.; Shankar, S.G.; Dhiva, S.; et al. Nanotoxicity studies of Azadirachta indica mediated silver nanoparticles against Eudrilus eugeniae, Danio rerio and its embryos. Biocatal. Agric. Biotechnol. 2023, 47, 102561. [Google Scholar] [CrossRef]
- Samrot, A.V.; Justin, C.; Padmanaban, S.; Burman, U. A study on the effect of chemically synthesized magnetite nanoparticles on earthworm: Eudrilus eugeniae. Appl. Nanosci. 2017, 7, 17–23. [Google Scholar] [CrossRef]
- Mahfouz, R.; Sharma, R.; Thiyagarajan, A.; Kale, V.; Gupta, S.; Sabanegh, E.; Agarwal, A. Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil. Steril. 2010, 94, 2141–2146. [Google Scholar] [CrossRef]
- Olugbodi, J.O.; David, O.; Oketa, E.N.; Lawal, B.; Okoli, B.J.; Mtunzi, F. Silver nanoparticles stimulates spermatogenesis impairments and hematological alterations in testis and epididymis of male rats. Molecules 2020, 25, 1063. [Google Scholar] [CrossRef]
- Sundarraj, K.; Manickam, V.; Raghunath, A.; Periyasamy, M.; Viswanathan, M.; Perumal, E. Repeated exposure to iron oxide nanoparticles causes testicular toxicity in mice. Environ. Toxicol. 2017, 32, 594–608. [Google Scholar] [CrossRef]
- Iyiola, O.; Olafimihan, T.F.; Sulaiman, F.A.; Anifowoshe, A.T. Genotoxicity and histopathological assessment of silver nanoparticles in Swiss albino mice. Cuad. Investig. UNED 2018, 10, 102–109. [Google Scholar] [CrossRef]
- Thakur, M.; Gupta, H.; Singh, D.; Mohanty, I.R.; Maheswari, U.; Vanage, G.; Joshi, D.S. Histopathological and ultra structural effects of nanoparticles on rat testis following 90 days (chronic study) of repeated oral administration. J. Nanobiotechnol. 2014, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Vaziri-Katehshori, N.; Noori, A. The effect of multi-wall carbon nanotubes on sex hormone levels and ovarian tissue in female Wistar rats. J. Kashan Univ. Med. Sci. 2018, 21, 525–533. [Google Scholar]
- Farshad, O.; Heidari, R.; Zamiri, M.J.; Retana-Márquez, S.; Khalili, M.; Ebrahimi, M.; Jamshidzadeh, A.; Ommati, M.M. Spermatotoxic Effects of Single-Walled and Multi-Walled Carbon Nanotubes on Male Mice. Front. Vet. Sci. 2020, 7, 591558. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Zhang, P.; Sun, F.; Chen, Z.; Ma, W.; Meng, F.; Hao, H.; Shang, X. Silica nanoparticles cause spermatogenesis dysfunction in mice via inducing cell cycle arrest and apoptosis. Ecotoxicol. Environ. Saf. 2022, 231, 113210. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, F.; Miao, Y.; Luo, X.; Dai, S.; Liu, J.; Niu, W.; Sun, Y. CdSe/ZnS quantum dots induced spermatogenesis dysfunction via autophagy activation. J. Hazard. Mater. 2020, 398, 122327. [Google Scholar] [CrossRef]
- Hong, F.; Si, W.; Zhao, X.; Wang, L.; Zhou, Y.; Chen, M.; Ge, Y.; Zhang, Q.; Wang, Y.; Zhang, J. TiO2 nanoparticle exposure decreases spermatogenesis via biochemical dysfunctions in the testis of male mice. J. Agric. Food Chem. 2015, 63, 7084–7092. [Google Scholar] [CrossRef]
- Mawed, S.A.; Marini, C.; Alagawany, M.; Farag, M.R.; Reda, R.M.; El-Saadony, M.T.; Elhady, W.M.; Magi, G.E.; Di Cerbo, A.; El-Nagar, W.G. ZnONanoparticles (ZnO-NPs) Suppress Fertility by Activating Autophagy, Apoptosis, and Oxidative Stress in the Developing Oocytes of Female Zebrafish. Antioxidants 2022, 11, 1567. [Google Scholar] [CrossRef]
- Ahmad, A. Safety and Toxicity Implications of Multifunctional Drug Delivery Nanocarriers on Reproductive Systems In Vitro and In Vivo. Front. Toxicol. 2022, 4, 895667. [Google Scholar] [CrossRef]
- Blum, J.L.; Xiong, J.Q.; Hoffman, C.; Zelikoff, J.T. Cadmium Associated with Inhaled Cadmium Oxide Nanoparticles Impacts Fetal and Neonatal Development and Growth. Toxicol. Sci. 2012, 126, 478–486. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Q.; Wang, Z.; Yan, B. Effects of nanotoxicity on female reproductivity and fetal development in animal models. Int. J. Mol. Sci. 2013, 14, 9319–9337. [Google Scholar] [CrossRef]
- Courbiere, B.; Auffan, M.; Rollais, R.; Tassistro, V.; Bonnefoy, A.; Botta, A.; Rose, J.; Orsière, T.; Perrin, J. Ultrastructural interactions and genotoxicity assay of cerium dioxide nanoparticles on mouse oocytes. Int. J. Mol. Sci. 2013, 14, 21613–21628. [Google Scholar] [CrossRef] [PubMed]
- Preaubert, L.; Courbiere, B.; Achard, V.; Tassistro, V.; Greco, F.; Orsiere, T.; Bottero, J.-Y.; Rose, J.; Auffan, M.; Perrin, J. Cerium dioxide nanoparticles affect in vitro fertilization in mice. Nanotoxicology 2016, 10, 111–117. [Google Scholar] [PubMed]
- Nabeh, M.; Taalab, Y.; El Wahab, D.A.; Asker, S.; Elbedwehy, A.; El Harouny, M. Silver Nanotoxicity on Kidneys and Ovaries of Female Albino Rats. Mansoura J. Forensic Med. Clin. Toxicol. 2020, 28, 340–358. [Google Scholar] [CrossRef]
- Larson, J.K.; Carvan, M., 3rd; Teeguarden, J.G.; Watanabe, G.; Taya, K.; Krystofiak, E.; Hutz, R.J. Low-dose gold nanoparticles exert subtle endocrine-modulating effects on the ovarian steroidogenic pathway ex vivo independent of oxidative stress. Nanotoxicology 2014, 8, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Katarzyńska-Banasik, D.; Grzesiak, M.; Kowalik, K.; Sechman, A. Administration of silver nanoparticles affects ovarian steroidogenesis and may influence thyroid hormone metabolism in hens (Gallus domesticus). Ecotoxicol. Environ. Saf. 2021, 208, 111427. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Yang, B.; Jiang, X.; Ma, X.; Lu, C.; Chen, C. Multiwalled carbon nanotubes inhibit steroidogenesis by disrupting steroidogenic acute regulatory protein expression and redox status. J. Nanosci. Nanotechnol. 2017, 17, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Holmannova, D.; Borsky, P.; Svadlakova, T.; Borska, L.; Fiala, Z. Reproductive and Developmental Nanotoxicity of Carbon Nanoparticles. Nanomaterials 2022, 12, 1716. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M.; Yoshida, T.; Ogura, T.; Nabeshi, H.; Nagano, K.; et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 2011, 6, 321–328. [Google Scholar] [CrossRef]
- Derksen, R.H.; Khamashta, M.A.; Branch, D.W. Management of the obstetric antiphospholipid syndrome. Arthritis Rheum. 2004, 50, 1028–1039. [Google Scholar] [CrossRef]
- Hillegass, J.M.; Shukla, A.; Lathrop, S.A.; MacPherson, M.B.; Fukagawa, N.K.; Mossman, B.T. Assessing nanotoxicity in cells in vitro. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 219–231. [Google Scholar] [CrossRef]
- Moussa, Z.; Judeh, Z.M.; Ahmed, S.A. Nonenzymatic exogenous and endogenous antioxidants. Free. Radic. Med. Biol. 2019, 1, 11–22. [Google Scholar]
- Puntmann, V.O. How-to guide on biomarkers: Biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad. Med. J. 2009, 85, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Assady, M.; Farahnak, A.; Golestani, A.; Esharghian, M. Superoxide Dismutase (SOD) Enzyme Activity Assay in Fasciola sp Parasites and Liver Tissue Extract. Iran. J. Parasitol. 2011, 6, 17–22. [Google Scholar]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Hadwan, M.H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef]
- Fanucchi, M.V. Development of antioxidant and xenobiotic metabolizing enzyme systems. In The Lung; Academic Press: Cambridge, MA, USA, 2004; pp. 177–185. [Google Scholar]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The impact of oxidative stress in male infertility. Front. Mol. Biosci. 2022, 8, 1344. [Google Scholar] [CrossRef]
- Il’yasova, D.; Scarbrough, P.; Spasojevic, I. Urinary biomarkers of oxidative status. Clin. Chim. Acta. 2012, 413, 1446–1453. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Samrot, A.V.; Shobana, N.; Jenna, R. Antibacterial and Antioxidant Activity of Different Staged Ripened Fruit of Capsicum annuum and Its Green Synthesized Silver Nanoparticles. Bionanoscience 2018, 8, 632–646. [Google Scholar] [CrossRef]
- Purayil, S.K.; Annley, C.; Ponnaiah, P.; Pattammadath, S.; Javad, P.T.M.; Selvarani, J.; Raji, P.; Thirumurugan, R.; Iyappan, P.; Samrot, A.V. Evaluation of Antioxidant and Antimicrobial Activity of Some Plants Collected from Malaysia. J. Pure Appl. Microbiol. 2019, 13, 2363–2374. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sean, T.C. Investigating the Antioxidant and Antimicrobial Activity of Artocarpus heterophyllus Lam. (Jackfruit) Latex. Biointerface Res. Appl. Chem. 2022, 12, 3019–3033. [Google Scholar]
- Selvarani, A.J.; Nishanthini, P.; Raji, P.; Samanvitha, K.S.; Paulraj, P.; Iyappan, P.; Chandramohan, M.; Samrot, A.V. Antioxidant and Quorum Quenching Activity against Pseudomonas aeruginosa SU-18 of some Edible Fruit Juices. J. Pure Appl. Microbiol. 2019, 13, 1863–1876. [Google Scholar]
- Ramana, K.V.; Reddy, A.; Majeti, N.V.; Singhal, S.S. Therapeutic potential of natural antioxidants. Oxidative Med. Cell. Longev. 2018, 2018, 9471051. [Google Scholar] [CrossRef]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Zhang, Y.; et al. Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 299–308. [Google Scholar] [CrossRef]
- Linnewiel, K.; Ernst, H.; Caris-Veyrat, C.; Ben-Dor, A.; Kampf, A.; Salman, H.; Danilenko, M.; Levy, J.; Sharoni, Y. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic. Biol Med. 2009, 47, 659–667. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Esmaeili, S.-A.; Abdollahi, E.; Sahebkar, A. A review on the pharmacology and toxicology of steviol glycosides extracted from stevia rebaudiana. Curr. Pharm. Des. 2017, 23, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Sonane, M.; Moin, N.; Satish, A. The role of antioxidants in attenuation of Caenorhabditis elegans lethality on exposure to TiO2 and ZnO nanoparticles. Chemosphere 2017, 187, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, R.; Qiao, Y.; Wang, D. Glycyrrhizic acid, active component from glycyrrhizae radix, prevents toxicity of graphene oxide by influencing functions of microRNAs in nematode Caenorhabditis elegans. Nanomedicine 2016, 12, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Xiaoming, Y.; Xiangmin, G.; Qiuli, W.; Yunli, Z.; Dayong, W. Vitamin E ameliorates neurodegeneration related phenotypes caused by neurotoxicity of Al2O3-nanoparticles in C. elegans. Toxicol. Res. 2015, 4, 1269–1281. [Google Scholar] [CrossRef]
- Scharf, A.; Gührs, K.H.; von Mikecz, A. Anti-amyloid compounds protect from silica nanoparticle-induced neurotoxicity in the nematode C. elegans. Nanotoxicology 2016, 10, 426–435. [Google Scholar] [CrossRef]
- Balkrishna, A.; Kumar, A.; Arya, V.; Rohela, A.; Verma, R.; Nepovimova, E.; Krejcar, O.; Kumar, D.; Thakur, N.; Kuca, K. Phytoantioxidant Functionalized Nanoparticles: A Green Approach to Combat Nanoparticle-Induced Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 3155962. [Google Scholar] [CrossRef]
- Subbaiya, R.; Selvam, M.M. Green synthesis of copper nanoparticles from Hibicus rosasinensis and their antimicrobial, antioxidant activities. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1183–1190. [Google Scholar]
- Ghosh, S.; More, P.; Nitnavare, R.; Jagta, S.; Chippalka, R.; Derl, A.; Kittue, R.; Aso, A.; Kale, S.; Singh, S.; et al. Antidiabetic and antioxidant properties of copper nanoparticles synthesized by medicinal plant Dioscorea bulbifera. J. Nanomed. Nanotechnol. 2015, 6. [Google Scholar] [CrossRef]
- Elemike, E.E.; Fayemi, O.E.; Ekennia, A.C.; Onwudiwe, D.C.; Ebenso, E.E. Silver nanoparticles mediated by Costus afer leaf Extract: Synthesis, Antibacterial, Antioxidant and electrochemical properties. Molecules 2017, 22, 701. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, S.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by _Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef]
- Gao, F.; Xiong, Z. Reactive oxygen species responsive polymers for drug delivery systems. Front. Chem. 2021, 9, 649048. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samrot, A.V.; Noel Richard Prakash, L.X. Nanoparticles Induced Oxidative Damage in Reproductive System and Role of Antioxidants on the Induced Toxicity. Life 2023, 13, 767. https://doi.org/10.3390/life13030767
Samrot AV, Noel Richard Prakash LX. Nanoparticles Induced Oxidative Damage in Reproductive System and Role of Antioxidants on the Induced Toxicity. Life. 2023; 13(3):767. https://doi.org/10.3390/life13030767
Chicago/Turabian StyleSamrot, Antony V., and Lawrence Xavier Noel Richard Prakash. 2023. "Nanoparticles Induced Oxidative Damage in Reproductive System and Role of Antioxidants on the Induced Toxicity" Life 13, no. 3: 767. https://doi.org/10.3390/life13030767
APA StyleSamrot, A. V., & Noel Richard Prakash, L. X. (2023). Nanoparticles Induced Oxidative Damage in Reproductive System and Role of Antioxidants on the Induced Toxicity. Life, 13(3), 767. https://doi.org/10.3390/life13030767






