Target Trial Emulation Using Hospital-Based Observational Data: Demonstration and Application in COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Emulated Trial Specification
2.2. Practical Implementation of Cloning, Censoring, and Weighting
2.3. Statistical Analysis of the Emulated Trial
2.3.1. Competing Risk Framework
2.3.2. Outcome Model: Cause-Specific Hazard Regression Model
2.3.3. Outcome Model: Cumulative Incidence Function
2.3.4. Additional and Naïve Statistical Analyses
3. Results
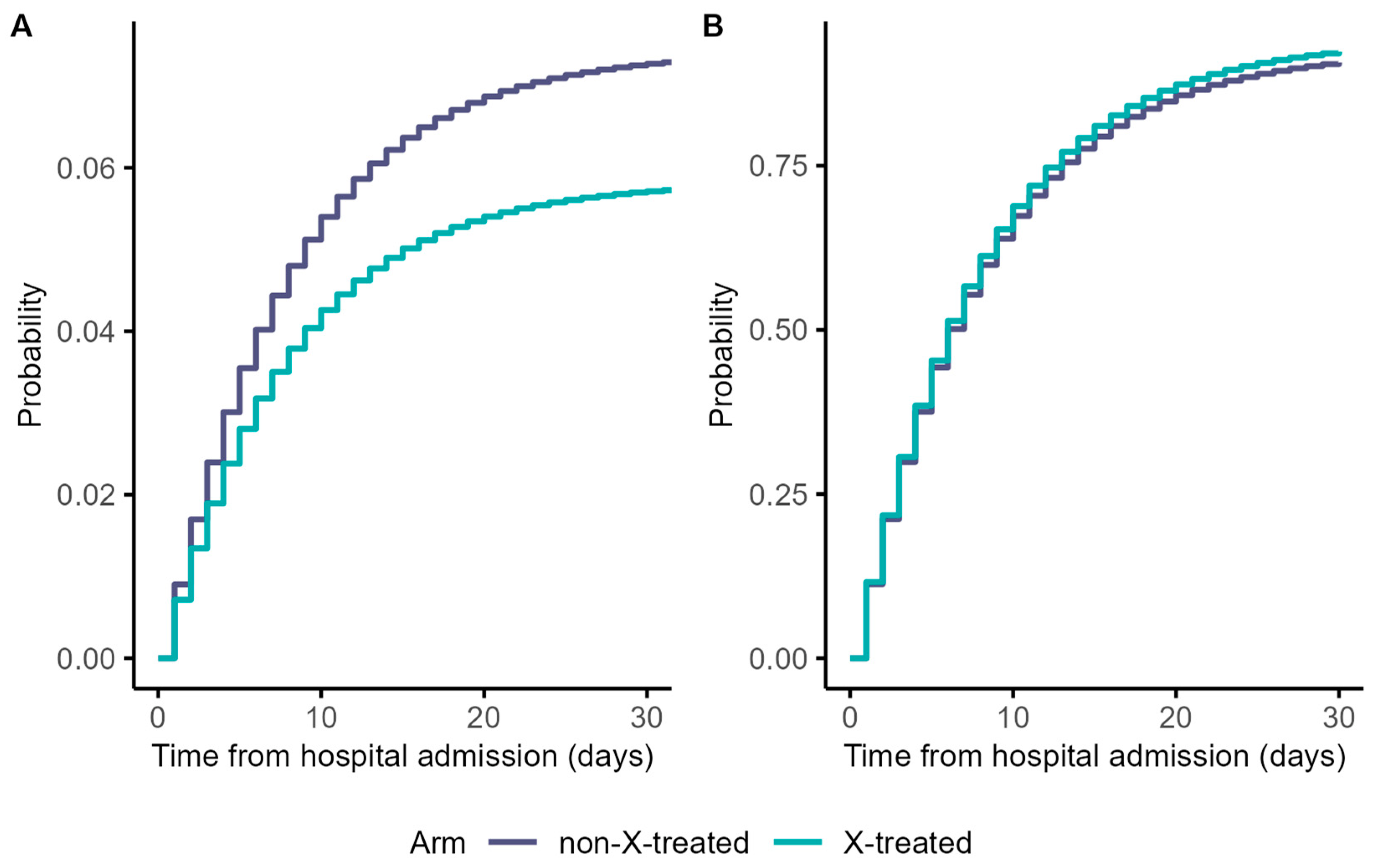
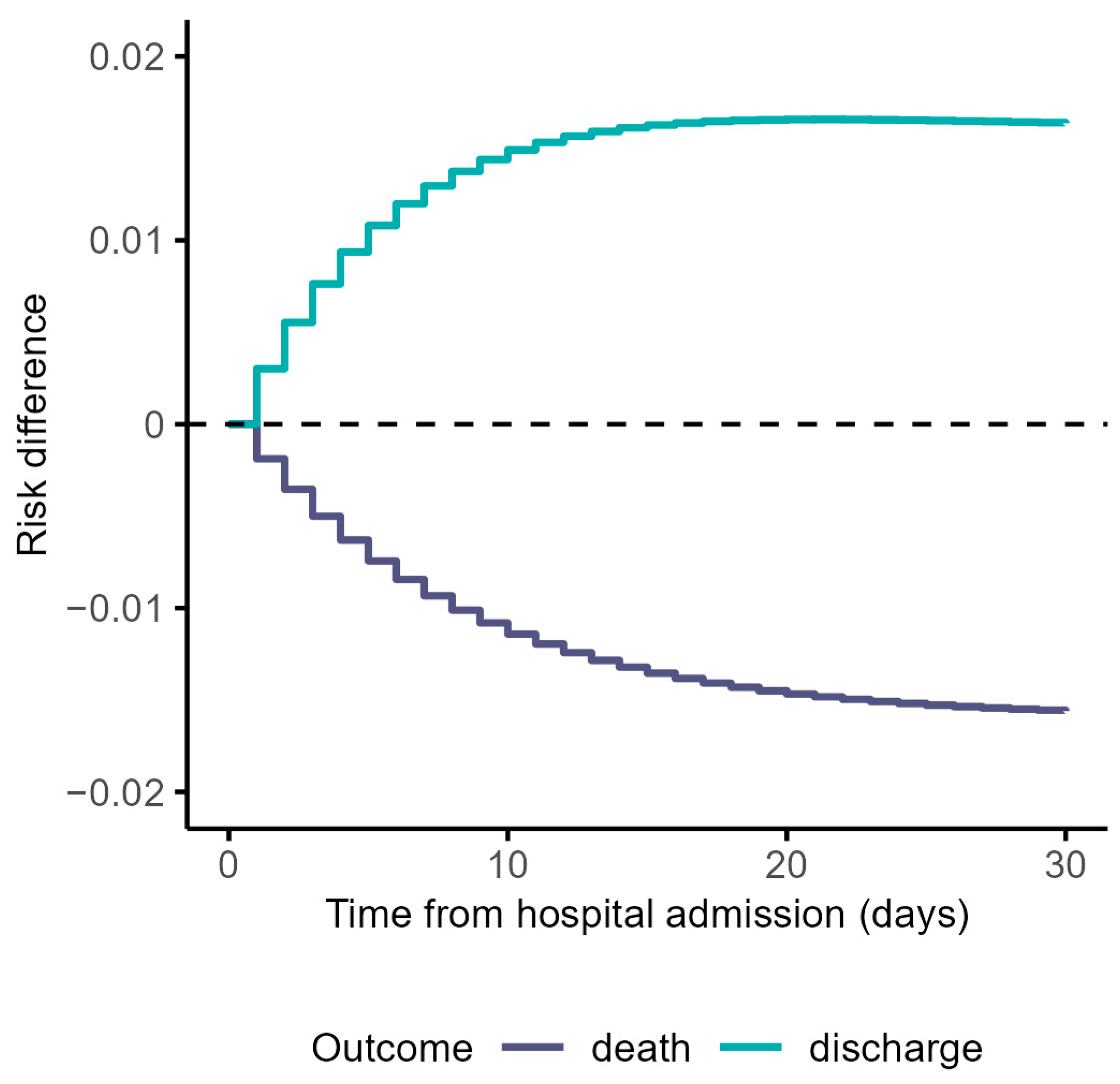
3.1. Cause-Specific Cumulative Hazards, Cumulative Incidence Functions, and Risk Differences Taking the Constant Hazards Approach
3.2. Additional and Naïve Analyses
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albhaisi, S.; Wenzel, R.P. The Value of Medical Registries and Observational Studies Early in Pandemics: The Coronavirus Disease 2019 (COVID-19) Experience. Clin. Infect. Dis. 2022, 74, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Desai, A.; Leyfman, Y.; Kuderer, N.M. Opportunities and Challenges of Observational Studies and Randomized Controlled Trials for Evaluating the Therapeutic Efficacy of COVID-19 Convalescent Plasma. Cancer Investig. 2021, 39, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Martinuka, O.; von Cube, M.; Wolkewitz, M. Methodological evaluation of bias in observational coronavirus disease 2019 studies on drug effectiveness. Clin. Microbiol. Infect. 2021, 27, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Renoux, C.; Azoulay, L.; Suissa, S. Biases in Evaluating the Safety and Effectiveness of Drugs for the Treatment of COVID-19: Designing Real-World Evidence Studies. Am. J. Epidemiol. 2021, 190, 1452–1456. [Google Scholar] [CrossRef]
- Fu, E.L.; van Diepen, M.; Xu, Y.; Trevisan, M.; Dekker, F.W.; Zoccali, C.; Jager, K.; Carrero, J.J. Pharmacoepidemiology for nephrologists (part 2): Potential biases and how to overcome them. Clin. Kidney J. 2021, 14, 1317–1326. [Google Scholar] [CrossRef]
- Cohen, J.B.; D′Agostino McGowan, L.; Jensen, E.T.; Rigdon, J.; South, A.M. Evaluating sources of bias in observational studies of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker use during COVID-19: Beyond confounding. J. Hypertens. 2021, 39, 795–805. [Google Scholar] [CrossRef]
- Suissa, S. Immortal time bias in pharmaco-epidemiology. Am. J. Epidemiol. 2008, 167, 492–499. [Google Scholar] [CrossRef]
- Austin, P.C.; Fine, J.P. Accounting for competing risks in randomized controlled trials: A review and recommendations for improvement. Stat. Med. 2017, 36, 1203–1209. [Google Scholar] [CrossRef]
- Wolkewitz, M.; Schumacher, M. Survival biases lead to flawed conclusions in observational treatment studies of influenza patients. J. Clin. Epidemiol. 2017, 84, 121–129. [Google Scholar] [CrossRef]
- Karim, M.E.; Gustafson, P.; Petkau, J.; Tremlett, H. Comparison of Statistical Approaches for Dealing With Immortal Time Bias in Drug Effectiveness Studies. Am. J. Epidemiol. 2016, 184, 325–335. [Google Scholar] [CrossRef]
- von Cube, M.; Grodd, M.; Wolkewitz, M.; Hazard, D.; Wengenmayer, T.; Canet, E.; Lambert, J. Harmonizing Heterogeneous Endpoints in Coronavirus Disease 2019 Trials Without Loss of Information. Crit. Care Med. 2021, 49, e11–e19. [Google Scholar] [CrossRef]
- van Walraven, C.; McAlister, F.A. Competing risk bias was common in Kaplan-Meier risk estimates published in prominent medical journals. J. Clin. Epidemiol. 2016, 69, 170–173.e8. [Google Scholar] [CrossRef]
- Andersen, P.K.; Geskus, R.B.; de Witte, T.; Putter, H. Competing risks in epidemiology: Possibilities and pitfalls. Int. J. Epidemiol. 2012, 41, 861–870. [Google Scholar] [CrossRef]
- Li, H.; Gleason, K.J.; Hu, Y.; Lovell, S.S.; Mukhopadhyay, S.; Wang, L.; Huang, B. Handling death as an intercurrent event in time to recovery analysis in COVID-19 treatment clinical trials. Contemp. Clin. Trials 2022, 119, 106758. [Google Scholar] [CrossRef]
- McCaw, Z.R.; Claggett, B.L.; Tian, L.; Solomon, S.D.; Berwanger, O.; Pfeffer, M.A.; Wei, L.-J. Practical Recommendations on Quantifying and Interpreting Treatment Effects in the Presence of Terminal Competing Risks: A Review. JAMA Cardiol. 2022, 7, 450–456. [Google Scholar] [CrossRef]
- Coemans, M.; Verbeke, G.; Döhler, B.; Süsal, C.; Naesens, M. Bias by censoring for competing events in survival analysis. BMJ 2022, 378, e071349. [Google Scholar] [CrossRef]
- Hernán, M.A.; Robins, J.M. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef]
- Cho, K.; Keithly, S.C.; Kurgansky, K.E.; Madenci, A.L.; Gerlovin, H.; Marucci-Wellman, H.; Doubleday, A.; Thomas, E.R.; Park, Y.; Ho, Y.-L.; et al. Early Convalescent Plasma Therapy and Mortality Among US Veterans Hospitalized With Nonsevere COVID-19: An Observational Analysis Emulating a Target Trial. J. Infect. Dis. 2021, 224, 967–975. [Google Scholar] [CrossRef]
- Breskin, A.; Wiener, C.; Adimora, A.A.; Brown, R.S.; Landis, C.; Reddy, K.R.; Verna, E.C.; Crawford, J.M.; Mospan, A.; Fried, M.W.; et al. Effectiveness of Remdesivir Treatment Protocols Among Patients Hospitalized with COVID-19: A Target Trial Emulation. Epidemiology 2023. [Google Scholar] [CrossRef]
- Hoffman, K.L.; Schenck, E.J.; Satlin, M.J.; Whalen, W.; Pan, D.; Williams, N.; Díaz, I. Comparison of a Target Trial Emulation Framework vs Cox Regression to Estimate the Association of Corticosteroids With COVID-19 Mortality. JAMA Netw. Open 2022, 5, e2234425. [Google Scholar] [CrossRef]
- Gupta, S.; Wang, W.; Hayek, S.S.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19. JAMA Intern. Med. 2021, 181, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Hayakawa, K.; Uemura, Y.; Shinozaki, T.; Matsunaga, N.; Terada, M.; Suzuki, S.; Asai, Y.; Kitajima, K.; Saito, S.; et al. Effectiveness of remdesivir in hospitalized nonsevere patients with COVID-19 in Japan: A large observational study using the COVID-19 Registry Japan. Int. J. Infect. Dis. 2022, 118, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alés, G.; Domingo-Relloso, A.; Quintana-Díaz, M.; Fernández-Capitán, C.; Hernán, M.A. Thromboprophylaxis with standard-dose vs. flexible-dose heparin for hospitalized COVID-19 patients: A target trial emulation. J. Clin. Epidemiol. 2022, 151, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Bajema, K.L.; Berry, K.; Streja, E.; Rajeevan, N.; Li, Y.; Yan, L.; Cunningham, F.; Hynes, D.M.; Rowneki, M.; Bohnert, A.; et al. Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among U.S. Veterans: Target trial emulation studies with one-month and six-month outcomes. medRxiv 2022. [Google Scholar] [CrossRef]
- Urner, M.; Barnett, A.G.; Bassi, G.L.; Brodie, D.; Dalton, H.J.; Ferguson, N.D.; Heinsar, S.; Hodgson, C.L.; Peek, G.; Shekar, K.; et al. Venovenous extracorporeal membrane oxygenation in patients with acute covid-19 associated respiratory failure: Comparative effectiveness study. BMJ 2022, 377, e068723. [Google Scholar] [CrossRef]
- Hajage, D.; Combes, A.; Guervilly, C.; Lebreton, G.; Mercat, A.; Pavot, A.; Nseir, S.; Mekontso-Dessap, A.; Mongardon, N.; Mira, J.P.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome Associated with COVID-19: An Emulated Target Trial Analysis. Am. J. Respir. Crit. Care Med. 2022, 206, 281–294. [Google Scholar] [CrossRef]
- Dickerman, B.A.; Gerlovin, H.; Madenci, A.L.; Kurgansky, K.E.; Ferolito, B.R.; Figueroa Muñiz, M.J.; Gagnon, D.R.; Gaziano, J.M.; Cho, K.; Casas, J.P.; et al. Comparative Effectiveness of BNT162b2 and mRNA-1273 Vaccines in U.S. Veterans. N. Engl. J. Med. 2022, 386, 105–115. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Locke, E.R.; Green, P.K.; Berry, K. Comparison of Moderna versus Pfizer-BioNTech COVID-19 vaccine outcomes: A target trial emulation study in the U.S. Veterans Affairs healthcare system. EClin.Med. 2022, 45, 101326. [Google Scholar] [CrossRef]
- Ben-Michael, E.; Feller, A.; Stuart, E.A. A Trial Emulation Approach for Policy Evaluations with Group-level Longitudinal Data. Epidemiology 2021, 32, 533–540. [Google Scholar] [CrossRef]
- Wang, J.; Peduzzi, P.; Wininger, M.; Ma, S. Statistical Methods for Accommodating Immortal Time: A Selective Review and Comparison. arXiv 2022, arXiv:2202.02369. [Google Scholar]
- Maringe, C.; Benitez Majano, S.; Exarchakou, A.; Smith, M.; Rachet, B.; Belot, A.; Leyrat, C. Reflection on modern methods: Trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data. Int. J. Epidemiol. 2020, 49, 1719–1729. [Google Scholar] [CrossRef]
- Sami, R.; Soltaninejad, F.; Amra, B.; Naderi, Z.; Haghjooy Javanmard, S.; Iraj, B.; Haji Ahmadi, S.; Shayganfar, A.; Dehghan, M.; Khademi, N.; et al. A one-year hospital-based prospective COVID-19 open-cohort in the Eastern Mediterranean region: The Khorshid COVID Cohort (KCC) study. PLoS ONE 2020, 15, e0241537. [Google Scholar] [CrossRef]
- Jernigan, D.B. Update: Public Health Response to the Coronavirus Disease 2019 Outbreak—United States, February 24, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 216–219. [Google Scholar] [CrossRef]
- Moura, L.M.; Westover, M.B.; Kwasnik, D.; Cole, A.J.; Hsu, J. Causal inference as an emerging statistical approach in neurology: An example for epilepsy in the elderly. Clin. Epidemiol. 2017, 9, 9–18. [Google Scholar] [CrossRef]
- Hernán, M.A.; Robins, J.M. Per-Protocol Analyses of Pragmatic Trials. N. Engl. J. Med. 2017, 377, 1391–1398. [Google Scholar] [CrossRef]
- Hernán, M.A.; Wang, W.; Leaf, D.E. Target Trial Emulation: A Framework for Causal Inference From Observational Data. JAMA 2022, 328, 2446–2447. [Google Scholar] [CrossRef]
- Hernán, M.A. How to estimate the effect of treatment duration on survival outcomes using observational data. BMJ 2018, 360, k182. [Google Scholar] [CrossRef]
- Willems, S.; Schat, A.; van Noorden, M.S.; Fiocco, M. Correcting for dependent censoring in routine outcome monitoring data by applying the inverse probability censoring weighted estimator. Stat. Methods Med. Res. 2018, 27, 323–335. [Google Scholar] [CrossRef]
- Austin, P.C.; Lee, D.S.; Fine, J.P. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016, 133, 601–609. [Google Scholar] [CrossRef]
- Wolkewitz, M.; von Cube, M.; Schumacher, M. Multistate Modeling to Analyze Nosocomial Infection Data: An Introduction and Demonstration. Infect. Control. Hosp. Epidemiol. 2017, 38, 953–959. [Google Scholar] [CrossRef]
- Xue, X.; Saeed, O.; Castagna, F.; Jorde, U.P.; Agalliu, I. The analysis of COVID-19 in-hospital mortality: A competing risk approach or a cure model? Stat. Methods Med. Res. 2022, 31, 1976–1991. [Google Scholar] [CrossRef] [PubMed]
- von Cube, M.; Schumacher, M.; Wolkewitz, M. Basic parametric analysis for a multi-state model in hospital epidemiology. BMC Med. Res. Methodol. 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Noordzij, M.; Leffondré, K.; van Stralen, K.J.; Zoccali, C.; Dekker, F.W.; Jager, K.J. When do we need competing risks methods for survival analysis in nephrology? Nephrol. Dial. Transpl. 2013, 28, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, J.; Allignol, A.; Schumacher, M. Competing Risks and Multistate Models with R; Springer: New York, NY, USA, 2012; ISBN 978-1-4614-2034-7. [Google Scholar]
- Wolkewitz, M.; Lambert, J.; von Cube, M.; Bugiera, L.; Grodd, M.; Hazard, D.; White, N.; Barnett, A.; Kaier, K. Statistical Analysis of Clinical COVID-19 Data: A Concise Overview of Lessons Learned, Common Errors and How to Avoid Them. Clin. Epidemiol. 2020, 12, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Ramzi, Z.S. Hospital readmissions and post-discharge all-cause mortality in COVID-19 recovered patients; A systematic review and meta-analysis. Am. J. Emerg. Med. 2022, 51, 267–279. [Google Scholar] [CrossRef]
- Latouche, A.; Allignol, A.; Beyersmann, J.; Labopin, M.; Fine, J.P. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J. Clin. Epidemiol. 2013, 66, 648–653. [Google Scholar] [CrossRef]
- Lin, H.-M.; Liu, S.T.H.; Levin, M.A.; Williamson, J.; Bouvier, N.M.; Aberg, J.A.; Reich, D.; Egorova, N. Informative Censoring-A Cause of Bias in Estimating COVID-19 Mortality Using Hospital Data. Life 2023, 13, 210. [Google Scholar] [CrossRef]
- Schuster, N.A.; Hoogendijk, E.O.; Kok, A.A.L.; Twisk, J.W.R.; Heymans, M.W. Ignoring competing events in the analysis of survival data may lead to biased results: A nonmathematical illustration of competing risk analysis. J. Clin. Epidemiol. 2020, 122, 42–48. [Google Scholar] [CrossRef]
- Bolch, C.A.; Chu, H.; Jarosek, S.; Cole, S.R.; Elliott, S.; Virnig, B. Inverse probability of treatment-weighted competing risks analysis: An application on long-term risk of urinary adverse events after prostate cancer treatments. BMC Med. Res. Methodol. 2017, 17, 93. [Google Scholar] [CrossRef]
- Young, J.G.; Stensrud, M.J.; Tchetgen Tchetgen, E.J.; Hernán, M.A. A causal framework for classical statistical estimands in failure-time settings with competing events. Stat. Med. 2020, 39, 1199–1236. [Google Scholar] [CrossRef]
- Petito, L.C.; García-Albéniz, X.; Logan, R.W.; Howlader, N.; Mariotto, A.B.; Dahabreh, I.J.; Hernán, M.A. Estimates of Overall Survival in Patients With Cancer Receiving Different Treatment Regimens: Emulating Hypothetical Target Trials in the Surveillance, Epidemiology, and End Results (SEER)-Medicare Linked Database. JAMA Netw. Open 2020, 3, e200452. [Google Scholar] [CrossRef]
- Hernán, M.; Robins, J.M. Causal Inference: What If; Chapman & Hall/CRC: Boca Raton, FL, USA, 2021; ISBN 1420076167. [Google Scholar]
- Pazzagli, L.; Linder, M.; Zhang, M.; Vago, E.; Stang, P.; Myers, D.; Andersen, M.; Bahmanyar, S. Methods for time-varying exposure related problems in pharmacoepidemiology: An overview. Pharmacoepidemiol. Drug Saf. 2018, 27, 148–160. [Google Scholar] [CrossRef]
- Stuart, E.A.; Lee, B.K.; Leacy, F.P. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J. Clin. Epidemiol. 2013, 66, S84–S90.e1. [Google Scholar] [CrossRef]

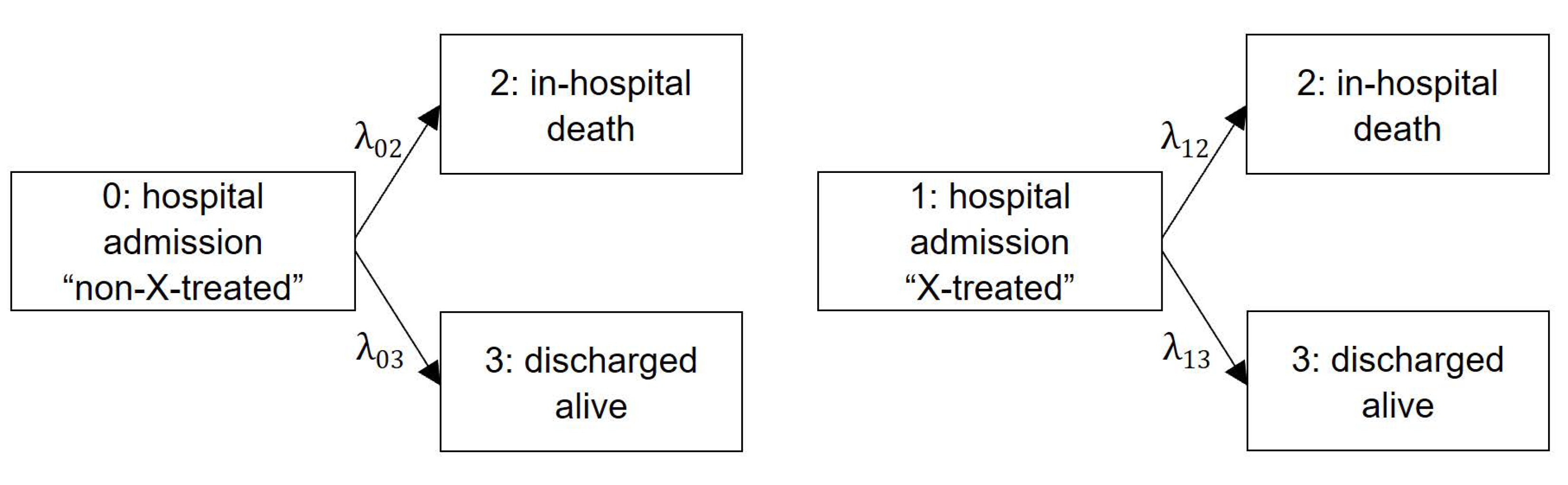
| Protocol Component | Description of Emulation |
|---|---|
| Research questions |
|
| Outcomes | In-hospital death and discharge alive (competing event) |
| Eligibility criteria |
|
| Exclusions | Any contraindication to ‘X’ antiviral treatment (e.g., liver dysfunction, kidney injury, cardiac arrhythmias, including QT prolongation) at hospital admission that made the patient unsuitable for receiving ‘X’ treatment |
| Treatment strategies |
|
| Treatment assignment | Non-randomized ‘X’ treatment assignment |
| Follow-up time | Begins with hospital admission, and treatment initiation must occur within the first two days after hospitalization and end at 60 days or in-hospital death or discharged alive |
| Grace period | First two days after hospital admission |
| Estimand | Difference in the risk for in-hospital death and discharge alive |
| Analysis plan |
|
| Adjustment variables |
|
| Corresponding Measure a | Mathematical Formulation |
|---|---|
| Constant hazards | |
| Death hazard w/o treatment | λ02 |
| Discharge hazard w/o treatment | λ03 |
| Hazard w/o treatment | |
| Death hazard with treatment | λ12 |
| Discharge hazard with treatment | λ13 |
| Hazard with treatment | |
| Mortality | |
| Mortality risk w/o treatment at the end of follow-up | |
| Mortality risk with treatment at the end of follow-up | |
| Mortality risk ratio at the end of follow-up | |
| Difference in mortality at the end of follow-up | |
| Hazards and cumulative incidence functions | |
| Hazard ratio of death (treatment vs. w/o treatment) at the end of follow-up | |
| Hazard ratio of discharge (treatment vs. w/o treatment) at the end of follow-up | |
| Cumulative risk of death w/o treatment at time t | |
| Cumulative risk of discharge w/o treatment at time t | |
| Cumulative risk of death with treatment at time t | |
| Cumulative risk of discharge with treatment at time t | |
| Risk differences and ratios | |
| Risk difference functions for death at time t | |
| Risk difference functions for discharge at time t | |
| Risk ratios for death at time t | |
| Risk ratios for discharge at time t | |
| Length of stay | |
| Length of stay w/o treatment | |
| Length of stay with treatment | |
| Difference in length of stay |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinuka, O.; Cube, M.v.; Hazard, D.; Marateb, H.R.; Mansourian, M.; Sami, R.; Hajian, M.R.; Ebrahimi, S.; Wolkewitz, M. Target Trial Emulation Using Hospital-Based Observational Data: Demonstration and Application in COVID-19. Life 2023, 13, 777. https://doi.org/10.3390/life13030777
Martinuka O, Cube Mv, Hazard D, Marateb HR, Mansourian M, Sami R, Hajian MR, Ebrahimi S, Wolkewitz M. Target Trial Emulation Using Hospital-Based Observational Data: Demonstration and Application in COVID-19. Life. 2023; 13(3):777. https://doi.org/10.3390/life13030777
Chicago/Turabian StyleMartinuka, Oksana, Maja von Cube, Derek Hazard, Hamid Reza Marateb, Marjan Mansourian, Ramin Sami, Mohammad Reza Hajian, Sara Ebrahimi, and Martin Wolkewitz. 2023. "Target Trial Emulation Using Hospital-Based Observational Data: Demonstration and Application in COVID-19" Life 13, no. 3: 777. https://doi.org/10.3390/life13030777
APA StyleMartinuka, O., Cube, M. v., Hazard, D., Marateb, H. R., Mansourian, M., Sami, R., Hajian, M. R., Ebrahimi, S., & Wolkewitz, M. (2023). Target Trial Emulation Using Hospital-Based Observational Data: Demonstration and Application in COVID-19. Life, 13(3), 777. https://doi.org/10.3390/life13030777








