The Connection between Immunocompetence and Reproduction in Wildlife
Abstract
:1. Introduction
2. The Concept of Immunocompetence and Reproduction in Animals
3. The Interplay of Physiology, Immunology, and Reproduction in Wildlife
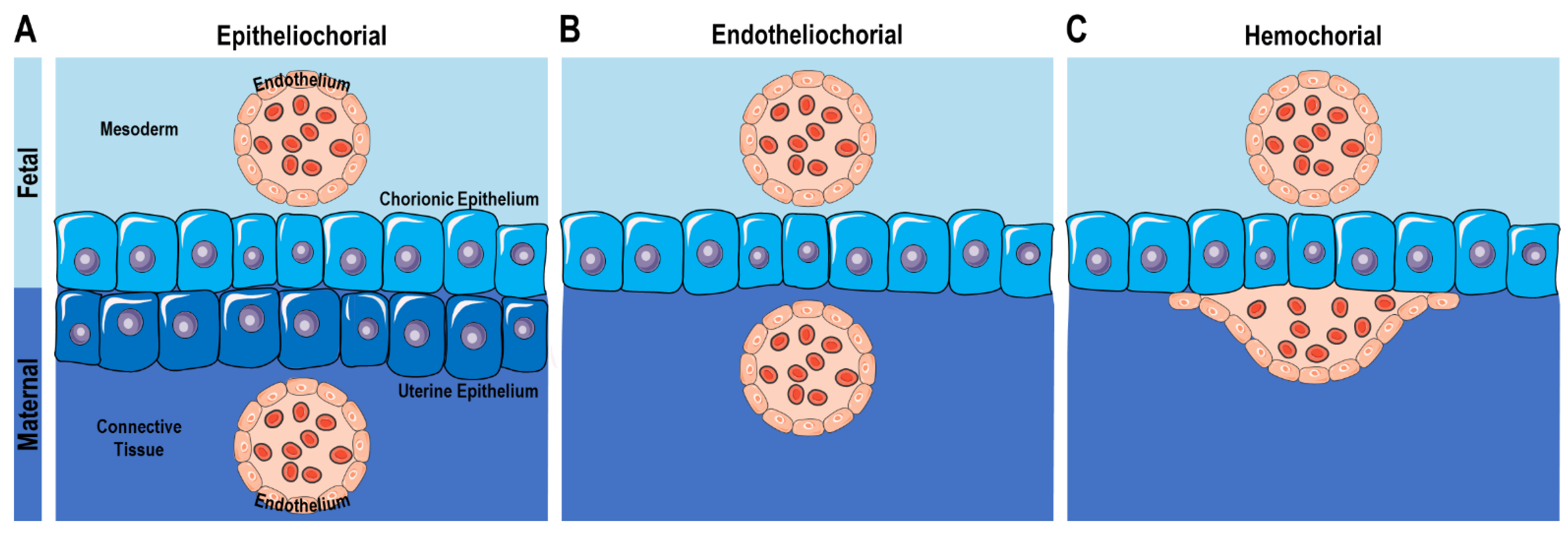
4. The Maternal–Fetal Interface in Live-Bearing Vertebrates
5. The Interaction of Reproductive Microbiome and Immunocompetence
6. The Detection of Molecular Markers of Immunocompetence during Gravidity
7. Infections and Parasite Infestations during Gravidity
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viney, M.E.; Riley, E.M.; Buchanan, K.L. Optimal immune responses: Immunocompetence revisited. Trends Ecol. Evol. 2005, 20, 665–669. [Google Scholar] [CrossRef]
- Lochmiller, R.L. Immunocompetence and animal population regulation. Oikos 1996, 76, 594–602. [Google Scholar] [CrossRef]
- Jeffery, C.A.; Forsberg, N.E. Influence of stress and nutrition on cattle immunity. Food Anim. Pract. 2007, 23, 105–149. [Google Scholar]
- McGlone, J.J.; Salak, J.L.; Lumpkin, E.A.; Nicholson, R.I.; Gibson, M.; Norman, R.L. Shipping stress and social status effects on pig performance, plasma cortisol, natural killer cell activity, and leukocyte numbers. J. Anim. Sci. 1993, 71, 888–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiner, G.; Willems, H.; Pesch, S.; Ohlinger, V.F. Varitation in resistance to the Porcine Reproductive and Respiratory Syndrome virus (PRRSV) in Pietrain and Miniature pigs. J. Anim. Breed. Genet. 2010, 127, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ballweg, I.C.; Frölich, K.; Fandrey, E.; Kliem, H.; Pfaffl, M.W. Comparison of the immune competence of Turopolje, German Landrace × Turopolje, and German Landrace × Pietrain pigs after PRRSV vaccination. Vet. Immunol. Immunopathol. 2016, 174, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.S.; Kumar, S.; Majumdar, S.; Sivaraman, G.K.; Shivakumar, B.M. Studies on immunocompetence status in two turkey varieties in India. Br. Poult. Sci. 2004, 45, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Hine, B.C.; Mallard, B.A.; Ingham, A.B.; Colditz, I.G. Immune competence in livestock. In Breeding Focus 2014—Resilience, 1st ed.; Hermesch, S., Dominik, S., Eds.; Animal Genetics and Breeding Unit, University of New England: Armidale, NSW, Australia; New England, ME, USA, 2014; pp. 49–196. [Google Scholar]
- Jolingi, P.; Mok, K.S.; de Vries Reilingh, G.; Wever, P.J.M.; Cornells, R.S.; Oskam, J.P.H.; Henken, A.M. An evaluation of immune competence in different swine breeds. Vet. Q. 1993, 15, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A review of antimicrobial resistance in poultry farming within low-resource settings. Animals 2020, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.B.; Babayan, S.A. Wild immunology. Mol. Ecol. 2011, 20, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Stope, M. Wild life raccoons in Germany as a reservoir for zoonotic agents. Eur. J. Wildl. Res. 2019, 65, 94. [Google Scholar] [CrossRef]
- Engels, M.; Gelderblom, H. Goat herpesviruses: Biological and physicochemical properties. J. Gen. Virol. 1983, 64, 2237–2247. [Google Scholar] [CrossRef]
- Owens, I.P.; Rowe, C.; Thomas, A.L. Sexual selection, speciation and imprinting: Separating the sheep from the goats. Trends Ecol. Evol. 1999, 14, 131–132. [Google Scholar] [CrossRef]
- Renfree, M.; Shaw, G. Reproduction in Monotremes and Marsupials. eLS 2001. [Google Scholar] [CrossRef]
- Freyer, C.; Renfree, M.B. The mammalian yolk sac placenta. J. Exp. Zool. Mol. Dev. Evol. 2009, 312, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Renfree, M.B. Review: Marsupials: Placental mammals with a difference. Placenta 2010, 31, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Mika, K.; Whittington, C.M.; McAllan, B.M.; Lynch, V.J. Gene expression phylogenies and ancestral transcriptome reconstruction resolves major transitions in the origins of pregnancy. eLife 2022, 11, e74297. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D. Endocrine and nutritional regulation of prenatal growth. Acta Paediatr. 1997, 86, 153–157. [Google Scholar] [CrossRef]
- Vernon, R.G.; Clegg, R.A.; Flint, D.J. Metabolism of sheep adipose tissue during pregnancy and lactation. Adapt. Regul. Biochem. J. 1981, 200, 307–314. [Google Scholar]
- Rogowitz, G.L.; McCLure, P.A. Energy export and offspring growth during lactation in Cotton Rats (Sigmodon hispidus). Funct. Ecol. 1995, 9, 143–150. [Google Scholar] [CrossRef]
- Rauw, W.M. Immune response from a resource allocation perspective. Front. Genet. 2012, 3, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keymer, A.E.; Read, A.F. Behavioural ecology: The impact of parasitism. In Parasite-Host Interaction: Coexistence or Conflict? 1st ed.; Oxford University Press: Oxford, UK, 1991; pp. 37–61. [Google Scholar]
- Sheldon, B.C.; Verhulst, S. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996, 11, 317–321. [Google Scholar] [CrossRef]
- Roberts, R.M.; Green, J.A.; Schulz, L.C. The evolution of the placenta. Reproduction 2016, 152, R179–R189. [Google Scholar] [CrossRef] [Green Version]
- Vogel, P. The current molecular phylogeny of Eutherian mammals challenges previous interpretations of placental evolution. Placenta 2005, 26, 591–596. [Google Scholar] [CrossRef]
- Murphy, W.J.; Eizirik, E.; O’Brien, S.J.; Madsen, O.; Scally, M.; Douady, C.J.; Teeling, E.; Ryder, O.A.; Stanhope, M.J.; de Jong, W.W.; et al. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science 2001, 294, 2348–2351. [Google Scholar] [CrossRef]
- Pravieux, J.J.; Poulet, H.; Charreyre, C.; Juillard, V. Protection of newborn animals through maternal immunization. J. Comp. Pathol. 2007, 137 (Suppl. 1), S32–S34. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, M.H.; Friess, A.E.; Hartmann, S.H. Ultrastructural evidence of transplacental transport of immunoglobulin G in bitches. J. Reprod. Fertil. 2000, 118, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Kwan, L.; Fris, M.; Rodd, F.H.; Rowe, L.; Tuhela, L.; Panhuis, T.M. An examination of the variation in maternal placentae across the genus Poeciliopsis (Poeciliidae). J. Morphol. 2015, 276, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Hamlett, W.C.; Eulitt, A.M.; Jarrell, R.L.; Kelly, M.A. Uterogestation and placentation in elasmobranchs. J. Exp. Zool. 1993, 266, 347–367. [Google Scholar] [CrossRef]
- Blackburn, D.G. Evolution of vertebrate viviparity and specializations for fetal nutrition: A quantitative and qualitative analysis. J. Morphol. 2015, 276, 961–990. [Google Scholar] [CrossRef]
- Blackburn, D.G.; Flemming, A.F. Invasive implantation and intimate placental associations in a placentotrophic African lizard, Trachylepis ivensi (scincidae). J. Morphol. 2012, 273, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Comizzoli, P.; Power, M.L.; Bornbusch, S.L.; Muletz-Wolz, C.R. Interactions between reproductive biology and microbiomes in wild animal species. Anim. Microbiome. 2021, 3, 87. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; de Agüero, M.G.; Ganal-Vonarburg, S.C. How nutrition and the maternal microbiota shape the neonatal immune system. Nat. Rev. Immunol. 2017, 17, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Goltsman, D.S.A.; Sun, C.L.; Proctor, D.M.; DiGiulio, D.B.; Robaczewska, A.; Thomas, B.C.; Shaw, G.M.; Stevenson, D.K.; Holmes, S.P.; Banfield, J.F.; et al. Metagenomic analysis with strain-level resolution reveals fine-scale variation in the human pregnancy microbiome. Genome. Res. 2018, 28, 1467–1480. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.G.; Ericsson, A.C.; Poock, S.E.; Melendez, P.; Lucy, M.C. Hot topic: 16S rRNA gene sequencing reveals the microbiome of the virgin and pregnant bovine uterus. J. Dairy Sci. 2017, 100, 4953–4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyman, C.C.; Holyoak, G.R.; Meinkoth, K.; Wieneke, X.; Chillemi, K.A.; DeSilva, U. Canine endometrial and vaginal microbiomes reveal distinct and complex ecosystems. PLoS ONE 2019, 14, e0210157. [Google Scholar] [CrossRef] [PubMed]
- Sluss, P.M.; Reichert, L.E. Presence of bacteria in porcine follicular fluid and their ability to generate an inhibitor of follicle-stimulating hormone binding to receptor. Biol. Reprod. 1983, 29, 335–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Cheng, G.; Li, C.; Yang, J.; Li, J.; Chen, D.; Zou, W.; Jin, S.; Zhang, H.; Li, D.; et al. The normal vaginal and uterine bacterial microbiome in giant pandas (Ailuropoda melanoleuca). Microbiol. Res. 2017, 199, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Zhai, Y.; Feng, L.; Bai, K.; Zhang, Z.; Huang, Y.; Li, T.; Li, D.; Li, H.; et al. Analysis of the vaginal microbiome of giant pandas using metagenomics sequencing. Microbiologyopen 2020, 9, e1131. [Google Scholar] [CrossRef]
- Uchihashi, M.; Bergin, I.L.; Bassis, C.M.; Hashway, S.A.; Chai, D.; Bell, J.D. Influence of age, reproductive cycling status, and menstruation on the vaginal microbiome in baboons (Papio anubis). Am. J. Primatol. 2015, 77, 563–578. [Google Scholar] [CrossRef] [Green Version]
- Yildrim, S.; Yeoman, C.J.; Jange, S.H.; Thomas, S.M.; Ho, M.; Leigh, S.R.; White, B.A.; Wilson, B.A.; Stumpf, R.M. Primate vaginal microbiomes exhibit species specifity without universal Lactobacillus dominance. ISME J. 2014, 8, 2431–2444. [Google Scholar] [CrossRef] [PubMed]
- Comizzoli, P.; Power, M. Reproductive Microbiomes in Wild Animal Species: A New Dimension in Conservation Biology. Adv. Exp. Med. Biol. 2019, 1200, 225–240. [Google Scholar] [PubMed]
- Hopkins, J. Molecular immunology-gene regulation and signal transduction. Vet. Immunol. Immunopathol. 2002, 87, 245–249. [Google Scholar] [CrossRef]
- Jackson, J.A.; Hall, A.J.; Friberg, I.M.; Ralli, C.; Lowe, A.; Zawadzka, M.; Turner, A.K.; Stewart, A.; Birtles, R.J.; Paterson, S.; et al. An immunological marker of tolerance to infection in wild rodents. PLoS Biol. 2014, 12, e1001901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, P.A.; Alvarez, C.A.; Saenz-Martinez, D.E.; Salinas-Parra, N.; Guzman, F.; Paradela, A.; Mercado, L. New insight to the rol of α-enolase (Eno-1) as immunological marker in rainbow trout fry. Dev. Comp. Immunol. 2021, 123, 104163. [Google Scholar] [CrossRef] [PubMed]
- Clerc, M.; Babayan, S.A.; Fenton, A.; Pedersen, A.B. Age affects antibody levels and anthelmintic treatment efficacy in a wild rodent. Int. J. Parasitol. Parasites Wildl. 2019, 8, 240–247. [Google Scholar] [CrossRef]
- Mutinati, M.; Piccinno, M.; Roncetti, M.; Campanile, D.; Rizzo, A.; Sciorsci, R. Oxidative stress during pregnancy in the sheep. Reprod. Domest. Anim. 2013, 48, 353–357. [Google Scholar] [CrossRef]
- Rizzo, A.; Roscino, M.T.; Binetti, F.; Sciorsci, R.L. Roles of reactive oxygen species in female reproduction. Reprod. Domest. Anim. 2012, 47, 344–352. [Google Scholar] [CrossRef]
- Vannucchi, C.I.; Jordao, A.A.; Vannucchi, H. Antioxidant compounds and oxidative stress in female dogs during pregnancy. Res. Vet. Sci. 2007, 83, 188–193. [Google Scholar] [CrossRef]
- Trillmich, F.; Guenther, A.; Jäckel, M.; Czirjak, G.A. Reproduction affects immune defenses in the guinea pig even under ad libitum food. PLoS ONE 2020, 15, e0230081. [Google Scholar] [CrossRef] [Green Version]
- Drazen, D.L.; Trasy, A.; Nelson, R.J. Photoperiod differentially affects energetics of immunity in pregnant and lactating Siberian hamsters (Phodopus sungorus). Can. J. Zool. 2003, 81, 1406–1413. [Google Scholar] [CrossRef] [Green Version]
- Deerenberg, C.; Arpanius, V.; Daan, S.; Bos, N. Reproductive effort decreases antibody responsivness. Proc. R. Soc. Lond. B 1997, 264, 1021–1029. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, A.; Roscino, M.T.; Minoia, G.; Trisolini, C.; Spedicato, M.; Mutinati, M.; Pantaleo, M.; Jirillo, F.; Sciorsci, R.L. Serum levels of reactive oxygen species (ROS) in the bitch. Immunopharmacol. Immunotoxicol. 2009, 31, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Colitti, M.; Stefanon, B.; Gabai, G.; Gelain, M.E.; Bonsembiante, F. Oxidative stress and nutraceuticals in the modulation of the immune function: Current knowledge in animals of veterinary interest. Antioxidants 2019, 8, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, G.; Plaza, A.; Schenffeldt, A.; Alarcon, P.; Gajardo, G.; Uberti, B.; Moran, G.; Henriquez, C. Equine bone marrow-derived mesenchymal stromal cells inhibit reactive oxygen species production by neutrophils. Vet. Immunol. Immunopathol. 2020, 221, 109975. [Google Scholar] [CrossRef]
- Rinaldi, M.; Moroni, P.; Paape, M.J.; Bannerman, D.D. Differential alterations in the ability of bovine neutrophils to generate extracellular and intracellular reactive oxygen species during the periparturient period. Vet. J. 2008, 178, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Waller, K.P. Mammary gland immunology around parturition. Influence of stress, nutrition and genetics. Adv. Exp. Med. Biol. 2000, 480, 231–245. [Google Scholar]
- Dimri, U.; Ranjan, R.; Sharma, M.C.; Varshney, V.P. Effect of vitamin E and selenium supplementation on oxidative stress indices and cortisol level in blood in water buffaloes during pregnancy and early postpartum period. Trop. Anim. Health Prod. 2010, 42, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Richner, H.; Christe, P.; Oppliger, A. Paternal investment affects prevalence of malaria. Proc. Natl. Acad. Sci. USA 1995, 92, 1192–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norris, K.; Anwar, M.; Read, A.F. Reproductive effort influences the prevalence of haematozoan parasites in great tits. J. Anim. Ecol. 1994, 63, 601–610. [Google Scholar] [CrossRef]
- Siikamaki, P.; Rätti, O.; Hovi, M.; Bennett, G.F. Association between haematozoan infections and reproduction in the pied flycatcher. Funct. Ecol. 1997, 11, 176–183. [Google Scholar] [CrossRef]
- Holmstad, P.R.; Hudson, P.J.; Skorping, A. The influence of a parasite community on the dynamics of a host population: A longitudinal study on willow ptarmigan and their parasites. Oikos 2005, 111, 377–391. [Google Scholar] [CrossRef]
- Del Cerro, S.; Merino, S.; La Martinez-de Puente, J.; Lobato, E.; Ruiz-de-Castaneda, R.; Rivero-de Aguilar, J.; Martinez, J.; Morales, J.; Tomas, G.; Moreno, J. Carotenoid-based plumage colouration is associated with blood parasite richness and stress protein levels in blue tits (Cyanistes caeruleus). Oecologia 2010, 162, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Apanius, V. Blood Parasitism, Immunity and Reproduction in the American Kestrel (Falco sparverius). Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 1991. [Google Scholar]
- Knowles, S.C.L.; Nakagawa, S.; Sheldon, B.C. Elevated reproductive effort increases blood parasitaemia and decreases immune function in birds: A meta-regression approach. Funct. Ecol. 2009, 23, 405–415. [Google Scholar] [CrossRef]
- Davidar, P.; Morton, E.S. Are multiple infections more severe for purple martins (Progne subis) than single infections? Auk 2006, 123, 141–147. [Google Scholar] [CrossRef]
- Wagner, R.H.; Davidar, P.; Schug, M.D.; Morton, E.S. Do blood parasites affect paternity, provisioning and mate-guarding in purple martins? Condor 1997, 99, 520–523. [Google Scholar] [CrossRef]
- Bordes, F.; Morand, S. The impact of multiple infections on wild animal hosts: A review. Infect. Ecol. Epidemiol. 2011, 1, 7346. [Google Scholar] [CrossRef] [Green Version]
- Lello, J.; Boag, B.; Hudson, P.J. The effect of single and concomitant pathogen infections on condition and fecundity of the wild rabbit (Oryctolagus cuniculus). Int. J. Parasitol. 2005, 35, 1509–1515. [Google Scholar] [CrossRef]
- Jolles, A.E.; Ezenwa, V.O.; Etienne, R.S.; Turner, W.C.; Olff, H. Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo. Ecology 2008, 89, 2239–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, D.O.; Messier, F. The effect of bovine tuberculosis and brucellosis on reproduction and survival of wood bison in Wood Buffalo National Park. J. Anim. Ecol. 2005, 74, 543–551. [Google Scholar] [CrossRef]
- Festa-Bianchet, M.J. Individual differences, parasites, and the cost of reproduction for bighorn ewes (Ovis canadensis). J. Anim. Ecol. 1997, 11, 176–183. [Google Scholar] [CrossRef]
- Hayward, A.D.; Pilkington, J.G.; Wilson, K.; McNeilly, T.N.; Watt, K.A. Reproductive effort influences intra-seasonal variation in parasite-specific antibody responses in wild Soay sheep. Funct. Ecol. 2019, 33, 1307–1320. [Google Scholar] [CrossRef]
- Gatmaitan, B.G.; Chason, J.L.; Lerner, A.M. Augmentation of the virulence of murine coxsackie-virus B-3 myocardiopathy by exercise. J. Exp. Med. 1970, 131, 1121–1136. [Google Scholar] [CrossRef] [Green Version]
- Levinson, S.O.; Milzer, A.; Lewin, P. Effect of fatigue, chilling and mechanical trauma on resistance to experimental poliomyelitis. Am. J. Hyg. 1945, 42, 204–213. [Google Scholar]
- Magnanou, E.; Fons, R.; Feliu, C.; Morand, S. Physiological responses of insular wild black rat (Rattus rattus) to natural infection by the digenean trematode Fasciola hepatica. Parasitol. Res. 2006, 99, 97–101. [Google Scholar] [CrossRef]
- Martin, L.B.; Scheuerlein, A.; Wikelski, M. Immune activity elevates energy expenditure of house sparrows: A link between direct and indirect costs? Proc. Biol. Sci. 2003, 270, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khokhlova, I.S.; Krasnov, B.R.; Kam, M.; Burdelova, N.I.; Degen, A.A. Energy cost of ectoparasitism: The flea Xenopsylla ramesis on the desert gerbil Gerbillus dasyurus. J. Zool. 2002, 258, 349–354. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stope, M.B. The Connection between Immunocompetence and Reproduction in Wildlife. Life 2023, 13, 785. https://doi.org/10.3390/life13030785
Stope MB. The Connection between Immunocompetence and Reproduction in Wildlife. Life. 2023; 13(3):785. https://doi.org/10.3390/life13030785
Chicago/Turabian StyleStope, Matthias Bernhard. 2023. "The Connection between Immunocompetence and Reproduction in Wildlife" Life 13, no. 3: 785. https://doi.org/10.3390/life13030785
APA StyleStope, M. B. (2023). The Connection between Immunocompetence and Reproduction in Wildlife. Life, 13(3), 785. https://doi.org/10.3390/life13030785




