The Effects of Glutathione on Clinically Essential Fertility Parameters in a Bleomycin Etoposide Cisplatin Chemotherapy Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. BEP and GSH Treatment Protocols
2.3. Tissue Removal and Sperm Collection
2.4. Serum Testosterone Level Measurement
2.5. Semen Analysis
2.6. Sperm DNA Fragmentation Analysis
2.7. Testicular Histomorphology
2.8. Statistical Analysis
3. Results
3.1. Effects of BEP and BEP + GSH on the Weights of the Rats and the Reproductive Organs
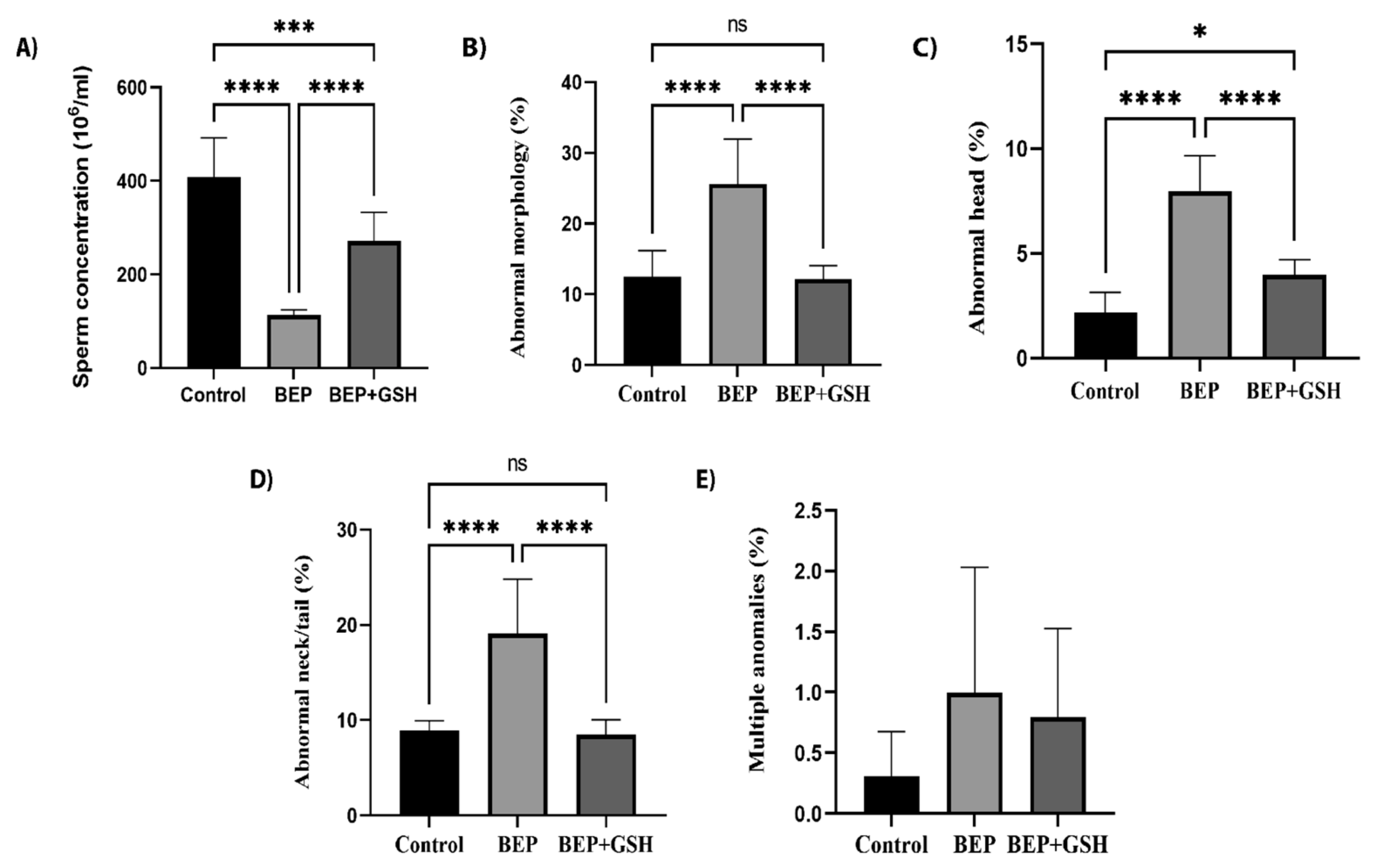
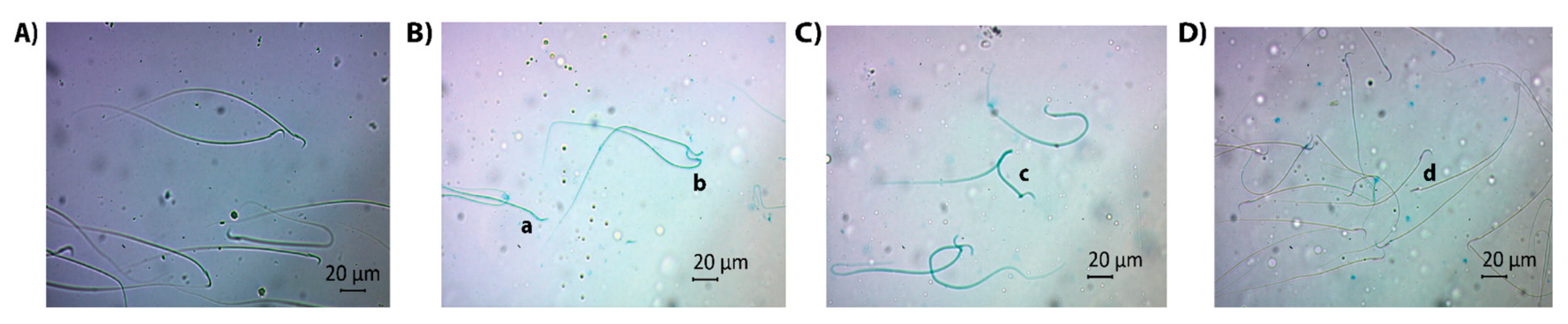
3.2. Effects of BEP and BEP + GSH Treatments on Sperm Count and Morphology
3.3. Effects of BEP and BEP + GSH on Sperm DNA Fragmentation
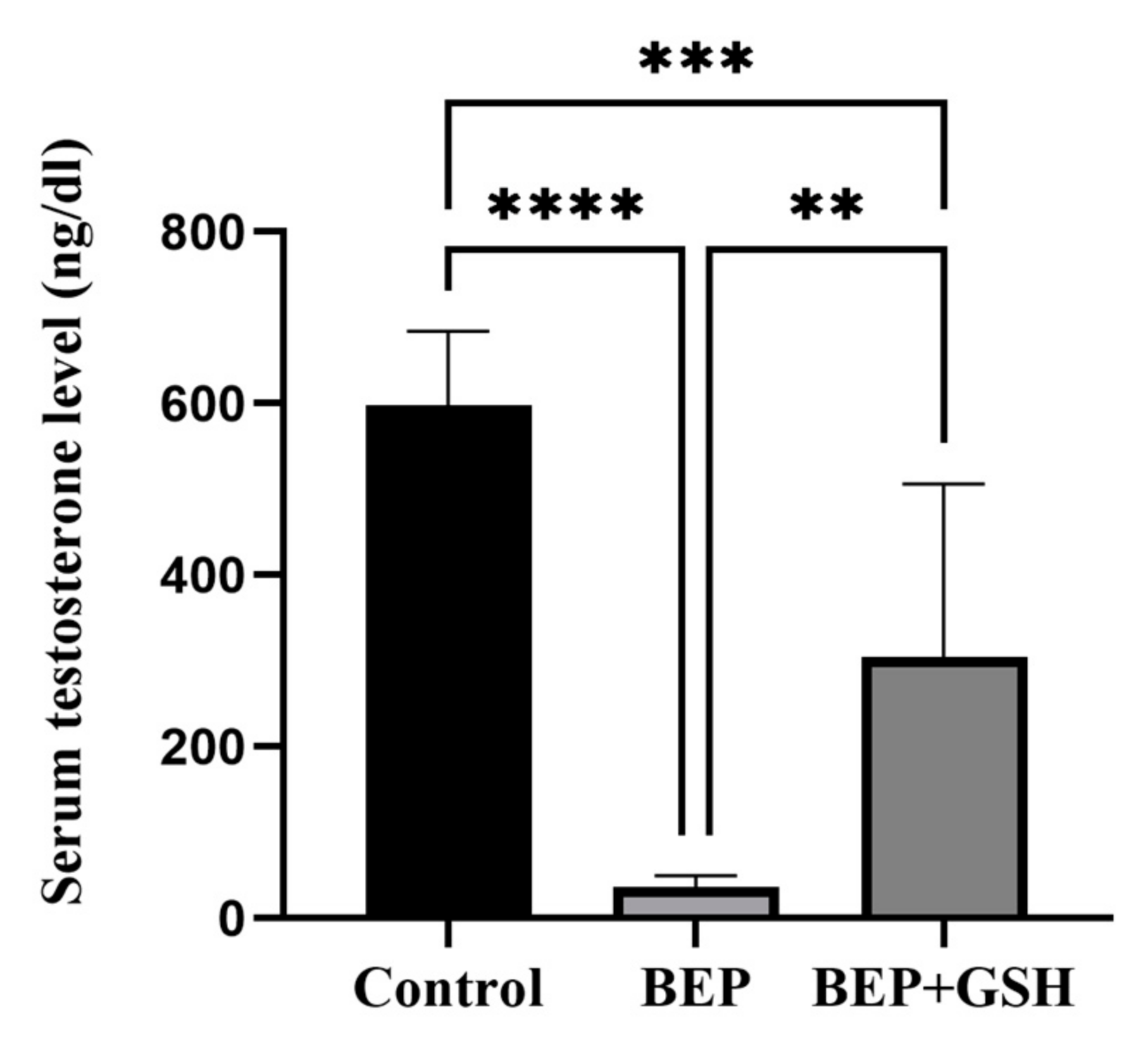
3.4. Effects of BEP and BEP + GSH on Serum Testosterone Levels
3.5. Effects of BEP and BEP + GSH on Testis Histomorphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michaelson, M.D. Epidemiology of and Risk Factors for Testicular Germ Cell Tumors. Shah, S., Lerner, S.P., Eds.; 2022. Available online: https://www.medilib.ir/uptodate/show/2997 (accessed on 23 January 2023).
- Gaddam, S.; Chesnut, G. Testicle Cancer; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Park, J.S.; Kim, J.; Elghiaty, A.; Ham, W.S. Recent Global Trends in Testicular Cancer Incidence and Mortality. Medicine 2018, 97, e12390. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.L.; Werntz, R.P.; Eggener, S.E. Testicular Cancer. Med. Clin. N. Am. 2018, 102, 251–264. [Google Scholar] [CrossRef] [PubMed]
- William, K.O. Overview of the Treatment of Testicular Germ Cell Tumors; Sonali, S., Jerome, P.R., Eds.; 2022; Available online: https://www.medilib.ir/uptodate/show/2960 (accessed on 23 January 2023).
- Jankilevich, G. BEP versus EP for Treatment of Metastatic Germ-Cell Tumours. Lancet Oncol. 2004, 5, 146. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Berretta, M.; Bottacin, A.; Palego, P.; Sartini, B.; Cosci, I.; Finos, L.; Selice, R.; Foresta, C.; Garolla, A. Impact of Bep or Carboplatin Chemotherapy on Testicular Function and Sperm Nucleus of Subjects with Testicular Germ Cell Tumor. Front. Pharmacol. 2016, 7, 122. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, C.; Hales, B.F.; Chan, P.; Robaire, B. Impact of Chemotherapeutics and Advanced Testicular Cancer or Hodgkin Lymphoma on Sperm Deoxyribonucleic Acid Integrity. Fertil. Steril. 2010, 94, 1374–1379. [Google Scholar] [CrossRef]
- Abram McBride, J.; Lipshultz, L.I. Male Fertility Preservation. Curr. Urol. Rep. 2018, 19, 49. [Google Scholar] [CrossRef]
- Petersen, P.M.; Hansen, S.W.; Giwercman, A.; Rørth, M.; Skakkebæk, N.E. Dose-Dependent Impairment of Testicular Function in Patients Treated with Cisplatin-Based Chemotherapy for Germ Cell Cancer. Ann. Oncol. 1994, 5, 355–358. [Google Scholar] [CrossRef]
- Brennemann, W.; Stoffel-Wagner, B.; Helmers, A.; Mezger, J.; Jager, N.; Klingmuller, D. Gonadal Function of Patients Treated With Cisplatin Based Chemotherapy for Germ Cell Cancer. J. Urol. 1997, 158, 844–850. [Google Scholar] [CrossRef]
- Bandak, M.; Jørgensen, N.; Juul, A.; Lauritsen, J.; Kier, M.G.G.; Mortensen, M.S.; Daugaard, G. Longitudinal Changes in Serum Levels of Testosterone and Luteinizing Hormone in Testicular Cancer Patients after Orchiectomy Alone or Bleomycin, Etoposide, and Cisplatin. Eur. Urol. Focus 2018, 4, 591–598. [Google Scholar] [CrossRef]
- Fosså, S.D.; Dahl, A.A.; Loge, J.H. Fatigue, Anxiety, and Depression in Long-Term Survivors of Testicular Cancer. JCO 2003, 21, 1249–1254. [Google Scholar] [CrossRef]
- Paoli, D.; Pallotti, F.; Lenzi, A.; Lombardo, F. Fatherhood and Sperm DNA Damage in Testicular Cancer Patients. Front. Endocrinol. 2018, 9, 506. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Ambar, R.F.; Agarwal, A.; Henkel, R. Etiologies of Sperm DNA Damage and Its Impact on Male Infertility. Andrologia 2021, 53, e13706. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, J.A.; Schlegel, P.N. Sperm DNA Damage and Its Relevance in Fertility Treatment: A Review of Recent Literature and Current Practice Guidelines. Int. J. Mol. Sci. 2023, 24, 1446. [Google Scholar] [CrossRef] [PubMed]
- Raeeszadeh, M.; Moradi, M.; Ayar, P.; Akbari, A. The Antioxidant Effect of Medicago sativa L. (Alfalfa) Ethanolic Extract against Mercury Chloride (HgCl2) Toxicity in Rat Liver and Kidney: An In Vitro and In Vivo Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 8388002. [Google Scholar] [CrossRef]
- Raeeszadeh, M.; Rezaee, M.; Akbari, A.; Khademi, N. The Comparison of the Effect of Origanum vulgare L. Extract and Vitamin C on the Gentamycin-Induced Nephrotoxicity in Rats. Drug Chem. Toxicol. 2022, 45, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as Precision Weapons in War against Cancer Chemotherapy Induced Toxicity—Exploring the Armoury of Obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Sridhar, C.; Reddy, Y.S.R.; De, B. Free Radicals, Antioxidants, Diseases and Phytomedicines: Current Status and Future Prospect. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 91–100. [Google Scholar]
- Wong, W.Y.; Merkus, H.M.W.M.; Thomas, C.M.G.; Menkveld, R.; Zielhuis, G.A.; Steegers-Theunissen, R.P.M. Effects of Folic Acid and Zinc Sulfate on Male Factor Subfertility: A Double-Blind, Randomized, Placebo-Controlled Trial. Fertil. Steril. 2002, 77, 491–498. [Google Scholar] [CrossRef]
- Yao, D.; Mills, J. Male Infertility: Lifestyle Factors and Holistic, Complementary, and Alternative Therapies. Asian J. Androl. 2016, 18, 410–418. [Google Scholar] [CrossRef]
- Showell, M.G.; Mackenzie-Proctor, R.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Hart, R.J. Antioxidants for Male Subfertility. Cochrane Database Syst. Rev. 2014, 2014, CD007411. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.M.; Mackenzie-Proctor, R.; Yazdani, A.; Stankiewicz, M.T.; Jordan, V.; Showell, M.G. Antioxidants for Male Subfertility. Cochrane Database Syst. Rev. 2019, 2019, CD007411. [Google Scholar] [CrossRef] [PubMed]
- de Ligny, W.; Smits, R.M.; Mackenzie-Proctor, R.; Jordan, V.; Fleischer, K.; de Bruin, J.P.; Showell, M.G. Antioxidants for Male Subfertility. Cochrane Database Syst. Rev. 2022, 2022, CD007411. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H. Glutathione and Its Role in Cellular Functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Garrido, N.; Meseguer, M.; Alvarez, J.; Simón, C.; Pellicer, A.; Remohí, J. Relationship among standard semen parameters, glutathione peroxidase/glutathione reductase activity, and mRNA expression and reduced glutathione content in ejaculated spermatozoa from fertile and infertile men. Fertil. Steril. 2004, 82, 1059–1066. [Google Scholar] [CrossRef]
- Lenzi, A.; Sgrò, P.; Salacone, P.; Paoli, D.; Gilio, B.; Lombardo, F.; Santulli, M.; Agarwal, A.; Gandini, L. A Placebo-Controlled Double-Blind Randomized Trial of the Use of Combined l-Carnitine and l-Acetyl-Carnitine Treatment in Men with Asthenozoospermia. Fertil. Steril. 2004, 81, 1578–1584. [Google Scholar] [CrossRef]
- Abdullah, F.; Khan Nor-Ashikin, M.; Agarwal, R.; Kamsani, Y.; Abd Malek, M.; Bakar, N.; Mohammad Kamal, A.-A.; Sarbandi, M.-S.; Abdul Rahman, N.-S.; Musa, N. Glutathione (GSH) Improves Sperm Quality and Testicular Morphology in Streptozotocin-Induced Diabetic Mice. Asian J. Androl. 2021, 23, 281–287. [Google Scholar] [CrossRef]
- Kilarkaje, N.; Mousa, A.M.; Al-Bader, M.M.; Khan, K.M. Antioxidants Enhance the Recovery of Three Cycles of Bleomycin, Etoposide, and Cisplatin–Induced Testicular Dysfunction, Pituitary-Testicular Axis, and Fertility in Rats. Fertil. Steril. 2013, 100, 1151–1159.e5. [Google Scholar] [CrossRef]
- Van Dam, P.S.; Van Asbeck, B.S.; Van Oirschot, J.F.L.M.; Biessels, G.J.; Hamers, F.P.T.; Marx, J.J.M. Glutathione and α-Lipoate in Diabetic Rats: Nerve Function, Blood Flow and Oxidative State: Glutathione and α-Lipoate in Diabetic Neuropathy. Eur. J. Clin. Investig. 2001, 31, 417–424. [Google Scholar] [CrossRef]
- Rat Sperm Morphological Assessment, Edition 1. 2000. Available online: http://www.irdg.co.uk/Sperm_morphology.pdf (accessed on 16 April 2022).
- Teixeira, T.A.; Pariz, J.R.; Dutra, R.T.; Saldiva, P.H.; Costa, E.; Hallak, J. Cut-off values of the Johnsen score and Copenhagen index as histopathological prognostic factors for postoperative semen quality in selected infertile patients undergoing microsurgical correction of bilateral subclinical varicocele. Transl. Androl. Urol. 2019, 8, 346–355. [Google Scholar] [CrossRef]
- de Vries, G.; Rosas-Plaza, X.; van Vugt, M.A.T.M.; Gietema, J.A.; de Jong, S. Testicular Cancer: Determinants of Cisplatin Sensitivity and Novel Therapeutic Opportunities. Cancer Treat. Rev. 2020, 88, 102054. [Google Scholar] [CrossRef]
- Alahmar, A. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the Role of Glutathione on Oxidative Stress and Infertility. JBRA Assist. Reprod. 2018, 22, 61–66. [Google Scholar] [CrossRef]
- Cilio, S.; Rienzo, M.; Villano, G.; Mirto, B.F.; Giampaglia, G.; Capone, F.; Ferretti, G.; Di Zazzo, E.; Crocetto, F. Beneficial Effects of Antioxidants in Male Infertility Management: A Narrative Review. Oxygen 2022, 2, 1–11. [Google Scholar] [CrossRef]
- Evans, E.P.P.; Scholten, J.T.M.; Mzyk, A.; Reyes-San-Martin, C.; Llumbet, A.E.; Hamoh, T.; Arts, E.G.J.M.; Schirhagl, R.; Cantineau, A.E.P. Male Subfertility and Oxidative Stress. Redox Biol. 2021, 46, 102071. [Google Scholar] [CrossRef] [PubMed]
- Khadivi, F.; Razavi, S.; Hashemi, F. Protective Effects of Zinc on Rat Sperm Chromatin Integrity Involvement: DNA Methylation, DNA Fragmentation, Ubiquitination and Protamination after Bleomycin Etoposide and Cis-Platin Treatment. Theriogenology 2020, 142, 177–183. [Google Scholar] [CrossRef]
- Moradi, M.; Goodarzi, N.; Faramarzi, A.; Cheraghi, H.; Hashemian, A.H.; Jalili, C. Melatonin Protects Rats Testes against Bleomycin, Etoposide, and Cisplatin-Induced Toxicity via Mitigating Nitro-Oxidative Stress and Apoptosis. Biomed. Pharmacother. 2021, 138, 111481. [Google Scholar] [CrossRef]
- Amirshahi, T.; Najafi, G.; Nejati, V. Protective Effect of Royal Jelly on Fertility and Biochemical Parameters in Bleomycin-induced Male Rats. Iran. J. Reprod. Med. 2014, 12, 209–216. [Google Scholar] [PubMed]
- Abdel-Latif, R.; Fathy, M.; Anwar, H.A.; Naseem, M.; Dandekar, T.; Othman, E.M. Cisplatin-Induced Reproductive Toxicity and Oxidative Stress: Ameliorative Effect of Kinetin. Antioxidants 2022, 11, 863. [Google Scholar] [CrossRef]
- Bieber, A.M.; Marcon, L.; Hales, B.F.; Robaire, B. Effects of Chemotherapeutic Agents for Testicular Cancer on the Male Rat Reproductive System, Spermatozoa, and Fertility. J. Androl. 2006, 27, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Türk, G.; Ateşşahin, A.; Sönmez, M.; Çeribaşi, A.O.; Yüce, A. Improvement of Cisplatin-Induced Injuries to Sperm Quality, the Oxidant-Antioxidant System, and the Histologic Structure of the Rat Testis by Ellagic Acid. Fertil. Steril. 2008, 89, 1474–1481. [Google Scholar] [CrossRef] [Green Version]
- Kirby, M. Testicular Cancer: Low Testosterone and the Metabolic Syndrome. Trends Urol. Men Health 2020, 11, 12–17. [Google Scholar] [CrossRef] [Green Version]
- García-Díaz, E.C.; Gómez-Quiroz, L.E.; Arenas-Ríos, E.; Aragón-Martínez, A.; Ibarra-Arias, J.A.; Retana-Márquez, M.d.S.I. Oxidative Status in Testis and Epididymal Sperm Parameters after Acute and Chronic Stress by Cold-Water Immersion in the Adult Rat. Syst. Biol. Reprod. Med. 2015, 61, 150–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakkas, D.; Alvarez, J.G. Sperm DNA Fragmentation: Mechanisms of Origin, Impact on Reproductive Outcome, and Analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Dorostghoal, M.; Kazeminejad, S.R.; Shahbazian, N.; Pourmehdi, M.; Jabbari, A. Oxidative Stress Status and Sperm DNA Fragmentation in Fertile and Infertile Men. Andrologia 2017, 49, e12762. [Google Scholar] [CrossRef]
- Tadokoro, Y.; Yomogida, K.; Ohta, H.; Tohda, A.; Nishimune, Y. Homeostatic Regulation of Germinal Stem Cell Proliferation by the GDNF/FSH Pathway. Mech. Dev. 2002, 113, 29–39. [Google Scholar] [CrossRef]
- Bilommi, R.; Nawas, B.A.; Kusmayadi, D.D.; Diposarosa, R.; Chairul, A.; Hernowo, B.S. The Effects of Glutathione on Malondialdehyde Expression and Seminiferous Tubule Damage in Experimental Testicular Torsion–Detorsion in Wistar Rats. J. Pediatr. Urol. 2013, 9, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, E.E.; Yahia, D.; El-Nisr, N.A. Chlorpyrifos Induced Testicular Damage in Rats: Ameliorative Effect of Glutathione Antioxidant: Chlorpyrifos Induced Testicular Damage in Rats. Environ. Toxicol 2014, 29, 1011–1019. [Google Scholar] [CrossRef]
- Hyman, M. Glutathione: The Mother of All Antioxidants. 2011. Available online: https://www.huffingtonpost.com/dr-mark-hyman/glutathione-the-mother-of_b_530494 (accessed on 7 February 2023).
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Hammad, A.A.; Orabi, S.H.; Elbaz, H.T.; Elweza, A.E.; Tahoun, E.A.; Elseehy, M.M.; El-Shehawi, A.M.; Mousa, A.A. Reproductive Injury in Male Rats from Acrylamide Toxicity and Potential Protection by Earthworm Methanolic Extract. Animals 2022, 12, 1723. [Google Scholar] [CrossRef]
- Chen, H.; Pechenino, A.S.; Liu, J.; Beattie, M.C.; Brown, T.R.; Zirkin, B.R. Effect of Glutathione Depletion on Leydig Cell Steroidogenesis in Young and Old Brown Norway Rats. Endocrinology 2008, 149, 2612–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, L.; Che, R.; He, L.; Gao, X.; Li, W.; Li, H. Role of Exogenous Glutathione in Alleviating Abiotic Stress in Maize (Zea mays L.). J. Plant Growth Regul. 2019, 38, 199–215. [Google Scholar] [CrossRef]


| Control | BEP | BEP + GSH | |
|---|---|---|---|
| Beginning body weight (g) | 323.5 ± 15.9 | 328.1 ± 11.9 | 338.13 ± 13.3 |
| Final body weight (g) | 398.0 ± 17.2 | 374.50 ± 11.8 | 380.6 ± 12.8 |
| Change in body weight (g) | 74.5 ± 10.6 | 46.4 ± 5.6 * | 49.1. ± 2.8 * |
| Right testicular weight (g) | 1.9 ± 0.06 | 1.6 ± 0.1 * | 1.4 ± 0.05 ** |
| Left testicular weight (g) | 1.9 ± 0.6 | 1.64 ± 0.10 * | 1.47 ± 0.058 * |
| Testes index a | 0.9 ± 0.03 | 0.8 ± 0.05 * | 0.8 ± 0.02 * |
| Right epididymis weight (g) | 0.8 ± 0.05 | 0.6 ± 0.05 * | 0.573 ± 0.02 ** |
| Left epididymis weight (g) | 0.8 ± 0.07 | 0.6 ± 0.04 * | 0.6 ± 0.02 * |
| Epididymis index b | 0.4 ± 0.02 | 0.33 ± 0.01 * | 0.30 ± 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayram, H.; Donmez Cakil, Y.; Sitar, M.E.; Demirel, G.; Selam, B.; Cincik, M. The Effects of Glutathione on Clinically Essential Fertility Parameters in a Bleomycin Etoposide Cisplatin Chemotherapy Model. Life 2023, 13, 815. https://doi.org/10.3390/life13030815
Bayram H, Donmez Cakil Y, Sitar ME, Demirel G, Selam B, Cincik M. The Effects of Glutathione on Clinically Essential Fertility Parameters in a Bleomycin Etoposide Cisplatin Chemotherapy Model. Life. 2023; 13(3):815. https://doi.org/10.3390/life13030815
Chicago/Turabian StyleBayram, Hale, Yaprak Donmez Cakil, Mustafa Erinc Sitar, Gamze Demirel, Belgin Selam, and Mehmet Cincik. 2023. "The Effects of Glutathione on Clinically Essential Fertility Parameters in a Bleomycin Etoposide Cisplatin Chemotherapy Model" Life 13, no. 3: 815. https://doi.org/10.3390/life13030815
APA StyleBayram, H., Donmez Cakil, Y., Sitar, M. E., Demirel, G., Selam, B., & Cincik, M. (2023). The Effects of Glutathione on Clinically Essential Fertility Parameters in a Bleomycin Etoposide Cisplatin Chemotherapy Model. Life, 13(3), 815. https://doi.org/10.3390/life13030815







