Melanin Stacking Differences in Pigmented and Non-Pigmented Melanomas: Quantitative Differentiation between Pigmented and Non-Pigmented Melanomas Based on Light-Scattering Properties
Abstract
:1. Introduction
1.1. Central Features in Early Diagnosis
1.2. Dermoscopy
1.3. Computer-Augmented Image Analysis
1.4. Digital Dermoscopy, AI, and Machine Learning
1.5. Differentiation between Pigmented Lesions
1.6. New Diagnostic Technologies Including Vibrational Optical Coherence Tomography (VOCT)
2. Methods
2.1. Subjects
2.2. Measurement of Resonant Frequency
2.3. Machine Learning Analysis
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silver, F.H.; Deshmukh, T.; Ryan, N.; Romm, A.; Nadiminti, H. “Fingerprinting” Benign and Cancerous Skin Lesions Using Vibrational Optical Coherence Tomography: Differentiation among Cancerous Lesion Types Based on the Presence of New Cells, Blood Vessels, and Fibrosis. Biomolecules 2022, 12, 1332. [Google Scholar] [CrossRef] [PubMed]
- Marghoob, N.G.; Liopyris, K.; Jaimes, N. Dermoscopy: A Review of the Structures That Facilitate Melanoma Detection. J. Am. Osteopat. Assoc. 2019, 119, 380–390. [Google Scholar] [CrossRef]
- Rigel, D.S.; Russak, J.; Friedman, R. The Evolution of Melanoma Diagnosis: 25 Years Beyond the ABCDs. CA Cancer J. Clin. 2010, 60, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, P.P. Cutaneous Malignant Melanoma: A Review of Early Diagnosis and Management. World J. Oncol. 2021, 12, 7–19. [Google Scholar] [CrossRef]
- Guy, G.P., Jr.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C.; Centers for Disease Control and Prevention (CDC). Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982–2030. MMWR. Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. [Google Scholar] [PubMed]
- Jones, O.T.; Jurascheck, L.C.; van Melle, M.; Hickman, S.; Burrows, N.P.; Hall, P.N.; Emery, J.; Walter, F. Dermoscopy for melanoma detection and triage in primary care: A systematic review. BMJ Open 2019, 9, e027529. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Mesica, A.; Gonzalez-Mercedes, M.; Deshmukh, T. Identification of Cancerous Skin Lesions Using Vibrational Optical Coherence Tomography (VOCT): Use of VOCT in Conjunction with Machine Learning to Diagnose Skin Cancer Remotely Using Telemedicine. Cancers 2023, 15, 156. [Google Scholar] [CrossRef]
- Petrie, T.; Samatham, R.; Witkowski, A.M.; Esteva, A.; Leachman, S.A. Melanoma Early Detection: Big Data, Bigger Picture. J. Investig. Dermatol. 2019, 139, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Sondermann, W.; Utikal, J.S.; Enk, A.H.; Schadendorf, D.; Klode, J.; Hauschild, A.; Weichenthal, M.; French, L.E.; Berking, C.; Schilling, B.; et al. Prediction of melanoma evolution in melanocytic nevi via artificial intelligence: A call for prospective data. Eur. J. Cancer 2019, 119, 30–34. [Google Scholar] [CrossRef]
- Fried, L.; Tan, A.; Bajaj, S.; Liebman, T.N.; Polsky, D.; Stein, J.A. Technological advances for the detection of melanoma: Advances in diagnostic techniques. J. Am. Acad. Dermatol. 2020, 83, 983–992. [Google Scholar] [CrossRef]
- Rigel, D.S.; Friedman, R.J.; Kopf, A.W.; Polsky, D. ABCDE—An Evolving Concept in the Early Detection of Melanoma. Arch. Dermatol. 2005, 141, 1032–1034. [Google Scholar] [CrossRef]
- Higgins, H.W., 2nd; Lee, K.C.; Galan, A.; Leffell, D.J. Melanoma in situ: Part I. Epidemiology, screening, and clinical features. J. Am. Acad. Dermatol. 2015, 73, 181–190. [Google Scholar] [CrossRef]
- Ferrari, B.; Pupelli, G.; Farnetani, F.; De Carvalho, N.T.; Longo, C.; Reggiani, C.; Argenziano, G.; Pellacani, G. Dermoscopic difficult lesions: An objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.; Cellura, A.P.; Hibler, B.; Burris, K. Computer-assisted diagnosis of melanoma. Semin. Cutan. Med. Surg. 2016, 35, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ji-Xu, A.; Dinnes, J.; Matin, R. Total body photography for the diagnosis of cutaneous melanoma in adults: A systematic review and meta-analysis. Br. J. Dermatol. 2021, 185, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gupta, S.K. Recent advancement in the early detection of melanoma using computerized tools: An image analysis perspective. Ski. Res. Technol. 2019, 25, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Celebi, M.E.; Garcia, I.F.; Ahmad, W. Melanoma recognition framework based on expert definition of ABCD for dermoscopic images. Ski. Res. Technol. 2013, 19, e93–e102. [Google Scholar] [CrossRef]
- Tomatis, S.; Carrara, M.; Bono, A.; Bartoli, C.; Lualdi, M.; Tragni, G.; Colombo, A.; Marchesini, R. Automated melanoma detection with a novel multispectral imaging system: Results of a prospective study. Phys. Med. Biol. 2005, 50, 1675. [Google Scholar] [CrossRef]
- Farina, B.; Bartoli, C.; Bono, A.; Colombo, A.; Lualdi, M.; Tragni, G.; Marchesini, R. Multispectral imaging approach in the diagnosis of cutaneous melanoma: Potentiality and limits. Phys. Med. Biol. 2000, 45, 1243. [Google Scholar] [CrossRef]
- Panjehpour, M.; Julius, C.E.; Phan, M.N.; Vo-Dinh, T.; Overholt, S. Laser-induced fluorescence spectroscopy for in vivo diagnosis of non-melanoma skin cancers. Lasers Surg. Med. 2002, 31, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Rajpara, S.; Botello, A.; Townend, J.; Ormerod, A. Systematic review of dermoscopy and digital dermoscopy/ artificial intelligence for the diagnosis of melanoma. Br. J. Dermatol. 2009, 161, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Curiel-Lewandrowski, C.; Novoa, R.A.; Berry, E.; Celebi, M.E.; Codella, N.; Giuste, F.; Gutman, D.; Halpern, A.; Leachman, S.; Liu, Y.; et al. Artificial Intelligence Approach in Melanoma. In Melanoma; Springer: New York, NY, USA, 2019; pp. 1–31. [Google Scholar] [CrossRef]
- Giavina-Bianchi, M.; de Sousa, R.M.; Paciello, V.Z.d.A.; Vitor, W.G.; Okita, A.L.; Prôa, R.; Severino, G.L.d.S.; Schinaid, A.A.; Santo, R.E.; Machado, B.S. Implementation of artificial intelligence algorithms for melanoma screening in a primary care setting. PLoS ONE 2021, 16, e0257006. [Google Scholar] [CrossRef]
- Vocaturo, E.; Perna, D.; Zumpano, E. Machine Learning Techniques for Automated Melanoma Detection. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019; pp. 2310–2317. [Google Scholar] [CrossRef]
- Menzies, S.W.; Westerhoff, K.; Rabinovitz, H.; Kopf, A.W.; McCarthy, W.H.; Katz, B. Surface Microscopy of Pigmented Basal Cell Carcinoma. Arch. Dermatol. 2000, 136, 1012–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feller, L.; Khammissa, R.A.G.; Kramer, B.; Altini, M.; Lemmer, J. Basal cell carcinoma, squamous cell carcinoma and melanoma of the head and face. Head Face Med. 2016, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Kato, J.; Horimoto, K.; Sato, S.; Minowa, T.; Uhara, H. Dermoscopy of Melanoma and Non-melanoma Skin Cancers. Front. Med. 2019, 6, 180. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.; Marsden, H.; Jaffe, W.; Matin, R.N.; Wali, G.N.; Greenhalgh, J.; McGrath, E.; James, R.; Ladoyanni, E.; Bewley, A.; et al. Assessment of Accuracy of an Artificial Intelligence Algorithm to Detect Melanoma in Images of Skin Lesions. JAMA Netw. Open 2019, 2, e1913436. [Google Scholar] [CrossRef] [Green Version]
- Silver, F.H.; Deshmukh, T.; Benedetto, D.; Kelkar, N. Mechano-vibrational spectroscopy of skin: Are changes in collagen and vascular tissue components early signs of basal cell carcinoma formation? Ski. Res. Technol. 2020, 27, 227–233. [Google Scholar] [CrossRef]
- Silver, F.H.; Deshmukh, T.; Kelkar, N.; Ritter, K.; Ryan, N.; Nadiminti, H. The “Virtual Biopsy” of Cancerous Lesions in 3D: Non-Invasive Differentiation between Melanoma and Other Lesions Using Vibrational Optical Coherence Tomography. Dermatopathology 2021, 8, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Shah, R.G.; Richard, M.; Benedetto, D. Comparative “virtual biopsies” of normal skin and skin lesions using vibrational optical coherence tomography. Ski. Res. Technol. 2019, 25, 743–749. [Google Scholar] [CrossRef]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef]
- Sadiq, I.; Kollias, N.; Baqer, A. Spectroscopic observations on human pigmentation. Photodermatol. Photoimmunol. Photomed. 2019, 35, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Traeg, J.; Duchstein, P.; Hennemann, M.; Clark, T.; Guldi, D.M.; Zahn, D. Size-Dependent Local Ordering in Melanin Aggregates and Its Implication on Optical Properties. J. Phys. Chem. A 2019, 123, 9403–9412. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Treatment—Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ensslin, C.J.; Hibler, B.P.; Lee, E.H.; Nehal, K.S.; Busam, K.J.; Rossi, A.M. Atypical Melanocytic Proliferations: A Review of the Literature. Dermatol. Surg. 2018, 44, 159–174. [Google Scholar] [CrossRef]
- Young, A.T.; Vora, N.B.; Cortez, J.; Tam, A.; Yeniay, Y.; Afifi, L.; Yan, D.; Nosrati, A.; Wong, A.; Johal, A.; et al. The role of technology in melanoma screening and diagnosis. Pigment. Cell Melanoma Res. 2020, 34, 288–300. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Chuchu, N.; di Ruffano, L.F.; Matin, R.N.; Thomson, D.R.; Wong, K.Y.; Aldridge, R.B.; Abbott, R.; Fawzy, M.; et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst. Rev. 2018, 2018, CD011902. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, G.; Passoni, E.; Pozzessere, P.; Maronese, C.A.; Marzano, A.V. Dermoscopy Use Leads to Earlier Cutaneous Mel-anoma Diagnosis in Terms of Invasiveness and Size? A Single-Center, Retrospective Experience. J. Clin. Med. 2022, 11, 4912. [Google Scholar] [CrossRef]


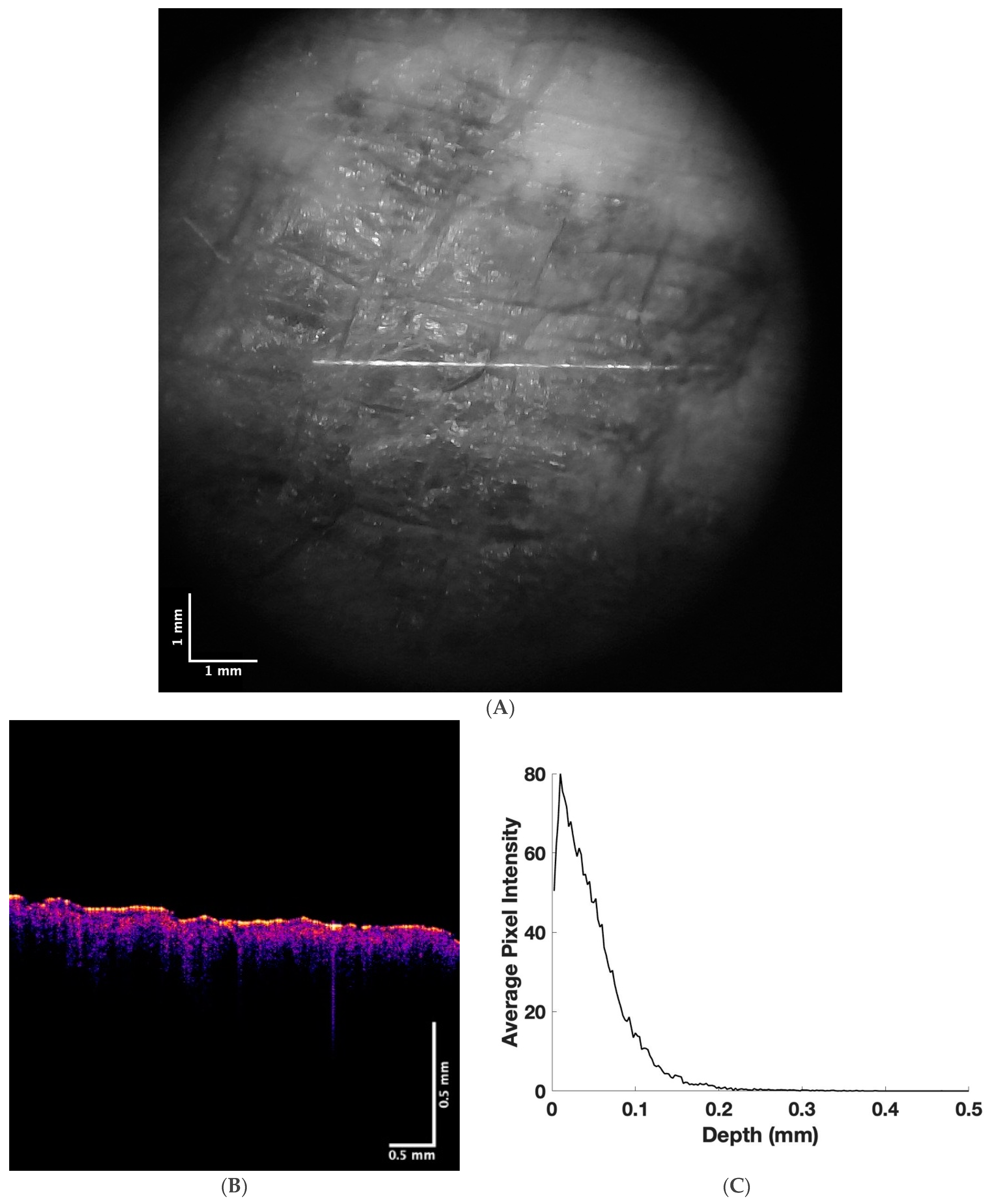
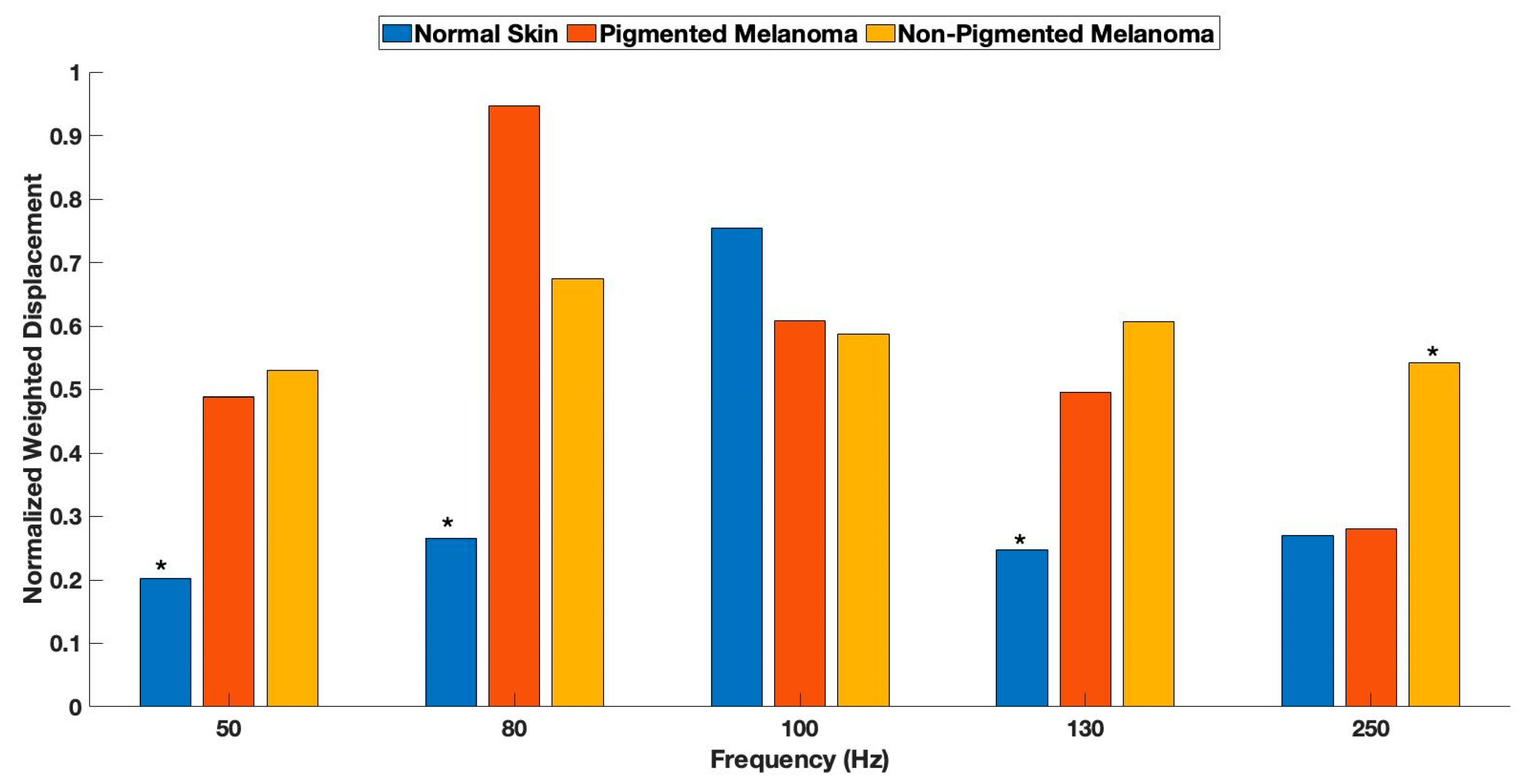
| Normal Skin | Pigmented Melanoma | Non-Pigmented Melanoma | |
|---|---|---|---|
| 50 Hz | |||
| Normal Skin | - | 0.051 | 0.00004 |
| Pigmented Melanoma | - | 0.31 | |
| 80 Hz | |||
| Normal Skin | - | 3 × 10−10 | 2.9 × 10−7 |
| Pigmented Melanoma | - | 1.4 × 10−5 | |
| 100 Hz | |||
| Normal Skin | - | 0.17 | 0.025 |
| Pigmented Melanoma | - | 0.23 | |
| 130 Hz | |||
| Normal Skin | - | 0.003 | 6.2 × 10−9 |
| Pigmented Melanoma | - | 0.1 | |
| 250 Hz | |||
| Normal Skin | - | 0.46 | 0.00002 |
| Pigmented Melanoma | - | 0.005 | |
| Average | SD | |
|---|---|---|
| Pigmented Melanoma | 256 μm | 29.4 μm |
| Non-Pigmented Melanoma | 277 μm | 30.1 μm |
| p-value: 0.007 | ||
| BCC | SCC | Melanoma | |
|---|---|---|---|
| Sensitivity | 90.9% | 91.6% | 83.33% |
| Specificity | 87.50% | 87.50% | 77.77% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silver, F.H.; Deshmukh, T.; Nadiminti, H.; Tan, I. Melanin Stacking Differences in Pigmented and Non-Pigmented Melanomas: Quantitative Differentiation between Pigmented and Non-Pigmented Melanomas Based on Light-Scattering Properties. Life 2023, 13, 1004. https://doi.org/10.3390/life13041004
Silver FH, Deshmukh T, Nadiminti H, Tan I. Melanin Stacking Differences in Pigmented and Non-Pigmented Melanomas: Quantitative Differentiation between Pigmented and Non-Pigmented Melanomas Based on Light-Scattering Properties. Life. 2023; 13(4):1004. https://doi.org/10.3390/life13041004
Chicago/Turabian StyleSilver, Frederick H., Tanmay Deshmukh, Hari Nadiminti, and Isabella Tan. 2023. "Melanin Stacking Differences in Pigmented and Non-Pigmented Melanomas: Quantitative Differentiation between Pigmented and Non-Pigmented Melanomas Based on Light-Scattering Properties" Life 13, no. 4: 1004. https://doi.org/10.3390/life13041004
APA StyleSilver, F. H., Deshmukh, T., Nadiminti, H., & Tan, I. (2023). Melanin Stacking Differences in Pigmented and Non-Pigmented Melanomas: Quantitative Differentiation between Pigmented and Non-Pigmented Melanomas Based on Light-Scattering Properties. Life, 13(4), 1004. https://doi.org/10.3390/life13041004







