Quantitative Evaluation of Oxygen Extraction Fraction Changes in the Monkey Brain during Acute Stroke by Using Quantitative Susceptibility Mapping
Abstract
1. Introduction
2. Methods and Materials
2.1. MRI Data Acquisition
2.2. MRI Data Processing
3. Results
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Rochefort, L.; Liu, T.; Kressler, B.; Liu, J.; Spincemaille, P.; Lebon, V.; Wu, J.; Wang, Y. Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: Validation and application to brain imaging. Magn. Reson. Med. 2010, 63, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Shmueli, K.; de Zwart, J.A.; van Gelderen, P.; Li, T.Q.; Dodd, S.J.; Duyn, J.H. Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn. Reson. Med. 2009, 62, 1510–1522. [Google Scholar] [CrossRef] [PubMed]
- Schweser, F.; Sommer, K.; Deistung, A.; Reichenbach, J.R. Quantitative susceptibility mapping for investigating subtle susceptibility variations in the human brain. Neuroimage 2012, 62, 2083–2100. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Kan, H.; Sakurai, K.; Oishi, K.; Matsukawa, N. Quantitative susceptibility mapping as an imaging biomarker for Alzheimer’s disease: The expectations and limitations. Front. Neurosci. 2022, 16, 938092. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, W.; Hu, J.; Nichol, H.; Haacke, E.M. Susceptibility weighted MRI pinpoints spontaneous intracerebral hemorrhage in stroke-prone spontaneously hypertensive rats. Magn. Reson. Imaging 2022, 93, 135–144. [Google Scholar] [CrossRef]
- Kou, Z.; Ye, Y.; Haacke, E.M. Evaluating the Role of Reduced Oxygen Saturation and Vascular Damage in Traumatic Brain Injury Using Magnetic Resonance Perfusion-Weighted Imaging and Susceptibility-Weighted Imaging and Mapping. Top. Magn. Reson. Imaging 2015, 24, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Vaas, M.; Deistung, A.; Reichenbach, J.R.; Keller, A.; Kipar, A.; Klohs, J. Vascular and tissue changes of magnetic susceptibility in the mouse brain after transient cerebral ischemia. Transl. Stroke Res. 2018, 9, 426–435. [Google Scholar] [CrossRef]
- Dimov, A.V.; Christoforidis, G.A.; Saadat, N.; Liu, M.M.; Jeong, Y.I.; Roth, S.; Niekrasz, M.; Carroll, T.J. QSM in canine model of acute cerebral ischemia: A pilot study. Magn. Reson. Med. 2021, 85, 1602–1610. [Google Scholar] [CrossRef]
- Uwano, I.; Kudo, K.; Sato, R.; Ogasawara, K.; Kameda, H.; Nomura, J.I.; Mori, F.; Yamashita, F.; Ito, K.; Yoshioka, K.; et al. Noninvasive Assessment of Oxygen Extraction Fraction in Chronic Ischemia Using Quantitative Susceptibility Mapping at 7 Tesla. Stroke 2017, 48, 2136–2141. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Tsai, C.Y.; Liao, M.C.; Yang, J.L.; Su, C.H.; Chen, J.H. Quantitative susceptibility mapping-based microscopy of magnetic resonance venography (QSM-mMRV) for in vivo morphologically and functionally assessing cerebromicrovasculature in rat stroke model. PLoS ONE 2016, 11, e0149602. [Google Scholar] [CrossRef]
- Fan, A.P.; Khalil, A.A.; Fiebach, J.B.; Zaharchuk, G.; Villringer, A.; Villringer, K.; Gauthier, C.J. Elevated brain oxygen extraction fraction measured by MRI susceptibility relates to perfusion status in acute ischemic stroke. J. Cereb. Blood Flow Metab. 2020, 40, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Kempf, D.J.; Tong, F.C.; Yan, Y.; Xu, Z.; Connor-Stroud, F.R.; Ford, B.D.; Howell, L.L.; Zhang, X. Longitudinal MRI Evaluation of Ischemic Stroke in the Basal Ganglia of a Rhesus Macaque (Macaca mulatta) with Seizures. Comp. Med. 2018, 68, 496–502. [Google Scholar] [CrossRef]
- Wang, S.; Gu, X.; Paudyal, R.; Wei, L.; Dix, T.A.; Yu, S.P.; Zhang, X. Longitudinal MRI evaluation of neuroprotective effects of pharmacologically induced hypothermia in experimental ischemic stroke. Magn. Reson. Imaging 2017, 40, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Paudyal, R.; Ford, B.D.; Zhang, X. Spatio-temporal assessment of the neuroprotective effects of neuregulin-1 on ischemic stroke lesions using MRI. J. Neurol. Sci. 2015, 357, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Jiang, Q.; Li, L.; Zhang, L.; Zhang, Z.G.; Ledbetter, K.A.; Panda, S.; Davarani, S.P.; Athiraman, H.; Li, Q.; et al. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J. Cereb. Blood Flow Metab. 2008, 28, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Meng, X.; Fisher, M.; Sotak, C.H.; Duong, T.Q. Pixel-by-pixel spatiotemporal progression of focal ischemia derived using quantitative perfusion and diffusion imaging. J. Cereb. Blood Flow Metab. 2003, 23, 1479–1488. [Google Scholar] [CrossRef]
- Neumann-Haefelin, T.; Wittsack, H.; Wenserski, F.; Siebler, M.; Seitz, R.; Modder, U.; Freund, H. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke 1999, 30, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lu, H. Cerebral oxygen extraction fraction MRI: Techniques and applications. Magn. Reson. Med. 2022, 88, 575–600. [Google Scholar] [CrossRef] [PubMed]
- Kudo, K.; Liu, T.; Murakami, T.; Goodwin, J.; Uwano, I.; Yamashita, F.; Higuchi, S.; Wang, Y.; Ogasawara, K.; Ogawa, A.; et al. Oxygen extraction fraction measurement using quantitative susceptibility mapping: Comparison with positron emission tomography. J. Cereb. Blood Flow Metab. 2016, 36, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, H.; Cho, J.; Mazerolle, E.L.; Wang, Y.; Pike, G.B. Cerebral OEF quantification: A comparison study between quantitative susceptibility mapping and dual-gas calibrated BOLD imaging. Magn. Reson. Med. 2020, 83, 68–82. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, Y.; Cho, J.; Shen, N.; Li, S.; Qin, Y.; Zhang, G.; Yan, S.; Xie, Y.; Zhang, S.; et al. The Spatiotemporal Evolution of MRI-Derived Oxygen Extraction Fraction and Perfusion in Ischemic Stroke. Front. Neurosci. 2021, 15, 716031. [Google Scholar] [CrossRef]
- Zhang, S.; Cho, J.; Nguyen, T.D.; Spincemaille, P.; Gupta, A.; Zhu, W.; Wang, Y. Initial Experience of Challenge-Free MRI-Based Oxygen Extraction Fraction Mapping of Ischemic Stroke at Various Stages: Comparison With Perfusion and Diffusion Mapping. Front. Neurosci. 2020, 14, 535441. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Sette, G.; Touzani, O.; Rioux, P.; Derlon, J.M.; MacKenzie, E.T.; Baron, J.C. Relationships between high oxygen extraction fraction in the acute stage and final infarction in reversible middle cerebral artery occlusion: An investigation in anesthetized baboons with positron emission tomography. J. Cereb. Blood Flow Metab. 1996, 16, 1176–1188. [Google Scholar] [CrossRef]
- Little, P.V.; Kraft, S.E.; Chireh, A.; Damberg, P.; Holmin, S. Oxygen metabolism MRI-A comparison with perfusion imaging in a rat model of MCA branch occlusion and reperfusion. J. Cereb. Blood Flow Metab. 2020, 40, 2315–2327. [Google Scholar] [CrossRef]
- Uchida, Y.; Kan, H.; Inoue, H.; Oomura, M.; Shibata, H.; Kano, Y.; Kuno, T.; Usami, T.; Takada, K.; Yamada, K.; et al. Penumbra Detection With Oxygen Extraction Fraction Using Magnetic Susceptibility in Patients With Acute Ischemic Stroke. Front. Neurol. 2022, 13, 752450. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.C.; Zhang, X.; Kempf, D.J.; Yepes, M.S.; Connor-Stroud, F.R.; Zola, S.; Howell, L. An enhanced model of middle cerebral artery occlusion in nonhuman primates using an endovascular trapping technique. AJNR Am. J. Neuroradiol. 2015, 36, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Belrose, J.C.; Noppens, R.R. Anesthesiology and cognitive impairment: A narrative review of current clinical literature. BMC Anesthesiol. 2019, 19, 241. [Google Scholar] [CrossRef]
- Seiler, A.; Deichmann, R.; Nöth, U.; Pfeilschifter, W.; Berkefeld, J.; Singer, O.C.; Klein, J.C.; Wagner, M. Oxygenation-Sensitive Magnetic Resonance Imaging in Acute Ischemic Stroke Using T2’/R2’ Mapping: Influence of Relative Cerebral Blood Volume. Stroke 2017, 48, 1671–1674. [Google Scholar] [CrossRef] [PubMed]
- Zaitsu, Y.; Kudo, K.; Terae, S.; Yazu, R.; Ishizaka, K.; Fujima, N.; Tha, K.K.; Haacke, E.M.; Sasaki, M.; Shirato, H. Mapping of cerebral oxygen extraction fraction changes with susceptibility-weighted phase imaging. Radiology 2011, 261, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Wisnieff, C.; Ramanan, S.; Olesik, J.; Gauthier, S.; Wang, Y.; Pitt, D. Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: Interpreting positive susceptibility and the presence of iron. Magn. Reson. Med. 2015, 74, 564–570. [Google Scholar] [CrossRef]
- Probst, J.; Rohner, M.; Zahn, M.; Piccirelli, M.; Pangalu, A.; Luft, A.; Deistung, A.; Klohs, J.; Wegener, S. Quantitative susceptibility mapping in ischemic stroke patients after successful recanalization. Sci. Rep. 2021, 11, 16038. [Google Scholar] [CrossRef]
- Borich, M.R.; Brown, K.E.; Boyd, L.A. Motor skill learning is associated with diffusion characteristics of white matter in individuals with chronic stroke. J. Neurol. Phys. Ther. 2014, 38, 151–160. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Hong, D.; Chen, F.; Ji, X.; Cao, G. White matter injury in ischemic stroke. Prog. Neurobiol. 2016, 141, 45–60. [Google Scholar] [CrossRef]
- Marin, M.A.; Carmichael, S.T. Stroke in CNS white matter: Models and mechanisms. Neurosci. Lett. 2018, 684, 193–199. [Google Scholar] [CrossRef]
- Cook, D.J.; Teves, L.; Tymianski, M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature 2012, 483, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Tymianski, M. Nonhuman Primate Models of Stroke for Translational Neuroprotection Research. Neurotherapeutics 2012, 9, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Jungreis, C.A.; Nemoto, E.; Boada, F.; Horowitz, M.B. Model of reversible cerebral ischemia in a monkey model. AJNR Am. J. Neuroradiol. 2003, 24, 1834–1836. [Google Scholar] [PubMed]
- Bihel, E.; Roussel, S.; Toutain, J.; Bernaudin, M.; Touzani, O. Diffusion tensor MRI reveals chronic alterations in white matter despite the absence of a visible ischemic lesion on conventional MRI: A nonhuman primate study. Stroke 2011, 42, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; D’Arceuil, H.E.; Westmoreland, S.; He, J.; Duggan, M.; Gonzalez, R.G.; Pryor, J.; de Crespigny, A.J. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke 2007, 38, 138–145. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Y.; Tong, F.; Li, C.; Jones, B.; Wang, S.; Meng, Y.; Muly, E.; Kempf, D.; Howell, L. Progressive Assessment of ischemic injury to white matter using diffusion tensor imaging: A preliminary study of a macaque model of stroke. Open Neuroimag. J. 2018, 12, 30–41. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Johnson, G.A.; Wu, B. High-field (9.4 T) MRI of brain dysmyelination by quantitative mapping of magnetic susceptibility. Neuroimage 2011, 56, 930–938. [Google Scholar] [CrossRef]
- Soni, N.; Vegh, V.; To, X.V.; Mohamed, A.Z.; Borges, K.; Nasrallah, F.A. Combined Diffusion Tensor Imaging and Quantitative Susceptibility Mapping Discern Discrete Facets of White Matter Pathology Post-injury in the Rodent Brain. Front. Neurol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Huang, P.; Zeng, Q.; Liu, C.; Wei, H.; Xuan, M.; Gu, Q.; Xu, X.; Wang, N.; Yu, X.; et al. Quantitative susceptibility mapping as a biomarker for evaluating white matter alterations in Parkinson’s disease. Brain Imaging Behav. 2019, 13, 220–231. [Google Scholar] [CrossRef]
- Li, C.X.; Meng, Y.; Yan, Y.; Kempf, D.; Howell, L.; Tong, F.; Zhang, X. Investigation of white matter and grey matter alteration in the monkey brain following ischemic stroke by using diffusion tensor imaging. Investig. Magn. Reson. Imaging. 2022, 26, 275–283. [Google Scholar] [CrossRef]
- Hirsch, N.M.; Toth, V.; Förschler, A.; Kooijman, H.; Zimmer, C.; Preibisch, C. Technical considerations on the validity of blood oxygenation level-dependent-based MR assessment of vascular deoxygenation. NMR Biomed. 2014, 27, 853–862. [Google Scholar] [CrossRef]
- Bauer, S.; Wagner, M.; Seiler, A.; Hattingen, E.; Deichmann, R.; Nöth, U.; Singer, O.C. Quantitative T2’-mapping in acute ischemic stroke. Stroke 2014, 45, 3280–3286. [Google Scholar] [CrossRef]
- Yu, Y.; Christensen, S.; Ouyang, J.; Scalzo, F.; Liebeskind, D.S.; Lansberg, M.G.; Albers, G.W.; Zaharchuk, G. Predicting Hypoperfusion Lesion and Target Mismatch in Stroke from Diffusion-weighted MRI Using Deep Learning. Radiology 2023, 307, e220882. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.; Ramadan, A.; Hillis, A.E.; Gottesman, R.F.; Leigh, R. Diffusion-perfusion mismatch: An opportunity for improvement in cortical function. Front. Neurol. 2014, 5, 280. [Google Scholar] [CrossRef] [PubMed]
- Spincemaille, P.; Anderson, J.; Wu, G.; Yang, B.; Fung, M.; Li, K.; Li, S.; Kovanlikaya, I.; Gupta, A.; Kelley, D.; et al. Quantitative Susceptibility Mapping: MRI at 7T versus 3T. J. Neuroimaging 2020, 30, 65–75. [Google Scholar] [CrossRef]
- Zhang, X. Editorial for “Cerebral Microbleeds Are Associated With Increased Brain Iron and Cognitive Impairment in Patients With Cerebral Small Vessel Disease-A Quantitative Susceptibility Mapping Study”. J. Magn. Reson. Imaging 2022, 56, 915–916. [Google Scholar] [CrossRef]
- Liu, C. Susceptibility tensor imaging. Magn. Reson. Med. 2010, 63, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarz, S.; Gottler, J.; Zimmer, C.; Hyder, F.; Preibisch, C. Characterizing white matter fiber orientation effects on multi-parametric quantitative BOLD assessment of oxygen extraction fraction. J. Cereb. Blood. Flow Metab. 2020, 40, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.; Chaurasia, B.; Fiorindi, A.; Umana, G.E.; Lu, B.; Montemurro, N. Ischemic Stroke and SARS-CoV-2 Infection: The Bidirectional Pathology and Risk Morbidities. Neurol. Int. 2022, 14, 391–405. [Google Scholar] [CrossRef] [PubMed]


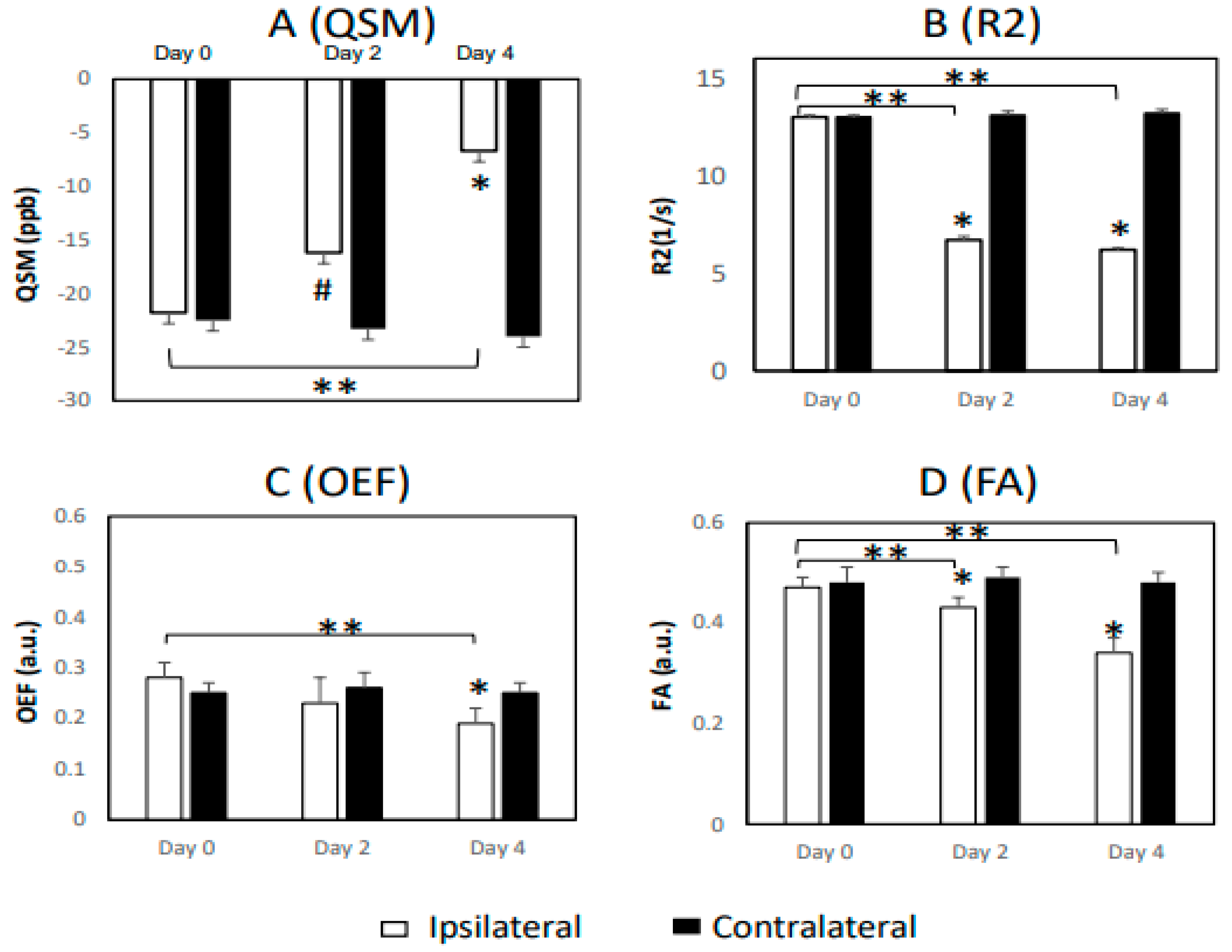
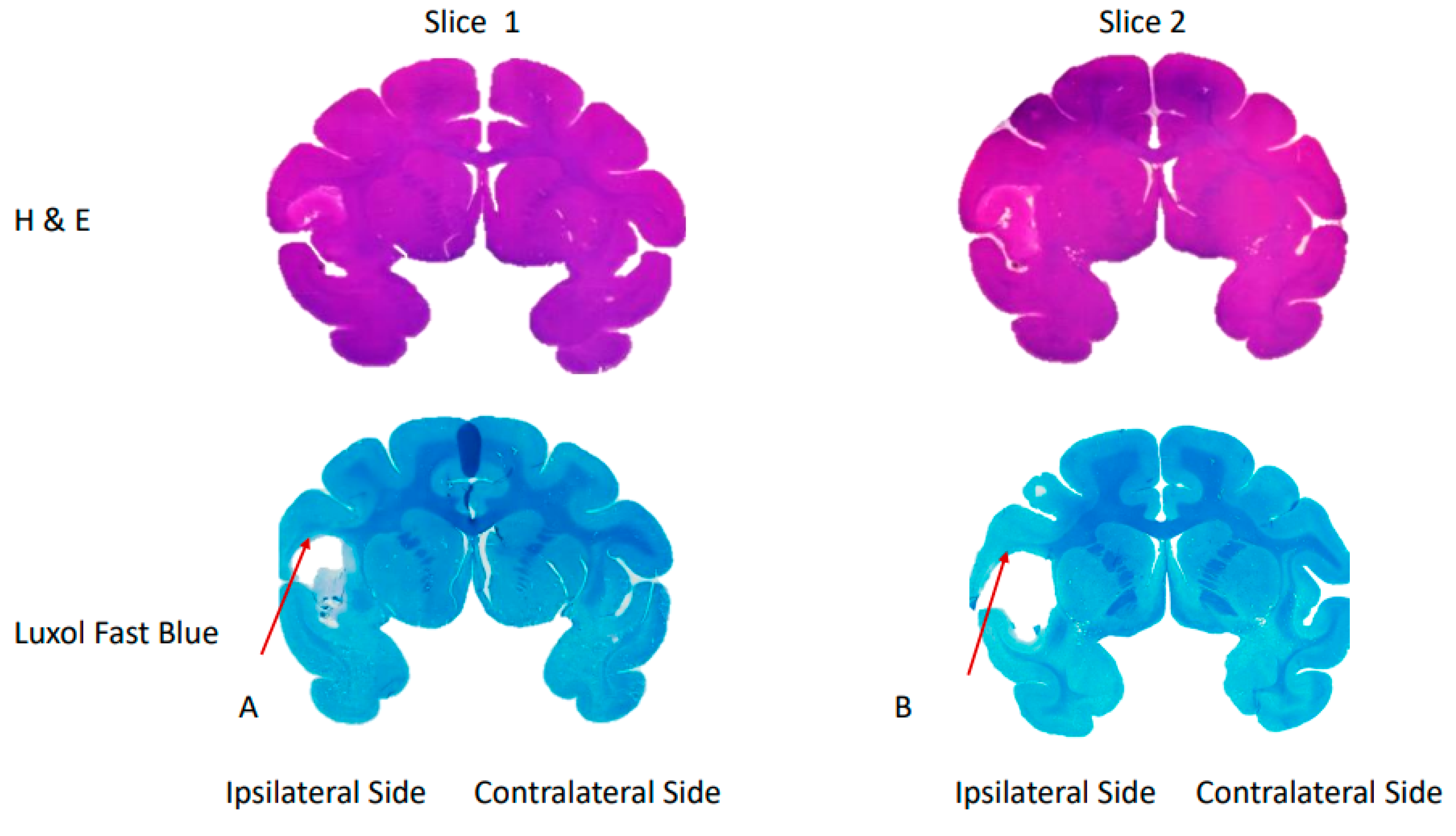
| Measurement | Day 0 | Day 2 | Day 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ips. | Con. | p | Ips. | Con. | p | Ips. | Con. | p | |
| WM, QSM | −21.8 ± 6.5 | −22.5 ± 4.5 | 0.91 | −16.2 ± 4.3 | −23.3 ± 2.0 | 0.08 | −6.7 ± 2.2 | −24.0 ± 3.6 | 0.003 * |
| WM, R2 | 13.0 ± 0.1 | 13.0 ± 0.1 | 0.57 | 6.7 ± 0.2 | 13.1 ± 0.2 | <0.001 * | 6.2 ± 0.1 | 13.2 ± 0.2 | <0.001 * |
| WM, R2’ | 12.1 ± 0.1 | 11.8 ± 0.1 | 0.005 * | 11.3 ± 0.2 | 11.8 ± 0.1 | 0.05 | 11.3 ± 0.1 | 11.8 ± 0.1 | 0.001 * |
| WM, OEF | 0.28 ± 0.03 | 0.25 ± 0.02 | 0.19 | 0.23 ± 0.05 | 0.26 ± 0.03 | 0.61 | 0.19 ± 0.03 | 0.25 ± 0.02 | 0.02 * |
| WM, FA | 0.47 ± 0.02 | 0.48 ± 0.03 | 0.47 | 0.43 ± 0.02 | 0.49 ± 0.02 | 0.001 * | 0.34 ± 0.03 | 0.48 ± 0.02 | 0.003 * |
| GM, QSM | 17.5 ± 1.6 | 14.1 ± 0.6 | 0.04 * | 8.8 ± 3.1 | 14.7 ± 1.7 | 0.13 | 8.4 ± 2.1 | 13.5 ± 1.8 | 0.02 * |
| GM, R2 | 10.2 ± 0.2 | 10.4 ± 0.1 | 0.19 | 7.0 ± 0.1 | 10.5 ± 0.1 | <0.001 * | 6.8 ± 0.3 | 10.4 ± 0.2 | <0.001 |
| GM, R2’ | 9.9 ± 0.3 | 9.7 ± 0.2 | 0.19 | 9.6 ± 0.2 | 9.6 ± 0.2 | 0.99 | 9.1 ± 0.2 | 9.6 ± 0.1 | 0.04 * |
| GM, OEF | 0.45 ± 0.08 | 0.36 ± 0.07 | 0.05 | 0.28 ± 0.03 | 0.35 ± 0.09 | 0.01 * | 0.30 ± 0.06 | 0.38 ± 0.03 | 0.04 * |
| GM, MD | 0.75 ± 0.03 | 0.84 ± 0.02 | 0.002 * | 0.66 ± 0.06 | 0.84 ± 0.02 | 0.001 * | 0.70 ± 0.05 | 0.85 ± 0.02 | 0.02 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Li, C.-X.; Zhang, X. Quantitative Evaluation of Oxygen Extraction Fraction Changes in the Monkey Brain during Acute Stroke by Using Quantitative Susceptibility Mapping. Life 2023, 13, 1008. https://doi.org/10.3390/life13041008
Meng Y, Li C-X, Zhang X. Quantitative Evaluation of Oxygen Extraction Fraction Changes in the Monkey Brain during Acute Stroke by Using Quantitative Susceptibility Mapping. Life. 2023; 13(4):1008. https://doi.org/10.3390/life13041008
Chicago/Turabian StyleMeng, Yuguang, Chun-Xia Li, and Xiaodong Zhang. 2023. "Quantitative Evaluation of Oxygen Extraction Fraction Changes in the Monkey Brain during Acute Stroke by Using Quantitative Susceptibility Mapping" Life 13, no. 4: 1008. https://doi.org/10.3390/life13041008
APA StyleMeng, Y., Li, C.-X., & Zhang, X. (2023). Quantitative Evaluation of Oxygen Extraction Fraction Changes in the Monkey Brain during Acute Stroke by Using Quantitative Susceptibility Mapping. Life, 13(4), 1008. https://doi.org/10.3390/life13041008








