Bryorutstroemia (Rutstroemiaceae, Helotiales), a New Genus to Accommodate the Neglected Sclerotiniaceous Bryoparasitic Discomycete Helotium fulvum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Observation
2.2. DNA Extraction, PCR Amplification and Sequencing
2.3. Phylogenetic Analysis
3. Results
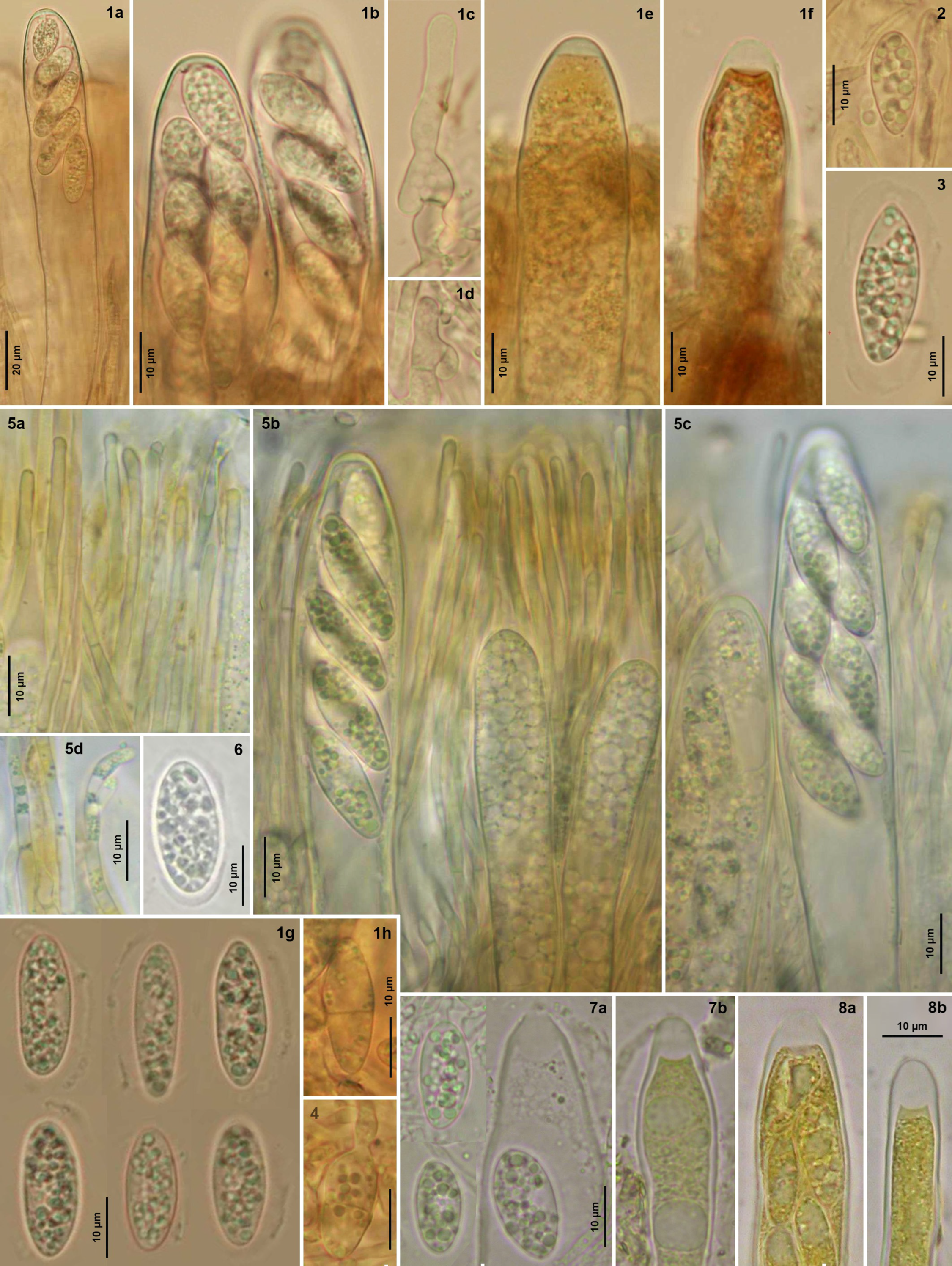
3.1. Taxonomy
3.2. Phylogeny
4. Discussion
4.1. Morphological Remarks
- 1. Asci (†) with prominent, inamyloid apical wall thickening; ascospores permanently hyaline; growing on bryophytes........ Bryorutstroemia
- 1. Ascus apex (†) with amyloid apical ring of the Sclerotinia-type, rarely faintly amyloid or inamyloid, but then only moderately thick-walled; growing on phanerogams........ 2
- 2. On monocotyledons........ Clarireedia
- 2. On dicotyledons or gymnosperms........ 3
- 3. Apothecia externally with prominent, septate, thick-walled setae........ Torrendiella
- 3. Apothecia without setae........ 4
- 4. Ascospores permanently hyaline........ Rutstroemia (including Dencoeliopsis), Lanzia
- 4. Ascospores turning brown with age, either within the living asci or when overmature........ Bicornispora, Lambertella, Martininia
4.2. Phylogenetic Remarks
4.3. Ecological Remarks
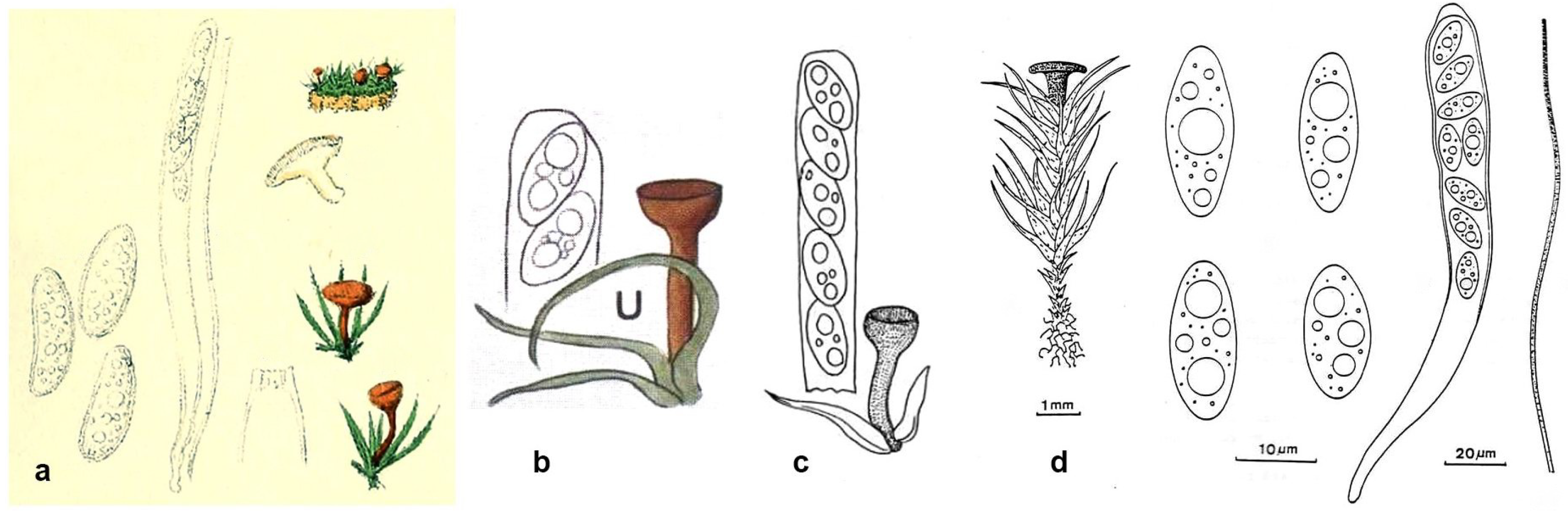









4.4. Literature Reports
4.5. Misinterpretations
4.6. Other Bryicolous Species of the Sclerotiniaceous Lineage
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boudier, J.L.É. Nouvelles espèces ou variétés de champignons de France. Bull. Soc. Mycol. Fr. 1897, 13, 11–18. [Google Scholar]
- Dennis, R.W.G. British Ascomycetes, 2nd ed.; J. Cramer: Vaduz, Liechtenstein, 1978; pp. 1–585. [Google Scholar]
- De Meulder, H. Helotium fulvum Boud., een bryofytische parasiet. AMK Meded. 1992, 92, 79–82. [Google Scholar]
- Schultheis, B.; Tholl, M.T. Journées luxembourgeoises de mycologie vernale 2001. Bull. Soc. Nat. Luxemb. 2003, 104, 21–39. [Google Scholar]
- Baral, H.O. Vital versus herbarium taxonomy: Morphological differences between living and dead cells of Ascomycetes, and their taxonomic implications. Mycotaxon 1992, 44, 333–390. [Google Scholar]
- Henriot, A.; Cheype, J.L. Piximètre: La Mesure de Dimensions Sur Images. Version 5.10 (R 1541). 2020. Available online: http://ach.log.free.fr/Piximetre (accessed on 4 December 2020).
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 1990, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov (accessed on 22 June 2022).
- FRDBI: Database of the British Mycological Society. Available online: https://basidiochecklist.science.kew.org/BritishFungi/FRDBI/FRDBIrecord.asp?intGBNum=10977 (accessed on 22 June 2022).
- Deutsche Gesellschaft für Mykologie. Available online: https://www.pilze-deutschland.de/organismen/helotium-fulvum-boud-1897-1 (accessed on 22 June 2022).
- Arnolds, E.; Kuyper, T.W.; Noordeloos, M.E. Overzicht van de Paddestoelen in Nederland; NMV: Wijster, The Netherlands, 1995; pp. 1–871. [Google Scholar]
- Jaklitsch, W.; Baral, H.O.; Lücking, R.; Lumbsch, H.T. Ascomycota. In Syllabus of Plant Families, 13th ed.; Frey, W., Ed.; Borntraeger: Stuttgart, Germany, 2016; pp. 1–322. [Google Scholar]
- Salgado-Salazar, C.; Beirn, L.A.; Ismaiel, A.; Boehm, M.J.; Carbone, I.; Putman, A.I.; Tredway, L.P.; Clarke, B.B.; Crouch, J.A. Clarireedia: A new fungal genus comprising four pathogenic species responsible for dollar spot disease of turfgrass. Fungal Biol. 2018, 122, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Pärtel, K.; Baral, H.-O.; Tamm, H.; Põldmaa, K. Evidence for the polyphyly of Encoelia and Encoelioideae with reconsideration of respective families in Leotiomycetes. Fungal Divers. 2016, 82, 183–219. [Google Scholar] [CrossRef]
- Baral, H.-O.; Rönsch, P.; Richter, U.; Urban, A.; Kruse, J.; Bemmann, M.; Kummer, V.; Valencia, F.J.; Huth, W. Schroeteria decaisneana, S. poeltii, and Ciboria ploettneriana (Sclerotiniaceae, Helotiales, Ascomycota), three parasites on Veronica seeds: First report of teleomorphs in Schroeteria. Mycol. Prog. 2022, 21, 359–407. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, Y.; Geng, J.; Dai, Y.; Ren, H.; Lamour, K. A new dollar spot disease of turfgrass caused by Clarireedia paspali. Mycol. Prog. 2019, 18, 1423–1435. [Google Scholar] [CrossRef]
- Holst-Jensen, A.; Kohn, L.M.; Schumacher, T. Nuclear rDNA phylogeny of the Sclerotiniaceae. Mycologia 1997, 89, 885–899. [Google Scholar] [CrossRef]
- Döbbeler, P. Belonioscyphella hypnorum (Helotiales, Ascomycetes), ein nekrotropher Parasit auf Laubmoosen. Ber. Bayer. Bot. Ges. 1986, 57, 153–158. [Google Scholar]
- Huhtinen, S.; Döbbeler, P. Bryoscyphus hyalotectus (Helotiales), a new polytrichicolous ascomycete from North America. Karstenia 2017, 57, 33–36. [Google Scholar] [CrossRef]
- Baral, H.O.; Krieglsteiner, L. Hymenoscyphus subcarneus, a little known bryicolous discomycete found in the Białowieża National Park. Acta Mycol. 2006, 41, 11–20. [Google Scholar] [CrossRef]
- GBIF: Global Biodiversity Information Facility Database. Available online: https://www.discoverlife.org/mp/20q?search=Helotium+fulvum (accessed on 22 June 2022).
- Bryoweb. Available online: https://botanika.prf.jcu.cz/bryoweb (accessed on 23 December 2022).
- Marstaller, R. Epigäische Moosgesellschaften im Buntsandsteingebiet des Saaletals zwischen Saalfeld und Jena. Hoppea Denkschr. Regensb. Bot. Ges. 2017, 78, 127–167. [Google Scholar]
- Ellis, M.B.; Ellis, J.P. Microfungi on Land Plants. An Identification Handbook; Croom Helm: London, UK; Sydney, Australia, 1985. [Google Scholar]
- Dennis, R.W.G. Remarks on the genus Hymenoscyphus S.F. Gray, with observations on sundry species referred by Saccardo and others to the genera Helotium, Pezizella or Phialea. Persoonia 1964, 3, 29–80. [Google Scholar]
- Velenovský, J. Monographia Discomycetum Bohemiae; Prague, Czech Republic, 1934; pars 1–2. [Google Scholar]
- Svrček, M. A taxonomic revision of inoperculate discomycetes described by J. Velenovský in the genus Helotium, preserved in National Museum, Prague. Sb. Nár. Mus. Praze B 1985, 40, 129–215. [Google Scholar]
- Svrček, M. New or less known Discomycetes. XVIII. Česká Mykol. 1988, 42, 137–148. [Google Scholar]
| Species | Collection Number | Country | Host | ITS | LSU | EF1α |
|---|---|---|---|---|---|---|
| Bicornispora seditiosa | AH 44702 T | Spain | Acer monspessulanum | KF499362 | KF499362 | MW001933 |
| Bryorutstroemia fulva | C.N. 103 | Hungary | Dicranum scoparium | OP035831 | OP035831 | OP058106 |
| Bryorutstroemia fulva | Z.S. 2/2021 | Czech Republic | Dicranella heteromalla | OP035812 | - | - |
| Bryorutstroemia fulva | Z.S. 7/2021 | Czech Republic | Dicranella heteromalla | OP035830 | OP035830 | OP058105 |
| Bryorutstroemia fulva | Z.S. 9/2021 | Czech Republic | Dicranella heteromalla | OP035829 | OP035829 | OP058104 |
| Bryorutstroemia fulva | Z.S. 19/2021 | Czech Republic | Dicranella heteromalla | OP035828 | OP035828 | OP058103 |
| “Cenangium” acuum | KL 243 | Germany | Pinus sylvestris | LT158439 | KX090822 | KX090674 |
| Chlorociboria glauca | KL 238 | France | Salix sp. | LT158438 | KX090821 | KX090673 |
| Ciboria amentacea | HR 98838 | Czech Republic | Alnus sp. | OP901951 | OP897698 | OP958788 |
| Ciboria americana | HR 102055 | Czech Republic | indet. gall | OP901952 | OP897699 | OP958784 |
| Ciboria betulae | 1145.P | Norway | Betula sp. | Z81427 | Z81403 | - |
| Ciboria conformata | HR B008890 | Czech Republic | Alnus glutinosa | OP902277 | OP897705 | OP958790 |
| Ciboria coryli | HR B008735 | Czech Republic | Corylus avellana | OP902275 | OP897703 | OQ023970 |
| Ciboria viridifusca | HR B006315 | Czech Republic | Alnus glutinosa | OP901954 | OP897702 | OP958783 |
| Clarireedia asphodeli | F142282 | Spain | Asphodelus fistulosus | KJ941085 | KJ941065 | - |
| Clarireedia bennettii | CBS 309.37 | unknown | indet. Poaceae | MF964321 | - | - |
| Clarireedia calopus | CBS 854.97 | Netherlands | indet. Poaceae | KF545314 | AB926155 | - |
| Clarireedia calopus # | CBS 465.73 | Great Britain | rabbit dung | KF588375 | MH878367 | - |
| Clarireedia gladioli | CBS 265.28 T | unkown | Gladiolus sp. | MH855008 | MH866477 | - |
| Clarireedia henningsiana | HR B013053 | Czech Republic | Scirpus sylvaticus | OP901955 | OP897706 | OP958787 |
| Clarireedia homoeocarpa | CBS 310.37 | Great Britain | Festuca sp. | MF964322 | MH867420 | - |
| Clarireedia maritima | H.B. 6860 | Spain | Ammophila arenaria | KF588372 | KJ941063 | - |
| Clarireedia narcissi | CBS 339.33 | Netherlands | Narcissus sp. | MH855451 | MH866916 | - |
| Clarireedia paspali | XC5 | China | Paspalum vaginatum | MH392087 | - | MH444193 |
| Clarireedia sp. | BVV | USA | Bromus tectorum | MT850272 | MG937748 | NJPS01000062 |
| Dumontinia tuberosa | TU109263 | Estonia | Anemone nemorosa | LT158412 | KX090843 | KX090697 |
| Encoelia furfuracea | KL 107 | Estonia | Corylus avellana | LT158416 | KX090798 | KX090653 |
| Hymenoscyphus scutula | G.M. 2014-12-25.2 | Luxembourg | indet. herb | MK674606 | MK674606 | - |
| Lambertella corni-maris | CLX4075 | USA | Malus sp. | KC958562 | KC964858 | - |
| Lambertella palmeri | AH 7655 | Spain | Quercus rotundifolia | KF499365 | KF499365 | - |
| Lambertella pyrolae | TNS-F 40132 T | Japan | Pyrola incarnata | AB926081 | AB926164 | - |
| Lambertella subrenispora | CBS 811.85 | Japan | Aster ageratoides | MH861915 | DQ470978 | DQ471101 |
| Lanzia allantospora | CBS 124334 | New Zealand | Agathis australis | AB926099 | AB926154 | - |
| Martininia panamaensis | CBS 207.47 | Panama | indet. log | MH856219 | MH867749 | - |
| Monilinia fructicola | 2014/FC48 | Hungary | Prunus persica | LT615175 | LT615175 | - |
| Monilinia oxycocci | 1087.P | Norway | Vaccinium oxycoccos | Z73789 | Z73754 | - |
| Piceomphale bulgarioides | HR B004019 | Czech Republic | Picea abies | OP901953 | OP897701 | OP958786 |
| Pycnopeziza sejournei | KL 267 | France | Hedera helix | LT158443 | KX090827 | KX090679 |
| Rutstroemia bolaris | 1526.P | Norway | Betula pubescens | Z80894 | Z81419 | - |
| Rutstroemia elatina | HR B000521 | Czech Republic | Abies sp. | OP902274 | OP897700 | OP958785 |
| Rutstroemia firma | KL 290 | Estonia | indet. angiosperm | LT158448 | KX090830 | KX090682 |
| Rutstroemia longipes | TNS: F-40097 | Japan | Daphniphyllum macropodum | AB926073 | AB926142 | - |
| Rutstroemia luteovirescens | HR B008840 | Czech Republic | Acer platanoides | OP902276 | OP897704 | OP958789 |
| Rutstroemia tiliacea | KL 160 | Germany | Tilia sp. | LT158423 | KX090808 | KX090661 |
| Schroeteria decaisneana | A.U. 2273 | Germany | Veronica hederifolia | MZ048345 | MZ048345 | - |
| Schroeteria delastrina | V.K. P1652-26 | Germany | Veronica arvensis | MW915645 | MW915645 | - |
| Sclerencoelia fraxinicola | KL 156 | Germany | Fraxinus excelsior | LT158420 | KX090805 | KX090659 |
| Scleromitrula shiraiana | Hirayama062001 | ? | ? | AY789408 | AY789407 | - |
| Sclerotinia sclerotiorum | 1980 UF-70 | USA | bean pods | CP017820 | CP017820 | - |
| Torrendiella setulata | H.B. 9775 | Canada | Acer spicatum | KF588367 | KJ941052 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baral, H.-O.; Sochorová, Z.; Sochor, M. Bryorutstroemia (Rutstroemiaceae, Helotiales), a New Genus to Accommodate the Neglected Sclerotiniaceous Bryoparasitic Discomycete Helotium fulvum. Life 2023, 13, 1041. https://doi.org/10.3390/life13041041
Baral H-O, Sochorová Z, Sochor M. Bryorutstroemia (Rutstroemiaceae, Helotiales), a New Genus to Accommodate the Neglected Sclerotiniaceous Bryoparasitic Discomycete Helotium fulvum. Life. 2023; 13(4):1041. https://doi.org/10.3390/life13041041
Chicago/Turabian StyleBaral, Hans-Otto, Zuzana Sochorová, and Michal Sochor. 2023. "Bryorutstroemia (Rutstroemiaceae, Helotiales), a New Genus to Accommodate the Neglected Sclerotiniaceous Bryoparasitic Discomycete Helotium fulvum" Life 13, no. 4: 1041. https://doi.org/10.3390/life13041041
APA StyleBaral, H.-O., Sochorová, Z., & Sochor, M. (2023). Bryorutstroemia (Rutstroemiaceae, Helotiales), a New Genus to Accommodate the Neglected Sclerotiniaceous Bryoparasitic Discomycete Helotium fulvum. Life, 13(4), 1041. https://doi.org/10.3390/life13041041






