Abstract
The present study reviewed the carbon-biogeochemistry-related observations concerning CO2 and CH4 dynamics in the estuaries adjoining the Indian Sundarbans mangrove ecosystem. The review focused on the partial pressure of CO2 and CH4 [pCO2(water) and pCH4(water)] and air–water CO2 and CH4 fluxes and their physical, biogeochemical, and hydrological drivers. The riverine-freshwater-rich Hooghly estuary has always exhibited higher CO2 emissions than the marine-water-dominated Sundarbans estuaries. The mangrove sediment porewater and recirculated groundwater were rich in pCO2(water) and pCH4(water), enhancing their load in the adjacent estuaries. Freshwater-seawater admixing, photosynthetically active radiation, primary productivity, and porewater/groundwater input were the principal factors that regulated pCO2(water) and pCH4(water) and their fluxes. Higher chlorophyll-a concentrations, indicating higher primary production, led to the furnishing of more organic substrates that underwent anaerobic degradation to produce CH4 in the water column. The northern Bay of Bengal seawater had a high carbonate buffering capacity that reduced the pCO2(water) and water-to-air CO2 fluxes in the Sundarbans estuaries. Several authors traced the degradation of organic matter to DIC, mainly following the denitrification pathway (and pathways between aerobic respiration and carbonate dissolution). Overall, this review collated the significant findings on the carbon biogeochemistry of Sundarbans estuaries and discussed the areas that require attention in the future.
1. Introduction
The term “global warming” and its connection with greenhouse gases and ocean circulations came to the limelight in 1975 [1], and the scientific community observed a consistent increase in the global temperature to cross the baseline values since 1976 [2]. In this regard, the Earth Summit in 1992 realized the need for a global partnership for sustainable development, ensuring environmental protection and improved human life. Later, in 2015, the UN Sustainable Development Summit created 17 sustainable development goals (SDGs) to achieve a world with reduced effects from climate change (https://sdgs.un.org/goals, accessed on 30 September 2022). Since the advent of global warming and climate change, countless studies have tried to understand the interactions between climate change and the Earth’s environmental systems. Throughout the industrial period, greenhouse gas emissions have increased significantly, and the environment and oceans have received increased amounts of carbon, nitrogen, and phosphorous. This excess carbon and nutrients affected the coastal zones, which include tidal wetlands, estuaries, and continental shelf waters [3]. This anthropogenic loading changes the nutrient cycling and greenhouse gas emissions from coastal systems [4].
Estuaries are dynamic and responsive systems with considerable environmental variability as they bridge the terrestrial sector with the oceans [5]. They also play a significant role in global carbon cycling. As per recent studies, the net carbon dioxide (CO2) emission from the estuaries (0.1 to 0.4 Pg C (carbon) yr−1) is equivalent to one-quarter of the net uptake of atmospheric CO2 by the open ocean [6,7,8,9]. Besides calculating the global carbon budget, coastal carbon cycles also help comprehend several ecological and geochemical phenomena, as carbon is the common currency often used to describe them [10]. Coastal issues such as hypoxia due to oxidation of organic carbon [11], acidification resulting from anthropogenic and organic CO2 invasion [12], and the loss of blue carbon due to human development and sea level rise [13] require a thorough understanding of the coastal carbon dynamics. In the face of rapid coastal development and accelerated climate change, it is essential to understand the role of nearshore ecosystems as carbon sources and sinks through coastal carbon cycle studies [10].
Among the greenhouse gases driving climate change, CO2 and methane (CH4) are the primary ones. CO2 and CH4 contribute 73% and 19%, respectively, of the total greenhouse gases emitted globally [14]. Coal combustion (39%), oil combustion (31%), and natural gas combustion (18%) are the primary anthropogenic sources of CO2. In contrast, cattle (21%), rice cultivation (10%), natural gas production (14%), oil production (9%), landfilling (10%), wastewater processing (11%), and coal mining (10%) are the primary CH4 sources in the same category [14]. Though the percentage contribution of CH4 is lower than CO2 in the total greenhouse gases, methane’s global warming potential is 34 times stronger than CO2. Hence, a slight change in atmospheric concentration has a more significant warming effect [15]. CH4 is the second most important greenhouse gas after CO2, contributing almost 20% of global warming since the pre-industrial era [16]. Hence, understanding the dynamics of both CO2 and CH4 emissions will help predict future climatic changes. CO2 and CH4 fluxes refer to the exchange rate of carbon dioxide and methane per unit area per unit time between different carbon reservoirs within the ecosphere through physicochemical, biological, or anthropogenic activities. Mangroves absorb CO2 from the immediate environment and convert it into biomass through photosynthetic activity. Depending on the biogeochemical processes, the stored CO2 may have different fates [17]. Primary consumers may directly or indirectly consume the biomass or deposit it deep into the sediments. The carbon in the biomass may be re-mineralized and emitted as CO2 or exported as dissolved inorganic carbon (DIC). The carbon in the biomass may also undergo export into an adjacent ecosystem as dissolved and particulate organic carbon (DOC and POC). These can either be precipitated in sediments or consumed by aquatic organisms.
The sources of CH4 emissions can be of three types: biogenic, thermogenic, and pyrogenic [15]. Biogenic sources include methanogenesis in wetlands, rice paddies, oxygen-poor reservoirs, the digestive systems of ruminants and termites, and waste deposit sites. Thermogenic CH4 forms over thousands of years through geological processes. CH4 comes into the open environment from the subsurface through natural features (terrestrial and marine seeps and mud volcanoes) and due to the exploitation of fossil fuels (coal, oil, natural gas, etc.). The pyrogenic sources refer to the incomplete combustion of biomass, soil carbon, biofuels, and fossil fuels [15]. Relatively fewer processes remove the CH4 released in the atmosphere. The primary sink of atmospheric CH4 is through chemical degradation, i.e., oxidation of CH4 by hydroxyl radicals (OH) in the troposphere, which accounts for 90% of the total global CH4 sink. The rest of the CH4 sinks are methanotrophic bacteria in the aerated soil [18,19], reactions with chlorine and atomic oxygen radicals in the stratosphere [20], and responses in the marine boundary layers [21].
Mangroves are among the most carbon-rich ecosystems on Earth [22,23], sequestering a large amount of carbon from the environment [24]. They are considered highly productive and carbon-dense ecosystems [25,26,27]. Globally, the rate of net primary production by the mangroves is 218 ± 72 Tg C yr−1 [28,29], approximately 1% of the carbon sequestration by the world’s forests [30]. Despite their limited distribution, mangroves play an enormous role in carbon sequestration with their high belowground root production and the ability to accumulate a large amount of organic matter in their substrates [31,32]. Hence, in the face of climate change and increased global CO2 and CH4 concentrations, the characterization of mangrove forests as carbon sources and sinks has gained added relevance [33]. Accurate quantification of the carbon pools and fluxes is crucial in developing mitigation strategies for reducing emissions from deforestation and forest degradation (REDD+) [34]. Several factors govern the net source or sink characteristics of a mangrove ecosystem. Transformation of the particulate and dissolved matter primarily creates a heterotrophic environment, eventually causing carbon emissions from the system [35,36,37,38,39]. However, net autotrophy in the New River Estuary of North Carolina, USA [40], and the estuaries on the southeast coast of Australia (viz. the Hastings River estuary, Camden Haven estuary, and Wallis Lake estuary) [41], caused these estuarine systems to behave as a net CO2 sink. Besides the biogeochemical characteristics such as trophic status [42] and net community production [43], the nature of geophysical processes in an estuary can also govern the CO2 flux. These include tidal amplitude [44,45], freshwater inflow [46,47], water residence time [48], connectivity of the system with salt marshes or tidal flats [8], and vertical stratification [49]. On the other hand, inundated wetlands rich in organic carbon tend to release a certain amount of CH4 into the surrounding environment. The emission rate depends on several climatic factors [50,51,52] along with some human-induced factors impacting methanogens and methanotrophs in the ecosystem [53,54,55]. Wetlands are the largest natural source of CH4, contributing about a third of the total global emissions (164 Tg yr−1) [50]. Soil temperature, water table, and the type of vegetation in the system often control the CH4 emissions from wetlands. Moreover, these relations vary across the wetland type (bog or swamp), the location of the wetland (temperate or tropical), and the level of disturbances the wetland experiences [51].
The Sundarbans mangrove ecosystem has always been a focal area of research in the recent past, considering the importance of mangrove forests in carbon sequestration discussed above. Overall, the CO2 and CH4 fluxes in the Indian Sundarbans come under the purview of three compartments, depending on the involvement of the carbon reservoirs and the direction of flux. They are: (i) atmosphere-biosphere flux (exchange of CO2 and CH4 between the mangrove forest and the atmosphere, i.e., above the forest canopy), (ii) soil-atmosphere flux (exchange of CO2 and CH4 between the exposed soil and the air below the canopy), and (iii) air–water flux (CO2 and CH4 exchange at the interface between the mangrove surrounding estuarine water and the atmosphere). Among these three types of fluxes, the present article reviewed the spatial and temporal dynamics of the air–water fluxes and the related carbon biogeochemistry of the estuarine water across the entire Sundarbans delta (Indian part).
Among the three types of carbon fluxes mentioned above, the air–water exchange is the most complex phenomenon as it involves several carbon parameters and their interplay with varied physicochemical, biogeochemical, and hydrological parameters. Unlike the mangrove canopy and the sediment surface, the aquatic column of the mangrove-adjacent estuaries furnishes a platform where several biogeochemical reactions coincide. Processes such as autotrophic utilization of aqueous CO2, degradation of POC and DOC, and the buffering capacity arising from the total alkalinity (TAlk) by DIC ratio can regulate the partial pressure of CO2 (pCO2) in water, which in turn governs the fluxes. Similarly, biochemical reactions carried out by methanogens and methanotrophs coupled with oxidation–reduction states of the water column control the partial pressure of CH4 (pCH4) in water and their fluxes. Aquatic carbon biogeochemistry has received substantially greater scientific attention in the Sundarbans estuaries owing to the intrinsic complexities of these concurrent mechanisms mentioned above. Many scholarly articles have come up in the past two decades in this regard to address multiple issues and research questions. Hence, we found it necessary to collate all this information acquired so far and synthesize the findings related to the carbon biogeochemistry of Sundarbans mangrove-dominated estuarine water, emphasizing the air–water CO2 and CH4 fluxes. The present review primarily focused on the physicochemical and a select few biological drivers that directly or indirectly govern these two fluxes. Our primary aim was to elucidate the spatiotemporal variability of the carbon biogeochemistry parameters and characterize the drivers of air–water CO2 and CH4 fluxes by reviewing the studies conducted in the Indian part of the Sundarbans (the estuaries adjoining the Indian Sundarbans).
2. Indian Sundarbans Mangrove Forest: A Brief Overview
Sundarbans is the world’s biggest continuous patch of mangrove forest, situated on the lower stretch of the Ganges–Brahmaputra–Meghna River delta system. It roughly covers an area of 10,200 km2, of which Bangladesh occupies 60% (Bangladesh Sundarbans Forest) and 40% remains in India (Sundarbans Biosphere Reserve) (UNESCO World Heritage Site: https://whc.unesco.org/en/list/798/, accessed on 2 October 2022). The Indian Sundarbans was declared a World Heritage Site and Biosphere Reserve by the International Union for Conservation of Nature (IUCN) and the United Nations Educational, Scientific, and Cultural Organization (UNESCO) in 1987 and 1989, respectively [56]. The Indian Sundarbans mangrove forest has one of the highest species diversity rates in the world, and it shelters several floras and faunas, including the iconic Royal Bengal Tiger. This mangrove forest provides many ecosystem services to the millions of people residing in its periphery. The reserve forest of the Indian Sundarbans covers an area of 4263 km2, including the core and buffer areas. The transitional zone covers an area of 5367 km2, densely populated with agricultural lands [57]. The soil texture in the Sundarbans varies from clayey to sandy loam to silty. The soil in the carbon-rich Sundarbans ecosystem accumulates 49–98% of the carbon storage within the depths of 0.5–3 m [26]. Several scholars have categorized the tropical climate in the Sundarbans as pre-monsoon (February to May), monsoon (June to September), and post-monsoon (October to January). The Sundarbans experience an average annual rainfall of ~1770 mm, 75% of which occurs during the monsoon. The ambient temperature shoots up to ~40 °C during the pre-monsoon season (in the summer months of May and June) and goes down to ~10 °C during the post-monsoon season (in the winter months of December and January). Samanta et al. [58] showed that between 2000 and 2020, the Indian Sundarbans lost 110 km2 of mangrove cover from the reserve forest area due to erosion. A genus composition study of the mangrove forest indicated a greater prevalence of salt-tolerant genera. The study showed an overall decline in forest cover and health conditions in the Indian Sundarbans. Increased salinity and temperature and reduced rainfall during pre-monsoon and post-monsoon periods were primarily responsible for such alterations in mangrove cover. Most rivers that fed this region with freshwater have become defunct, including Ichhamati, Jamuna, Bidyadhari, Noai, Suti, Kumarjol, Ghagramari, Karati, Thakuran, Bidya, Saptamukhi, and Matla [59,60]. The Hooghly River maintained the perennial freshwater flow into the Indian Sundarbans; however, the water flow is insufficient to rejuvenate the already decayed distributaries [59]. The Matla estuary is located in the central part of the Indian Sundarbans and flows through mangrove forests. The monsoon runoff and the tidal activity help to maintain its estuarine characteristics [61]. Both the Hooghly and Matla estuaries are meso-macro tidal, experiencing semidiurnal tides [62]. Besides the Hooghly and Matla Rivers, several other rivers flow through the Indian Sundarbans mangrove system, from west to east, such as the Mooriganga, Saptamukhi, Thakuran, and Bidya.
3. Overview of the Carbon Biogeochemistry Research in Indian Sundarbans
The Hooghly and Matla estuaries are the most studied regions in the Indian Sundarbans, a mangrove-dominated estuarine system (Figure 1). Mukhopadhyay et al. [63] studied the monthly variation of CO2 fluxes between the surface water of the Hooghly River and the associated atmosphere during 1999 (Table S1). Apart from CO2 fluxes, they also measured TAlk, salinity, dissolved oxygen (DO), gross primary production (GPP), and community respiration (CR). Later, Padhy et al. [64] developed an algorithm to compute the pCO2 and CO2 flux from remotely sensed data on sea surface temperature (SST) and chlorophyll a (Chl-a) concentration and in situ measurements in the Hooghly estuary. Several studies characterized the spatial and temporal (seasonal and diurnal) variations in CO2 fluxes in this region [65,66,67]. Akhand et al. [68] and Dutta et al. [69] compared the CO2 flux dynamics between the Hooghly and the Matla estuaries. The role of groundwater on inorganic carbon dynamics and pCO2 in the Hooghly and Sundarbans estuarine systems was revealed by Akhand et al. [70] using a coupled automated 222Rn and pCO2(water) measurement system. All these studies also measured associated carbon parameters such as dissolved inorganic carbon (DIC) (Table S2), dissolved organic carbon (DOC), and particulate organic carbon (POC) (Table S3), along with pH, salinity, SST, TAlk, wind speed, and Chl-a. Besides the Hooghly and Matla estuaries, the Mooriganga, Saptamukhi, and Thakuran estuaries also received substantial attention regarding carbon biogeochemistry [71,72,73,74,75]. Several researchers also covered parts of Edward Creek, Herobhanga River, and Lothian Island [73,76] (Table S4). Relatively fewer studies are available on CH4 dynamics in the Indian Sundarbans (Table S5). Biswas et al. [77] studied the CH4 flux dynamics covering the Sundarbans estuaries (Muriganga, Saptamukhi, and Thakuran Rivers), Lothian Island, and the Hooghly estuary (Diamond Harbour, Kachuberia, Beguakhali) in 2003. This study reported the spatial and temporal changes in the CH4 fluxes. Neetha [78] compared the CH4 flux between Jharkhali Island (Sundarbans estuary) and the Hooghly estuary. Later, Dutta et al. [69,79,80,81,82] studied the CH4 flux around Lothian Island, the Hooghly estuary, and the Saptamukhi estuary.
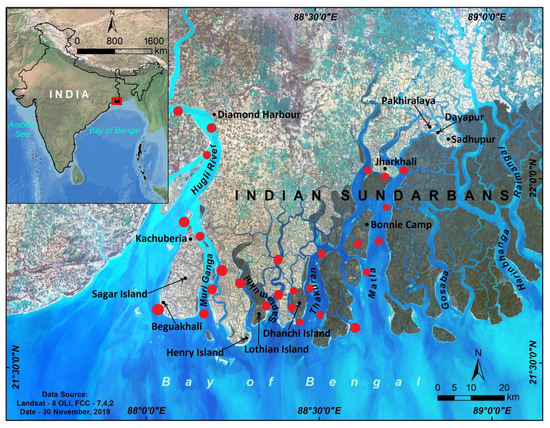
Figure 1.
The Indian Sundarbans mangrove ecosystem and the adjacent estuaries. Black dots refer to the study locations, and the red dots denote the sites sampled in different studies and considered in this review article. The inset map shows the location of the Indian Sundarbans.
4. Carbon Parameters and Total Alkalinity in Estuarine Water
Carbon parameters (DIC, DOC, and POC concentrations), TAlk, and other physicochemical parameters can regulate CO2 and CH4 fluxes. Researchers have extensively used the bulk formula method to estimate air–water CO2 and CH4 exchange rates in the Indian Sundarbans [83,84]. Computation of pCO2(water) requires SST and sea surface salinity data, along with knowledge of DIC and TAlk (expressed in moles per kilogram of seawater), because the concentration of DIC and TAlk remains constant with changes in water temperature and pressure [85]. Different biogeochemical processes can influence the concentration of DIC in an aquatic system. Cycling of DIC is related to the production and utilization of CO2 through photosynthesis and respiration, i.e., the metabolic state of an estuary [86].
Apart from the heterogenic activities in a system, DIC can originate from riverine and groundwater inputs, inputs from inter-tidal wetlands, and photodegradation of dissolved organic matter [87,88,89,90]. Hence, to understand the net autotrophy (sink) or heterotrophy (source) nature of an estuary or an open water system, analysis of CO2 saturation is insufficient [91]. Thus, a detailed understanding of the sources, cycling patterns, and fate of DIC in the estuaries is needed to analyze the biogeochemical cycling of carbon and develop carbon budgets for coastal systems.
A significant fraction of the organic matter in estuarine and oceanic water remains dissolved [92,93,94]. Estuaries receive organic matter from adjacent land masses and local sources [95]. In the estuarine mixing processes, the terrestrial organic carbon undergoes rapid removal and decomposition [96]. Biodegradation is one of the primary processes responsible for removing DOC from estuarine and marine waters [97,98,99]. In many estuarine systems, respiration exceeds primary production [35,100], and the available organic matter is wholly or partially mineralized [98,99,101]. Thus, higher DOC may correlate with higher pCO2 concentrations [102].
Globally, most of the estuaries are net sources of CO2, and along with DIC and DOC, land-derived POC plays a vital role in determining that characteristic [103]. Annually, mangroves export 28 Tg C of POC to the sea. Plant debris, phytoplankton, and microphytobenthos generate this POC [104]. This POC in estuarine water causes heterotrophic activity and influences the DIC composition in seawater through mineralization [35,105].
The TAlk represents the inorganic carbon content in the aquatic body. It is the sum of bicarbonate, carbonate, borate, and hydroxide ions [83]. TAlk, when produced more than DIC, is often considered to increase the sinking potential for atmospheric CO2 since it increases the carbonate buffering capacity of seawater [106]. Rivers are the primary transporters of inorganic carbon [107]; moreover, they are a potent source of the same [48,108,109,110]. The coastal ocean’s source/sink characteristics depend on carbonate buffering capacity. Freshwater discharge, agricultural runoff, and groundwater discharge primarily regulate this buffering capacity [111,112]. Human activities such as deforestation, excessive agricultural practices, and events such as acid rain deposition cause a reduction in the carbonate buffering capacity of coastal systems [113,114]. Hence, along with other carbon parameters, the TAlk dynamics study is crucial in understanding coastal water’s CO2 source/sink behavior.
4.1. Spatial Variability
This region exhibited significant spatial variability in the carbon parameters and TAlk. Several studies have compared the DIC content of the anthropogenically disturbed Hooghly estuary with the less influenced Saptamukhi, Thakuran, and Matla estuaries (the Sundarbans estuary). They all reported higher DIC content in the Hooghly estuary than the others (Table S2). Ray et al. [84] observed similar findings from the Hooghly estuary and Lothian Island. Akhand et al. [70], while studying the influence of submarine groundwater discharge (SGD) on the DIC of estuarine water, reported about four times higher DIC content in the groundwater compared to the estuarine surface water. Though DIC has been well sampled throughout the spatial extent of this region, more endeavors are needed to measure the stable isotopic signatures of DIC to develop a better understanding of the DIC sources in this estuarine complex.
Compared to the DIC, fewer studies report DOC dynamics from the Indian Sundarbans region (Table S3). Ray et al. [73,84], Dutta et al. [67,69], and Akhand et al. [66,70] compared the DOC values for the Hooghly and Sundarbans estuaries. All of the studies documented similar values of DOC for the Hooghly and Sundarbans (Saptamukhi, Thakuran, and Matla) estuaries. According to the study by Ray et al. [73], rather than the DOC formation and decomposition rates, the lower residence time of water in the mangroves was responsible for the low value of DOC in the Hooghly and Sundarbans estuaries. Akhand et al. [70] reported the surface water and groundwater DOC in the Hooghly and Matla estuaries. In the Matla estuary, DOC in groundwater was significantly lower than the surface water. Thus, groundwater did not prove to be a significant source of DOC in the Sundarbans estuarine system. As mangrove habitats are considered the primary source of DOC in mangrove-adjacent estuaries, studies focusing on DOC variability from the mangrove island periphery to open estuarine channels are required to understand the rate of DOC dilution in these waters.
Ray et al. [73,84] compared POC between the Hooghly and Saptamukhi estuaries (Table S3). The studies suggested that POC in the Saptamukhi estuary has an autochthonous origin and that the Hooghly estuary is the primary source of terrestrial organic matter draining into Saptamukhi. The relatively higher POC in the Hooghly estuary compared to the Sundarbans estuaries (Saptamukhi, Matla) was attributed to increased freshwater flow in the estuary from upstream of the Hooghly River [66]. However, such measurements are required in all the major estuaries of this region to generate a more holistic scenario on this issue.
Several researchers studied the buffering capacity of the mangrove-dominated estuarine waters of the Indian Sundarbans (Table S2). Akhand et al. [76] studied the spatial variability of TAlk in the inner (Herobhanga), middle (Thakuran), and outer (Edward) estuaries. They did not observe variations in the TAlk values between the inner and middle estuaries; however, the outer estuary exhibited lower TAlk values. Akhand et al. [68] also reported similar results, where inner estuarine stations showed higher TAlk values than outer estuarine stations. Ray et al. [73] studied TAlk variability along the salinity gradient of the Hooghly estuary, the mangrove-dominated Saptamukhi estuary’s adjacent coastal waters of the Bay of Bengal, in 2014. However, they reported a narrow range of TAlk for both regions. Ghosh et al. [83] studied the Hooghly estuary (from the upper to the lower estuary) and the adjacent coastal waters during 2015–2016. They observed TAlk values decreasing from the upper to the lower estuary. Dutta et al. [67,69] studied TAlk variations in the Hooghly and Sundarbans estuaries and reported no spatial differences. However, the Hooghly estuary exhibited wider variations. Akhand et al. [70] compared the TAlk in the surface water and groundwater between the Hooghly and Matla estuaries. Both surface water and groundwater TAlk were marginally higher in the Hooghly estuary. Compared to surface water, TAlk was significantly higher in groundwater for both regions. Acharya et al. [71] studied TAlk in the Saptamukhi, Thakuran, and Matla estuaries, and they did not record any significant variation.
Several researchers have studied pCO2 in the Indian Sundarbans (Table S4). Sea surface temperature (SST) influences the pCO2(water) by decreasing the solubility of CO2 in water [115]. The pCO2(water) in the estuary decreased with proximity to the open sea [76]. pCO2(water) in Edward creek (0 km from the shoreline), Thakuran River (31 km from the shoreline), and Herobhanga River (55 km from the shoreline) were 507.98 ± 73.69 µatm, 518.84 ± 71.94 µatm, and 234.34 ± 64.08 µatm, respectively; whereas, pCO2(air) showed comparatively less variability (386.73 ± 1.97 µatm, 386.80 ± 5.05 µatm, and 376.26 ± 2.75 µatm, respectively). Ray et al. [84] studied the pCO2 in the riverine (992.5 µatm) and marine parts (372 µatm) of the Hooghly estuary and reported similar findings. Akhand et al. [68] compared the pCO2(water) in the Hooghly and Matla estuaries during 2013–2014. Being an anthropogenically influenced estuary with a high river discharge, the annual mean pCO2(water) value for the Hooghly estuary (~2200 µatm) was higher than the comparatively less anthropogenically disturbed Matla estuary (annual mean ~530 µatm). Dutta et al. [67] reported similar observations in the Hooghly (267 µatm to 4678 µatm) and Sundarbans estuaries (376 µatm to 561 µatm). pCO2 for the Hooghly estuary reduces gradually towards the offshore region. However, they did not report any spatial variation for the Sundarbans estuary [67]. Acharya et al. [71] studied the Saptamukhi, Thakuran, and Matla estuaries (Sundarbans estuary) from 2016 to 2020 and reported an average annual value of 993 ± 898 µatm. Though a substantial number of studies have been carried out in this regard, most endeavors indirectly estimated pCO2(water), which adds to the uncertainties in flux computation. More endeavors are needed to measure pCO2(water) directly and continuously through the deployment of automated sensors.
Several researchers characterized the spatial variation in CO2 flux between air and water (Table S4). Padhy et al. [64] measured the CO2 flux covering the entire Hooghly estuary. They observed significant variation in CO2 flux from the seaside to the upstream region due to the estuary’s north–south orientation. The estuary acted as a source with variable magnitude (90 mol C m−2 year−1 during summer and 0.5 mol C m−2 year−1 during winter). According to Akhand et al. [76], the inner (Edward Creek) and middle estuary (Thakuran River) acted as net sources (29.69 and 23.62 mg CO2 m−2 day−1 respectively), while the outer estuary acted as a net sink (−33.37 mg CO2 m−2 day−1). The heterotrophic nature of the water column and the sediments [116,117] added to community respiration in the mangrove estuary, which largely contributed to the CO2 source behavior of the inner and middle estuaries. At the same time, the dominant autotrophic characteristics of the outer estuary govern its sink behavior. Akhand et al. [68] compared the water-to-air CO2 emissions from the Hooghly and Matla estuaries. They reported that the annual mean CO2 emission from the Hooghly estuary was 14 times higher than that of the Matla estuary. The increased river runoff due to high river discharge was primarily responsible for the increased CO2 efflux from the Hooghly estuary during the last decade [68]. Dutta et al. [67,69,72] reported similar observations on CO2 efflux while working in the Hooghly and Sundarbans estuaries. Bacterial abundance in the highly nutrient-rich water of the urbanized river stretches triggered higher respiration and rapid mineralization [118], which in turn caused CO2 supersaturation and higher CO2 efflux [119,120]. Akhand et al. [65] compared the nature of CO2 flux between the creeks of Dhanchi Island and the adjacent estuary. According to the study, the creeks acted as net sources of CO2, while the estuary was a net sink for CO2. Similar to pCO2(water), fluxes are estimated in most studies, which leaves a substantial scope of uncertainties. Direct flux measurements should be undertaken by future researchers by deploying chamber techniques to reduce the uncertainty in flux estimations.
Compared to other carbon parameters, fewer studies exist on the CH4 dynamics and air–water flux in Sundarbans estuarine water (Table S5). Biswas et al. [77] studied the spatial dynamics of CH4 in the Hooghly River estuary; in the stations, Diamond Harbour (inner estuary), Kachuberia (tip of Sagar Island, mid estuary), and Beguakhali (sea end/river mouth/outer estuary). Saptamukhi, Mooriganga, Thakuran, and the adjacent waters of the Lothian Island, were also studied. pCH4 in the Hooghly estuary was lower than in the Saptamukhi, Mooriganga, and Thakuran river systems, indicating the latter received a higher input of methane-rich waters from the surrounding mangroves. Dutta et al. [80] measured pCH4 in the surface water and porewater in the Saptamukhi estuary adjacent to Lothian Island. Compared to the groundwater, pCH4 was marginally higher in the surface water. Dissolved CH4 in water and CH4 flux were compared between the Hooghly and Sundarbans estuaries by Dutta et al. [69] during the pre-monsoon of 2016. The CH4 concentration in the Hooghly estuary varied over a broader range than in the Sundarbans estuary. In both estuaries, air–water CH4 flux was always positive, suggesting efflux from water to air. In the Hooghly estuary, maximum and minimum CH4 flux was reported from the freshwater and marine zones, respectively.
Table 1 summarizes the range of all the parameters reviewed in this study in the inner, middle, and outer estuaries of the Indian Sundarbans. TAlk and DIC exhibited almost similar spatial variability in these estuaries. The minimum observed concentrations of these two parameters were similar in the inner, middle, and outer estuarine reaches. However, the maximum values decreased from the inner to the outer estuarine regions. The decrease in DIC from the inner to outer regions was higher than the corresponding decrease in TAlk. This observation again indicates the influence of the coastal waters having a high TAlk/DIC ratio, implying a high buffering capacity. The DOC range in the inner estuarine region was significantly higher than the middle and outer reaches, primarily due to the allochthonous anthropogenic inputs from the upper reaches. The POC, on the contrary, showed higher ranges in the outer estuarine reaches. This observation indicates the presence of autochthonous POC derived from the mangrove environment and allochthonous POC outflown, primarily through the Hooghly estuary. pCO2(water) and air–water CO2 flux mirrored each other in their spatial variabilities, which indicates that pCO2(water) is the fundamental parameter that regulates the air-water CO2 fluxes in these estuaries. The maximum values of pCO2(water) and air–water CO2 flux steadily decreased from the inner estuarine reaches to the outer region. This observation again unequivocally indicates that the seawater low in pCO2(water) having a high buffering capacity leads to lower air-water CO2 fluxes in the outer regions compared to the inner reaches, where freshwater plays a dominant role. CH4 concentration in water and air–water CH4 flux showed a similar spatial trend. The inner estuarine region, having higher organic carbon than the middle and outer reaches, provided ample substrate for methanogenic activities, which led to higher dissolved CH4 levels in the water and eventually higher air–water CH4 fluxes.

Table 1.
The range (minimum to maximum) of TAlk, DIC, DOC, POC, pCO2(water), air–water CO2 flux, CH4 concentration in water, and air–water CH4 flux observed in the inner, middle, and outer estuarine reaches of the Indian Sundarbans.
4.2. Temporal Variability
This section discusses the diurnal and seasonal variabilities of the carbon biogeochemistry-related parameters reported by several studies. Padhy et al. [64] studied the DIC in the Hooghly estuary (Table S2) and the adjacent coastal waters following an empirical equation [121]. They reported lower values in May and November 2014 (990 ± 27 μmol kg−1 and 1130 ± 29 μmol kg−1, respectively) and relatively higher values in January, February, and September 2014 (1310 ± 107 μmol kg−1, 1711 ± 19 μmol kg−1, and 1697 ± 77 μmol kg−1, respectively). The relative riverine water fluxes, which bring terrestrial organic sources into the estuarine system, influenced the lower and higher DIC in the respective months. Dutta et al. [67,69] studied DIC variability in the Hooghly and Sundarbans estuaries during pre-monsoon and post-monsoon. A 6% increase in DIC was observed during pre-monsoon in the Hooghly estuary, while DIC did not vary much in the Sundarbans estuary seasonally. Ray et al. [84] also reported marginally higher values of DIC in the Hooghly estuary during pre-monsoon than post-monsoon. However, Akhand et al. [66] showed that pre-monsoon DIC concentrations were significantly higher than the monsoon and post-monsoon concentrations.
Dutta et al. [72] studied the diurnal dynamics of DOC in the Sundarbans estuary. DOC showed non-conservative behavior during low tide and conservative behavior during high tide (Table S3). The moderately significant relation between DOC and salinity indicated that the DOC in the Sundarbans estuarine system remains regulated by processes other than estuarine mixing. The biogeochemical and physiological processes, along with the photo-oxidation in the mangrove system, were suggested to regulate the diurnal fluctuations in DOC. Dutta et al. [67,69] reported the seasonal changes in DOC during the pre-monsoon and post-monsoon seasons. In the Hooghly and Sundarbans estuaries, the pre-monsoon DOC concentrations were 48% and 45% higher than the post-monsoon DOC concentrations. However, Akhand et al. [66] did not observe any such trend in the DOC.
Dutta et al. [69,72] reported the seasonal and diel changes in POC, respectively (Table S3). In the Hooghly estuary, POC was higher by 52% during pre-monsoon than post-monsoon, while in the Sundarbans estuary, POC was higher during post-monsoon (by 62%) when compared to pre-monsoon. The diurnal study showed marginally higher POC in the Sundarbans estuary during the daytime. A seasonal study by Ray et al. [84] also reported higher POC in the waters surrounding Lothian Island during post-monsoon (45.4 ± 7.5 µM) than pre-monsoon (28.0 ± 8.6 µM). The diurnal variation study of POC [84] showed an increasing POC trend with increasing water height. Like DIC, Akhand et al. [66] reported significantly higher POC levels in the Matla estuary during the pre-monsoon season compared to the other two seasons.
The temporal variability of TAlk is a relatively less documented topic in the carbonate chemistry research in the Indian Sundarbans (Table S2). Akhand et al. [68] studied the seasonal variation of TAlk in the Hooghly and Matla estuaries, which showed lower values of TAlk during the monsoon in the inner estuarine stations from both study areas. Ghosh et al. [83] reported similar observations from the Hooghly estuary (from the upper to the lower estuary) and the adjacent coastal waters. The TAlk values were highest during the pre-monsoon and lowest during the monsoon. Dutta et al. [72] studied the diurnal variability of TAlk in the Sundarbans estuary (Saptamukhi, Thakuran, and Matla estuaries), which varied over a narrow range (2.19 to 2.58 m equiv/l). Akhand et al. [66] also reported similar observations from the Matla estuary (2178 to 2273 μmol kg−1). They sampled twice during the two consecutive pre-monsoon seasons of 2017 and 2018 and observed significantly higher mean TAlk than in the monsoon and post-monsoon seasons.
Biswas et al. [74] measured the diurnal variation of pCO2 in the Mooriganga, Saptamukhi, and Thakuran estuaries in 2001 (Table S4). pCO2(air) varied with the time of day (minimum at 1200 h and maximum at 2100 h) and ranged between 403 µatm and 635 µatm, respectively. The pCO2(water) was highest at 0600 h (1062 µatm) and lowest at 1500 h (416 µatm). While mangrove plant cover, wind velocity, and turbulence influenced the pCO2(air), tide-driven changes in salinity regulated the pCO2(water) variation. Padhy et al. [64] studied pCO2 in the Hooghly estuary and the adjacent coastal waters during the winter and summer of 2008. During winter, pCO2(water) ranged between 320 µatm and 500 µatm in the coastal domain and 340 µatm to 375 µatm in the upstream river. However, during summer, pCO2(water) was spatially uniform (about 450 µatm). Changes in tidal circulations and physicochemical properties along the estuary (from the upstream river to the offshore estuary) caused spatial variation in pCO2(water). Ray et al. [84] studied pCO2 in the adjacent waters of Lothian Island and throughout the Hooghly River (from the upstream riverine part to the river mouth) during the pre-monsoon and post-monsoon of 2014. The study reported comparatively higher values of pCO2 during the pre-monsoon season. Akhand et al. [66] reported the highest mean pCO2(water) during the monsoon and the lowest during the post-monsoon season.
Mukhopadhyay et al. [63] studied the air–water CO2 flux along the Hooghly estuary in 1999. The study indicated the estuary as a source of CO2 during the pre-monsoon (the maximum emission rate being 84.4 mmol m−2 d−1), and the role reversed during the monsoon (the maximum influx rate being −2.78 mmol m−2 d−1). (Table S4). Biswas et al. [74] studied the diurnal variation of air–water CO2 flux in the Sundarbans estuary (Mooriganga, Saptamukhi, and Thakuran estuaries) in 2001. The study showed a distinct diurnal variation in the CO2 flux (from −16.2 to 49.9 µmol m−2 h−1). Ambient and sea surface temperatures, daylight hours, and the rate of photosynthetic activity influenced the rate of CO2 flux. Akhand et al. [76] studied the diurnal variation in CO2 flux in the Edward Creek, Thakuran River, and Herobhanga River estuaries for their distance from the coastal waters. The study provided insight into the changing behavior of the estuarine system in terms of CO2 flux from the inner to outer estuarine regions. The findings about the diurnal variability were similar to those observed by Biswas et al. [74]; however, apart from the photosynthetic activity and respiration, the semidiurnal tidal cycles were responsible for the variations. Chen et al. [122] also observed that the strong CO2 source nature in the upper estuaries often turns into a CO2 sink character in the outer estuarine river plumes. In line with the pCO2(water)-related observations made by Akhand et al. [66], they reported air–water CO2 fluxes decreasing from monsoon to pre-monsoon, followed by post-monsoon seasons.
Biswas et al. [77] measured the seasonal changes in CH4 concentration in water and air–water CH4 flux in the Hooghly estuary (from the inner riverine zone to the river mouth), the Saptamukhi, Mooriganga, and Thakuran estuaries, and the adjacent waters of Lothian Island. Seasonal variability of CH4(air) was prominent; however, it spanned over a narrow range (Table S5). The CH4(water) was maximum during the post-monsoon and minimum during the monsoon season. Increased dissolved CH4 concentration was during high tide and vice versa. As observed by other researchers, the CH4 concentration in estuarine rivers generally shows a decreasing trend from freshwater to saltwater regions [123,124,125]. However, in the Hooghly estuary, the CH4 concentration was observed to increase in the lower stretch of the estuary. Apart from the riverine source, the mangrove ecosystem in the lower stretch of the estuary acted as an additional source of CH4; hence, the CH4 concentration increased. The primary peak in CH4 flux was during the post-monsoon, and a secondary peak was during the monsoon. The residence time of CH4-rich riverine water in the estuary plays an essential role in CH4 flux. Dutta et al. [81,82,85,86] recorded similar results from the Saptamukhi estuary. CH4 concentration was maximum during the post-monsoon and minimum during the pre-monsoon. CH4 flux was water-to-air throughout the year, and maximum efflux occurred during the monsoon and minimum during the pre-monsoon. Padhy et al. [126] studied the CH4 emissions from the surrounding waters of degraded mangroves. Based on remote sensing analysis, they considered Sadhupur, Pakhiralaya, and Dayapur as degraded mangrove sites for their study. They measured air–water CH4 fluxes in stagnant water, during tidewater, and after tidewater. In all the locations, the CH4 concentration and the CH4 flux were higher in stagnant water during the monsoon.
Though an adequate number of studies characterized the seasonal variability of the carbon biogeochemistry parameters discussed above, long-term temporal monitoring over the course of decades at a fixed location is absent from this region. Systematic monitoring of these parameters in high and low-tide sessions during spring tide and neap tide phases over multiple years would enable us to characterize the changes in the concentrations of these parameters over time. Such prolonged monitoring would allow us to correlate the changes in concentrations of these parameters with climate change indicators and/or anthropogenic activities. In the absence of such long-term monitoring, the role of climate change and/or anthropogenic activities in governing the carbon biogeochemistry in these regions remains unanswered.
Table 2 summarizes the range of all the parameters reviewed in this study in the pre-monsoon, monsoon, and post-monsoon seasons. The maximum concentrations of TAlk and DIC occurred during the pre-monsoon season, followed by the post-monsoon and the monsoon seasons. This observation indicates that these two parameters remained high during the dry seasons (pre-monsoon and post-monsoon) and underwent dilution in the monsoon season. DOC and POC also showed similar trends, which indicates that during the dry season, when evaporation rates remain high with minimal freshwater discharge from the upper reaches, the estuarine water mass remains enriched with both of these forms of organic carbon. However, during the monsoon, these values remain lower primarily due to the dilution effect in the presence of voluminous freshwater flow. pCO2(water), on the contrary, exhibited higher concentration ranges during the monsoon season. The dominance of riverine freshwater and catchment flow triggered by the monsoon-induced rainfall reduces the pH of the estuarine water column, which facilitates a higher pCO2(water) than that observed in the dry seasons. Air–water CO2 fluxes reciprocated accordingly, and the highest flux ranges occurred during the monsoon, followed by the pre-monsoon and post-monsoon seasons. The pre-monsoon season, which coincides with the summer months, experiences enhanced temperatures that reduce the solubility of CO2 in the estuarine water and give rise to substantially higher magnitudes of air–water CO2 flux. However, the effect of enhanced temperature (during the pre-monsoon season) could not overrule the effect of lowered pH levels (during the monsoon season) in regulating pCO2(water). In the case of CH4 concentrations, however, the pre-monsoon season exhibited higher ranges, as did the air–water CH4 fluxes. This observation showed that higher temperatures activated methanogenic activities more in the pre-monsoon season than in the other two seasons.

Table 2.
The range (minimum to maximum) of TAlk, DIC, DOC, POC, pCO2(water), air–water CO2 flux, CH4 concentration in water, and air–water CH4 flux observed in the pre-monsoon, monsoon, and post-monsoon seasons in the Indian Sundarbans.
5. Factors Regulating Air-Water CO2 and CH4 Flux
Various factors governed the air–water CO2 and CH4 flux in the Indian Sundarbans mangrove system. For ease of discussion, we categorized them into three major types: physical, biogeochemical, and hydrological.
5.1. Role of Physical Factors
Water temperature, wind speed, tidal activity, rainfall, and photosynthetically active radiation (PAR) are critical in regulating CO2 and CH4 concentrations in water and, subsequently, the CO2 and CH4 fluxes. Water temperature is known to influence CO2 solubility in seawater [127] and, subsequently, the variations of pCO2(water) [128]. Almost all the aforementioned studies that monitored the seasonal variability of pCO2(water) and air–water CO2 fluxes observed higher magnitudes of both these parameters during the pre-monsoon season compared to the post-monsoon season [66,68]. However, attributing only temperature as the sole factor in regulating pCO2(water) would be erroneous, as several other interlinked physical factors play crucial roles.
The availability of PAR regulates autotrophic activities in estuarine water [129]. Reduced PAR causes dominance of heterotrophy and induces CO2 efflux [130]. Increased turbidity or total suspended matter in the seawater reduces PAR penetration in the water column [131], increasing the CO2 source potential of seawater. Akhand et al. [68] measured PAR in the Hooghly and Matla estuaries. They reported a significant difference in PAR between the two estuaries, with substantially lower PAR in the Hooghly compared to the Matla estuary. This low PAR was mainly due to the high turbidity levels in Hooghly compared to Matla. Sadhuram et al. [132] reported that the estuarine water column of the Hooghly remains highly turbid almost throughout the year due to strong tidal and wave actions, which leads to the bottom-churning of sediments. Tidal cycles also play a crucial role in regulating pCO2(water) as they cause the physical mixing of the seawater with the riverine freshwater. Akhand et al. [65,68] observed that freshwater from upstream had a higher pCO2(water), whereas the seawater encroaching during the high tide had a low pCO2(water). Thus, it is an essential driving factor for CO2 flux. During high tide phases, due to the dominance of seawater in the estuary, CO2 efflux remains low.
The CH4 concentration in estuarine water also varies with tidal cycles. During high tides, flushing the mangrove swamp brings large quantities of CH4 into the estuary, causing an increased CH4 concentration in the water [77]. Wind speed is another physical forcing factor that regulates flux magnitude. Higher wind speed facilitates CO2/CH4 exchange at the air–water interface, increasing flux magnitude [76]. Rainfall also plays a vital role in regulating flux in the estuarine region. Increased rainfall leads to increased river runoff and more drainage of mangrove porewater into the estuary. Thus, higher CO2 efflux generally prevailed during the monsoon seasons [66,68]. During the dry seasons, high levels of air pollution from the urban sectors are advected into the coastal regions, causing a gradual decrease in CH4 concentration in the air due to atmospheric oxidation [77].
5.2. Role of Biogeochemical Factors
Salinity is one of the significant factors that govern CO2 flux. Higher salinity means the dominance of seawater, where pCO2 is low [133]. Thus, CO2 efflux from the seawater also remains low. Decreased salinity in a coastal region refers to an increased river runoff or pore water discharge, rich in pCO2, promoting CO2 efflux [134]. The DIC in water mainly remains in carbonate and bicarbonate forms. Lower pH induces excess DIC to transform into a gaseous form, which eventually releases into the surrounding atmosphere through efflux. Akhand et al. [66] and Dutta et al. [72] observed a significant negative correlation between pCO2(water) and pH.
The carbonate buffering capacity of estuaries depends on the TAlk/DIC ratio [135]. The higher this ratio, the lower the potential of CO2 emissions from the estuaries. Akhand et al. [65] observed that the northern Bay of Bengal waters has high TAlk/DIC, which enhances the carbonate buffering capacity within the estuaries during the high tide. This phenomenon prevents the conversion of carbonate and bicarbonate in water into excess DIC, reducing CO2 efflux from water to air. Chen et al. [136] also reported that mixing with seawater is the primary cause for the pCO2 to decrease near the river mouths. Akhand et al. [65] also noted that DOC and POC degradation to DIC occurs in the Sundarbans estuaries primarily through denitrification. The mangrove pedosphere offers a platform where organic matter degradation eventually enhances the DIC levels in the adjacent estuary, reducing carbonate buffering capacity [116].
Salinity shows a strong negative correlation with CH4 concentration in water, indicating riverine freshwater is the primary source of CH4 [137]. In several instances, higher chlorophyll concentrations also lead to higher levels of dissolved CH4 in water. Higher chlorophyll ensures increased productivity (provided all the other parameters such as water surface temperature, PAR, salinity, and pH are optimum), which eventually increases the organic matter supply to the sediments, and this causes increased methane production by methanogens [138].
5.3. Role of Hydrological Factors (Pore Water, Groundwater, and Freshwater Discharge)
Compared to the surface water, pCO2 and pCH4 in the pore water remain several folds higher [119]. Pore water and groundwater are often responsible for the high pCO2 and pCH4 in mangrove estuaries during low tide [139]. Microbial and root respiration in the rhizosphere and anaerobic methane emission driven by methanogens enhance the CO2 and CH4 levels in the porewater [9,79]. Das et al. [140] reported that porewater from the mangrove sediments of the Sundarbans has substantial potential to enhance the pCO2 and pCH4 in the adjacent estuaries. Studies indicate that pore water outflux from the sediments caused pCO2 to be high in the creeks during low tide [65]. However, this effect of pore water is not visible in the estuaries, a) due to the higher volume of water in the estuaries, which dilutes the pore water, and b) because the higher residence time of water in the creeks causes organic matter degradation, which further increases pCO2. Higher CH4 concentration in the estuarine water during post-monsoon is caused by the flux of pore water from the mangrove swamp sediments after monsoon rainfall and higher litter fall during post-monsoon [77]. This pore water laden with CH4 increases the CH4 concentration in estuarine water, causing maximum CH4 flux in the post-monsoon season.
Groundwater discharge is another process that influences the pCO2 in estuarine and oceanic waters [141]. Groundwaters are generally rich in CO2 [142]. Thus, it can significantly increase the pCO2(water) and CO2 efflux in the discharged area. Groundwaters are also laden with nutrients and organic matter, which further increase the organic load in the water, increasing pCO2. However, Akhand et al. [70] did not observe any significant difference in DOC between groundwater and surface water in the Hooghly and Sundarbans estuaries. Groundwaters are also high in TAlk and DIC content, adding to CO2 flux. Akhand et al. [70] found TAlk and DIC in recirculated and fresh groundwaters four times higher than the surface waters in the Hooghly and Matla estuaries. They also observed that low-tide sessions had higher Radon-222 signatures, indicating higher groundwater contribution, which coincided with higher pCO2(water). Akhand et al. [70] could not quantify the submarine groundwater discharge in the Sundarbans estuaries and, hence, could not assess the exact contribution of groundwater in enhancing estuarine pCO2(water). However, their findings strongly indicate that groundwater seepage in these estuaries could play a pivotal role in regulating its pCO2(water) and air–water CO2 fluxes, as groundwater samples had a significantly higher TAlk/DIC ratio than surface waters, which can significantly increase the estuarine water’s buffering capacity as well. Freshwater discharge from the upper reaches is crucial in regulating the pCO2 dynamics in estuarine regions worldwide [143]. In the Indian part of the Sundarbans (where carbon-biogeochemistry-related studies mostly concentrate), freshwater mainly comes from the Hooghly estuary on the western margin and the Raimangal in the east. The estuaries that flow through the central part of this mangrove forest receive minimal freshwater and retain their estuarine character mainly through monsoon-induced rainfall and runoff from upper catchment areas [61]. Akhand et al. [68] explicitly portrayed that higher riverine discharge into the Hooghly estuary led to four times higher pCO2(water) and fourteen times higher CO2 emission in the Hooghly compared to the Matla estuary, which does not receive much freshwater and flows through the central part of the Sundarbans. Akhand et al. [68] also correlated the observed monthly mean pCO2(water) and air–water CO2 flux in the Hooghly estuary with the monthly mean freshwater discharge reported by Rudra [144]. They observed that both mean pCO2(water) and air–water CO2 flux increased with freshwater discharge. The highest CO2 effluxes were during the monsoon season, when the discharge peaked, and vice versa. Akhand et al. [68] concluded an undeniable role of riverine allochthonous DIC and organic matter input that enhanced pCO2(water) with increasing riverine freshwater discharge. The same holds for Sundarbans estuaries, as well; however, riverine freshwater input data for the Sundarbans estuaries are unavailable, which barred several researchers from quantifying the role of riverine freshwater in governing the carbon dynamics in these estuaries.
The existing set of studies was, to a large extent, successful in characterizing the role of physical, biogeochemical, and hydrological factors in regulating CO2 and CH4 fluxes; however, future research should strive to acquire more data, as sampling number and frequency are the two major constraints at present. Given the vast expanse of this estuarine complex, the sampling stations should be randomly distributed throughout the estuarine system, and future research should focus on high-temporal-resolution sampling from each station to increase the number of observations that can reduce the uncertainties in the observed relationships.
5.4. Role of the Microbiomes in Carbon Dynamics of Sundarban
Microbiomes play a crucial role in carbon dynamics and biogeochemistry through the degradation of organic matter and through mineralization pathways. The CO2 and CH4 evasion caused by the microbiome-dependent processes mainly facilitates offsetting the carbon sequestration ability in the mangrove ecosystem, especially in the adjacent soil and water. In the Sundarbans, the microbiomes concerning carbon dynamics and sequestration have been relatively poorly studied compared to other counterparts of the mangrove ecosystem. Ghosh and Bhadury [145] studied coastal bacterioplankton diversity concerning biogeochemical cycling on the largest island of the Indian Sundarban delta, Sagar Island, using a 16S rRNA clone library and Illumina MiSeq techniques. They found several sequences belonging to Sphingomonadales, Chromatiales, Alteromonadales, Oceanospirillales, and Bacteroidetes, which might have a regulatory role in coastal carbon cycling. Among the bacterioplankton, an abundance of Synechococcus sp. during the monsoon period signified the role of oxygenic photoautotrophs in the coastal carbon cycling of the Sundarbans. Bhadury and Singh [146] further confirmed the dominance of Synechococcus-like 16S rRNA sequences and the influence of small-sized picocyanobacterial cells in regulating carbon export in the Sundarbans’ mangrove ecosystems while working in the Mooriganga estuary.
They also reported other cyanobacterial sequences showing taxonomic affiliation with members of the Chroococcales, Pleurocapsales, Oscillatoriales, and Stigonematales. Das et al. [147,148] reported that organic carbon was the most significant factor that regulated the total microbial population in the mangrove-adjacent sediment. They also depicted that cellulose-degrading bacteria dominated throughout the year in the sediment, which might have a decisive role in the mineralization and decomposition processes, majorly affecting the carbon biogeochemistry of the Sundarbans. Mukherjee et al. [149] conducted a post-monsoonal study in the Saptamukhi and Thakuran estuaries of the Sundarbans on bacterioplankton concerning inorganic nutrients and carbonate variables. They stated that Proteobacteria dominated the bacterioplankton, with a contribution from Bacteroidetes in the Saptamukhi and Cyanobacteria and Actinobacteria in the Thakuran; the interactions between physicochemical parameters, nutrient levels, and estuarine carbonate chemistry controlled post-monsoonal bacterioplankton abundance. Dhal et al. [150] revealed microbial communities in the waters of the “Island of Sundarban Mangroves” and “Open Marine Water” of the Thakuran-Matla River estuarine complex adjacent to the Maipith coastal area. They reported dominance of marine hydrocarbon-degrading bacteria under families Oceanospirillaceae and Spongiibacteraceae, where the most abundant bacterial family Rhodobacteracea almost equally dominated in both study sites. Their investigation further reported Actinobacteria, which is known to be associated with carbon cycling to decompose the plant biomass via degrading the cellulose and hemicellulose materials (a dominant resource material in mangrove plants).
6. The Lateral Flux of Carbon to the Bay of Bengal
The last two decades witnessed several studies that characterized the lateral fluxes of carbon from the terrestrial sectors to the adjacent ocean through the estuaries [8,151,152]. Recent developments in understanding estuarine carbon biogeochemistry indicate that long-term carbon sinks should encompass the laterally exported DIC from the estuaries to the nearshore coastal oceans [153]. The lateral export of organic matter and nutrients from the mangrove sediments to the estuarine waters plays a vital role in regulating the carbon flux. In this regard, Mukhopadhyay et al. [87] were the first to quantify the lateral fluxes of nutrients and DIC from the Hooghly estuary to the adjacent northern Bay of Bengal by deploying a biogeochemical mass balance model. They reported a lateral flux of DIC of 2.3 × 1011 mol per year while working from 1999 to 2001. Mukhopadhyay et al. [87] observed that 7.5% of the DIC was removed from the system as air–water CO2 flux during this lateral transport. Ghosh et al. [83], during 2015–16, observed that annually, the Hooghly estuary outfluxes around 4.45 ± 1.90 × 1011 mol and 4.59 ± 1.70 × 1011 mol of TAlk and DIC, respectively, to the adjacent coastal ocean. They concluded that the enhanced discharge load of inorganic and organic matter in the upper reaches of the Hooghly River led to an increased lateral flux of DIC and DOC, compared to the observations of Mukhopadhyay et al. [87]. Ghosh et al. [83] also inferred that the lateral DIC fluxes to the coastal ocean were 30 to 60 times higher than the air–water CO2 fluxes from the Hooghly estuary towards the atmosphere. Ray et al. [84] quantified the lateral fluxes of DIC, DOC, and POC from the Hooghly estuary and the Sundarbans estuaries as baseline data. They reported a lateral DIC, DOC, and POC flux of 3.45 × 1011 mol, 0.28 × 1011 mol, and 0.05 × 1011 mol per year, respectively, from the Hooghly estuary. In contrast, the Sundarbans estuaries outwelled 3.07 × 1011 mol, 2.52 × 1011 mol, and 0.48 × 1011 of DIC, DOC, and POC mol per year, respectively, with a total of 7.3 Tg C yr−1. Thus, Ray et al. [84] concluded that the mangrove-adjacent waters of the Sundarbans estuaries outwelled slightly less DIC but nine times more DOC and 9.6 times more POC than the Hooghly estuary. One of the constraints of this study [84] was the use of river discharge data from the Hooghly estuary to compute the lateral fluxes from the Sundarbans estuaries, which might lead to substantial uncertainties. Thus, further studies are of the utmost necessity to characterize the overall carbon budget of the Sundarbans estuaries.
7. Summary and Conclusions
Collating all the observations considered in this review, we can conclude that the pCO2(water) and pCH4(water) in the Sundarbans estuaries remain supersaturated compared to the atmospheric concentrations. Hence, these estuaries act as sources of CO2 and CH4. Figure 2 summarizes the entire review in a nutshell. The inner estuarine regions emit more CO2 and CH4 than the outer reaches close to the northern Bay of Bengal. Some studies indicated that the outer reaches could sometimes act as a sink for CO2, which is unusual for mangrove-adjacent waters. Analyzing the reasons behind this observation, several authors noted that the water from the adjacent Bay of Bengal is primarily responsible for enhancing the carbonate buffering capacity of these estuaries, especially in regions with an adequate quantity of riverine freshwater that does not end up in the upper reaches. Among the physical drivers, water temperature and photosynthetically active radiation play a pivotal role in governing CO2 emissions. Some significant biogeochemical and hydrological factors that governed the CO2 fluxes were salinity, porewater, groundwater, and freshwater discharge. The degree of primary productivity was the main regulating factor for air–water CH4 fluxes.
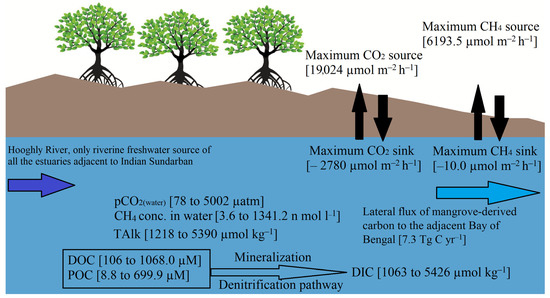
Figure 2.
Schematic diagram of the ranges of different carbon parameters [particulate organic carbon (POC), dissolved organic carbon (DOC), and dissolved inorganic carbon (DIC)], total alkalinity (TAlk), partial pressure of CO2 (pCO2), and concentration of CH4 in the Indian Sundarbans’ adjoining estuaries according to different studies. Hooghly River is the only source of riverine freshwater in the Indian Sundarbans. The major organic matter degradation/ mineralization pathway is denitrification. The mangrove surrounding waters of these estuaries acted as a source and sink for CO2 and CH4 depending on the seasonality and spatiality; the source and sink capacity varied over a wide range. These estuaries also transfer a substantial amount of carbon to the adjacent Bay of Bengal, mainly in POC, DOC, and DIC forms.
This review indicates that a substantial number of studies characterizing the air–water CO2 and CH4 fluxes exist in the estuaries of the Sundarbans. However, all these studies concentrated only on the estuaries that flow through the Indian counterpart of the Sundarbans. An adequate number of studies in the Bangladesh Sundarbans would enable us to draw a scenario about the entire Sundarbans estuarine network. Each major estuary flowing through the Sundarbans has unique freshwater–seawater admixing dynamics and a varying influence of adjacent mangroves. The present observations indicate that such subtle differences in the salinity regime and mangrove influence might lead to varying partial pressures of CO2 and CH4 [pCO2(water) and pCH4(water)] and air–water CO2 and CH4 fluxes. Thus, sampling all the major waterways in the vast Sundarbans is necessary. Among the Indian estuaries, the Hooghly (flowing through the western boundary and carrying the bulk load of freshwater) and the Matla (flowing through the central part with minimal freshwater from the upper reaches) have received the most scientific attention. Other estuaries such as Raimangal, Bidya, Saptamukhi, and Thakuran should receive similar attention in the future.
The studies conducted so far have had varying sampling strategies. Some studies concentrated on fewer sampling points but stressed high temporal resolution, whereas others tried to cover more locations with a lower temporal resolution. In a dynamic estuarine environment such as the Sundarbans, tide-induced variability in carbon-biogeochemistry-related parameters poses a challenge to characterizing the mean and range of a particular parameter. Thus, more studies focusing simultaneously on the spatial extent and temporal resolution can alleviate the uncertainties in the data range. Besides the estuaries of the Bangladesh Sundarbans, which have yet to receive proper scientific attention in this regard, the estuarine waters flowing through the core area of the Indian Sundarbans also need rigorous sampling in the future, or else a substantial part of this unique eco-region remains undersampled. Whatever data on freshwater discharge are available at present mainly focus on the Hooghly estuary. Future researchers should model the estuarine flow through the various other estuaries of the Sundarbans that do not have a proper connection with the upper reaches. This would help in delineating the lateral fluxes of various forms of carbon from this system to the adjacent ocean and vice versa. Most of the studies accomplished so far have relied on discrete sampling techniques, which constrained the number of observations, as they require manual presence. Automated sensors on fixed platforms and the deployment of buoys should be on future research agendas. Otherwise, characterizing the temporal changes in the behavior of these estuaries with the changing climate and anthropogenic influences upstream would be highly challenging.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life13040863/s1, Table S1. The list of studies on air-water CO2 and CH4 fluxes, along with other related carbon-biogeochemistry parameters carried out in the Indian Sundarbans estuaries. Table S2. List of observations on TAlk and DIC from several studies on the Indian Sundarbans estuaries. The results are displayed either as mean ± standard deviation from the mean or as the range (minimum to maximum). NA denotes not available. Table S3. List of observations on DOC and POC from several studies on the Indian Sundarbans estuaries. The results are displayed either as mean ± standard deviation from the mean or as the range (minimum to maximum). Single magnitudes, in some instances, represent the mean (without any reported standard deviation). Table S4. List of observations on pCO2(water) and air-water CO2 fluxes from several studies on the Indian Sundarbans estuaries. The results are displayed either as mean ± standard deviation from the mean or as the range (minimum to maximum). Single magnitudes, in some instances, represent the mean (without any reported standard deviation). Table S5. List of observations on CH4 concentration in water and air-water CH4 fluxes from several studies on the Indian Sundarbans estuaries. The results are displayed either as mean ± standard deviation from the mean or as the range (minimum to maximum). Single magnitudes, in some instances, represent the mean (without any reported standard deviation).
Author Contributions
Conceptualization, S.H., A.C. and A.A.; formal analysis, I.D.; data curation, I.D.; writing—original draft preparation, I.D.; writing—review and editing, A.A. and A.C.; visualization, A.C.; supervision, S.H.; project administration, S.H.; funding acquisition, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NATIONAL REMOTE SENSING CENTRE (NRSC), grant number CAP-IGBP/BOD/CSG/ECSA/24/08/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the supplementary material.
Acknowledgments
Authors are grateful to the funding agency of this work, the National Remote Sensing Centre (NRSC), Indian Space Research Organization (ISRO), Department of Space, Government of India. The authors are also indebted to the anonymous reviewers for their constructive suggestions that helped the authors to enrich the quality of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Broecker, W.S. Climatic change: Are we on the brink of a pronounced global warming? Science 1975, 189, 460–463. [Google Scholar] [CrossRef] [PubMed]
- de Menocal, P. Wallace Smith Broecker. Nature 2019, 568, 34. [Google Scholar] [CrossRef]
- Chen, C.T.A.; Tsunogai, S. Carbon and nutrients in the ocean. In Asian Change in the Context of Global Change; Galloway, J.N., Melillio, J.M., Eds.; Cambridge University Press: Cambridge, UK, 1998; pp. 271–307. [Google Scholar]
- Chen, C.T.; Huang, T.H.; Chen, Y.C.; Bai, Y.; He, X.; Kang, Y. Air–sea exchanges of CO2 in the world’s coastal seas. Biogeosciences 2013, 10, 6509–6544. [Google Scholar] [CrossRef]
- Dyer, K.R. Response of estuaries to climate change. In Climate Change: Impact on Coastal Habitation; Eisma, D., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 85–110. [Google Scholar]
- Borges, A.V.; Delille, B.; Frankignoulle, M. Budgeting sinks and sources of CO2 in the coastal ocean: Diversity of ecosystems counts. Geophys. Res. Lett. 2005, 32, L14601. [Google Scholar] [CrossRef]
- Borges, A.; Abril, G. Carbon dioxide and methane dynamics in estuaries. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D.S., Eds.; Academic Press: Waltham, MA, USA, 2010; Volume 5, pp. 119–161. [Google Scholar] [CrossRef]
- Cai, W.J. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annu. Rev. Mar. Sci. 2011, 3, 123–145. [Google Scholar] [CrossRef]
- Chen, X.; Santos, I.R.; Call, M.; Reithmaier, G.M.; Maher, D.; Holloway, C.; Wadnerkar, P.D.; Gómez-Álvarez, P.; Sanders, C.J.; Li, L. The mangrove CO2 pump: Tidally driven pore-water exchange. Limnol. Oceanogr. 2021, 66, 1563–1577. [Google Scholar] [CrossRef]
- Najjar, R.G.; Herrmann, M.; Alexander, R.; Boyer, E.W.; Burdige, D.J.; Butman, D.; Cai, W.J.; Canuel, E.A.; Chen, R.F.; Friedrichs, M.A.; et al. Carbon budget of tidal wetlands, estuaries, and shelf waters of Eastern North America. Glob. Biogeochem. Cycles 2018, 32, 389–416. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Cai, W.J.; Hu, X.; Huang, W.J.; Murrell, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.C.; Zhai, W.; Hollibaugh, J.T.; Wang, Y.; et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.; Craft, C.; Fourqurean, J.W.; Kauffman, J.B.; Marbà, N.; et al. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 2012, 7, e43542. [Google Scholar] [CrossRef]
- Olivier, J.G.J.; Peters, J.A.H.W. Trends in Global CO2 and Total Greenhouse Gas Emissions: 2020 Report; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2020. [Google Scholar]
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Canadell, J.G.; Dlugokencky, E.J.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.; et al. Three decades of global methane sources and sinks. Nat. Geosci. 2013, 6, 813–823. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambrige, UK, 2014. [Google Scholar]
- IUCN. The management of natural coastal carbon sink. In IUCN World Commission on Protected Areas; Laffoley, D., Drimsditch, G., Eds.; Natural England, UNEP: York, UK, 2009. [Google Scholar]
- Curry, C.L. Modeling the soil consumption of atmospheric methane at the global scale. Glob. Biogeochem. Cycles 2007, 21, GB4012. [Google Scholar] [CrossRef]
- Zhuang, Q.; Melillo, J.M.; Kicklighter, D.W.; Prinn, R.G.; McGuire, A.D.; Steudler, P.A.; Felzer, B.S.; Hu, S. Methane fluxes between terrestrial ecosystems and the atmosphere at northern high latitudes during the past century: A retrospective analysis with a process-based biogeochemistry model. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Cicerone, R.J.; Oremland, R.S. Biogeochemical aspects of atmospheric methane. Glob. Biogeochem. Cycles 1988, 2, 299–327. [Google Scholar] [CrossRef]
- Allan, W.; Struthers, H.; Lowe, D.C. Methane carbon isotope effects caused by atomic chlorine in the marine boundary layer: Global model results compared with Southern Hemisphere measurements. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Duarte, C.M.; Middelburg, J.J.; Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Perera, K.A.R.S.; Amarasinghe, M.D. Carbon sequestration capacity of mangrove soils in micro tidal estuaries and lagoons: A case study from Sri Lanka. Geoderma 2019, 347, 80–89. [Google Scholar] [CrossRef]
- Nagarajan, B.; Pandiarajan, C.; Krishnamoorthy, M.; Sophia, P. Reproductive fitness and success in mangroves: Implication on conservation. In Proceedings of the Taal the 12th World Lake Conference, Jaipur, India, 28 October–2 November 2007. [Google Scholar]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Giovanonni, L.; Kelly, J.; Dunstan, N.; Borde, A.; Diefenderfer, H.; Cornu, C.; Janousek, C.; Apple, J.; Brophy, L. Total ecosystem carbon stocks at the marine-terrestrial interface: Blue carbon of the Pacific Northwest Coast, United States. Glob. Change Biol. 2020, 26, 5679–5692. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Cabezas, A.; Mitsch, W.J.; MacDonnell, C.; Zhang, L.; Bydałek, F.; Lasso, A. Methane emissions from mangrove soils in hydrologically disturbed and reference mangrove tidal creeks in southwest Florida. Ecol. Eng. 2018, 114, 57–65. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon sequestration in mangrove forests. Carbon Manag. 2012, 3, 313–322. [Google Scholar] [CrossRef]
- Komiyama, A.; Havanond, S.; Srisawatt, W.; Mochida, Y.; Fujimoto, K.; Ohnishi, T.; Ishihara, S.; Miyagi, T. Top/root biomass ratio of a secondary mangrove (Ceriops tagal (Perr.) CB Rob.) forest. For. Ecol. Manag. 2000, 139, 127–134. [Google Scholar] [CrossRef]
- Khan, M.; Islam, N.; Suwa, R.; Hagihara, A. Biomass and aboveground net primary production in a subtropical mangrove stand of Kandelia obovata (S.; L.) Yong at Manko Wetland, Okinawa, Japan. Wetl. Ecol. Manag. 2009, 17, 585–599. [Google Scholar] [CrossRef]
- Eong, O.J. Mangroves-a carbon source and sink. Chemosphere 1993, 27, 1097–1107. [Google Scholar] [CrossRef]
- Gurney, A.; Ahammad, H.; Ford, M. The economics of greenhouse gas mitigation: Insights from illustrative global abatement scenarios modelling. Energy Econ. 2009, 31, S174–S186. [Google Scholar] [CrossRef]
- Gattuso, J.P.; Frankignoulle, M.; Wollast, R. Carbon and carbonate metabolism in coastal aquatic ecosystems. Annu. Rev. Ecol. Syst. 1998, 29, 405–434. [Google Scholar] [CrossRef]
- Bianchi, T.S. Biogeochemistry of Estuaries; Oxford University Press Inc.: Oxford, UK, 2007. [Google Scholar]
- Testa, J.M.; Kemp, W.M.; Hopkinson, C.S.; Smith, S.V. Ecosystem Metabolism. In Estuarine Ecology, 1st ed.; Day, J.W., Crump, B.C., Kemp, W.M., Yáñez-Arancibia, A., Eds.; Wiley, Inc.: Hoboken, NJ, USA, 2012; pp. 381–416. [Google Scholar] [CrossRef]
- Laruelle, G.G.; Dürr, H.H.; Slomp, C.P.; Borges, A.V. Evaluation of sinks and sources of CO2 in the global coastal ocean using a spatially-explicit typology of estuaries and continental shelves. Geophys. Res. Lett. 2010, 37, L15607. [Google Scholar] [CrossRef]
- Laruelle, G.G.; Dürr, H.H.; Lauerwald, R.; Hartmann, J.; Slomp, C.P.; Goossens, N.; Regnier, P.A.G. Global multi-scale segmentation of continental and coastal waters from the watersheds to the continental margins. Hydrol. Earth Syst. Sci. 2013, 17, 2029–2051. [Google Scholar] [CrossRef]
- Crosswell, J.R.; Anderson, I.C.; Stanhope, J.W.; Van Dam, B.; Brush, M.J.; Ensign, S.; Piehler, M.F.; McKee, B.; Bost, M.; Paerl, H.W. Carbon budget of a shallow, lagoonal estuary: Transformations and source-sink dynamics along the river-estuary-ocean continuum. Limnol. Oceanogr. 2017, 62, S29–S45. [Google Scholar] [CrossRef]
- Maher, D.T.; Eyre, B.D. Carbon budgets for three autotrophic Australian estuaries: Implications for global estimates of the coastal air-water CO2 flux. Glob. Biogeochem. Cycles 2012, 26. [Google Scholar] [CrossRef]
- Cotovicz, L.C., Jr.; Knoppers, B.A.; Brandini, N.; Costa Santos, S.J.; Abril, G. A strong CO2 sink enhanced by eutrophication in a tropical coastal embayment (Guanabara Bay, Rio de Janeiro, Brazil). Biogeosciences 2015, 12, 6125–6146. [Google Scholar] [CrossRef]
- Jiang, Z.P.; Cai, W.J.; Lehrter, J.; Chen, B.; Ouyang, Z.; Le, C.; Roberts, B.J.; Hussain, N.; Scaboo, M.K.; Zhang, J.; et al. Spring net community production and its coupling with the CO2 dynamics in the surface water of the northern Gulf of Mexico. Biogeosciences 2019, 16, 3507–3525. [Google Scholar] [CrossRef]
- Joesoef, A.; Huang, W.J.; Gao, Y.; Cai, W.J. Air–water fluxes and sources of carbon dioxide in the Delaware Estuary: Spatial and seasonal variability. Biogeosciences 2015, 12, 6085–6101. [Google Scholar] [CrossRef]
- Crosswell, J.R.; Wetz, M.S.; Hales, B.; Paerl, H.W. Air-water CO2 fluxes in the microtidal Neuse River estuary, North Carolina. J. Geophys. Res. Oceans 2012, 117, C08017. [Google Scholar] [CrossRef]
- Yao, H.; McCutcheon, M.R.; Staryk, C.J.; Hu, X. Hydrologic controls on CO2 chemistry and flux in subtropical lagoonal estuaries of the northwestern Gulf of Mexico. Limnol. Oceanogr. 2020, 65, 1380–1398. [Google Scholar] [CrossRef]
- Li, X.; Han, G.; Liu, M.; Song, C.; Zhang, Q.; Yang, K.; Liu, J. Hydrochemistry and dissolved inorganic carbon (DIC) cycling in a tropical agricultural river, Mun River Basin, Northeast Thailand. Int. J. Environ. Res. Public Health 2019, 16, 3410. [Google Scholar] [CrossRef]
- Joesoef, A.; Kirchman, D.L.; Sommerfield, C.K.; Cai, W.J. Seasonal variability of the inorganic carbon system in a large coastal plain estuary. Biogeosciences 2017, 14, 4949–4963. [Google Scholar] [CrossRef]
- Koné, Y.M.; Borges, A.V. Dissolved inorganic carbon dynamics in the waters surrounding forested mangroves of the Ca Mau Province (Vietnam). Estuar. Coast. Shelf Sci. 2008, 77, 409–421. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Change Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef] [PubMed]
- Turetsky, M.R.; Kotowska, A.; Bubier, J.; Dise, N.B.; Crill, P.; Hornibrook, E.R.; Minkkinen, K.; Moore, T.R.; Myers-Smith, I.H.; Nykänen, H.; et al. A synthesis of methane emissions from 71 northern, temperate, and subtropical wetlands. Glob. Chang. Biol. 2014, 20, 2183–2197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zimmermann, N.E.; Stenke, A.; Li, X.; Hodson, E.L.; Zhu, G.; Huang, C.; Poulter, B. Emerging role of wetland methane emissions in driving 21st century climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 9647–9652. [Google Scholar] [CrossRef]
- Arai, H.; Yoshioka, R.; Hanazawa, S.; Minh, V.Q.; Tuan, V.Q.; Tinh, T.K.; Phu, T.Q.; Jha, C.S.; Rodda, S.R.; Dadhwal, V.K.; et al. Function of the methanogenic community in mangrove soils as influenced by the chemical properties of the hydrosphere. Soil Sci. Plant Nutr. 2016, 62, 150–163. [Google Scholar] [CrossRef]
- Zheng, X.; Guo, J.; Song, W.; Feng, J.; Lin, G. Methane emission from mangrove wetland soils is marginal but can be stimulated significantly by anthropogenic activities. Forests 2018, 9, 738. [Google Scholar] [CrossRef]
- He, Y.; Guan, W.; Xue, D.; Liu, L.; Peng, C.; Liao, B.; Hu, J.; Yang, Y.; Wang, X.; Zhou, G.; et al. Comparison of methane emissions among invasive and native mangrove species in Dongzhaigang, Hainan Island. Sci. Total Environ. 2019, 697, 133945. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.B.; Choudhury, A.; Hussain, Z.; Acharya, G. Mangroves of the Sundarbans: India; IUCN: Bangkok, Thailand, 1994; Volume 1, p. 247. ISBN 13 9782831702094. [Google Scholar]
- Mandal, D.B. Man in Biosphere: A Case Study of Sundarbans Biosphere Reserve, 1st ed.; Gyan Publishing House: New Delhi, India, 2007. [Google Scholar]
- Samanta, S.; Hazra, S.; Mondal, P.P.; Chanda, A.; Giri, S.; French, J.R.; Nicholls, R.J. Assessment and attribution of mangrove Forest changes in the Indian Sundarbanss from 2000 to 2020. Remote Sens. 2021, 13, 4957. [Google Scholar] [CrossRef]
- Bhadra, T.; Mukhopadhyay, A.; Hazra, S. Identification of River Discontinuity Using Geo-Informatics to Improve Freshwater Flow and Ecosystem Services in Indian Sundarbans Delta. In Environment and Earth Observation; Hazra, S., Mukhopadhyay, A., Ghosh, A., Mitra, D., Dadhwal, V., Eds.; Springer Remote Sensing/Photogrammetry; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Roshith, C.M.; Meena, D.K.; Manna, R.K.; Sahoo, A.K.; Swain, H.S.; Raman, R.K.; Sengupta, A.; Das, B.K. Phytoplankton community structure of the Gangetic (Hooghly-Matla) estuary: Status and ecological implications in relation to eco-climatic variability. Flora 2018, 240, 133–143. [Google Scholar] [CrossRef]
- Chatterjee, M.; Shankar, D.; Sen, G.K.; Sanyal, P.; Sundar, D.; Michael, G.S.; Chatterjee, A.; Amol, P.; Mukherjee, D.; Suprit, K.; et al. Tidal variations in the Sundarbans estuarine system, India. J. Earth Syst. Sci. 2013, 122, 899–933. [Google Scholar] [CrossRef]
- De, T.K.; De, M.; Das, S.; Chowdhury, C.; Ray, R.; Jana, T.K. Phytoplankton abundance in relation to cultural eutrophication at the land-ocean boundary of Sunderbans, NE Coast of Bay of Bengal, India. J. Environ. Sci. Stud. 2011, 1, 169–180. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.K.; Biswas, H.; De, T.K.; Sen, S.; Jana, T.K. Seasonal effects on the air–water carbon dioxide exchange in the Hooghly estuary, NE coast of Bay of Bengal, India. Environ. Monit. Assess. 2002, 4, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Padhy, P.C.; Nayak, R.K.; Dadhwal, V.K.; Salim, M.; Mitra, D.; Chaudhury, S.B.; Rao, P.R.; Rao, K.H.; Dutt, C.B.S. Estimation of partial pressure of carbon dioxide and air-sea fluxes in Hooghly estuary based on in situ and satellite observations. J. Indian Soc. Remote Sens. 2016, 44, 135–143. [Google Scholar] [CrossRef]
- Akhand, A.; Chanda, A.; Watanabe, K.; Das, S.; Tokoro, T.; Chakraborty, K.; Hazra, S.; Kuwae, T. Low CO2 evasion rate from the mangrove-surrounding waters of the Sundarbans. Biogeochemistry 2021, 153, 95–114. [Google Scholar] [CrossRef]
- Akhand, A.; Chanda, A.; Watanabe, K.; Das, S.; Tokoro, T.; Hazra, S.; Kuwae, T. Reduction in Riverine Freshwater Supply Changes Inorganic and Organic Carbon Dynamics and Air-Water CO2 Fluxes in a Tropical Mangrove Dominated Estuary. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG006144. [Google Scholar] [CrossRef]
- Dutta, M.K.; Kumar, S.; Mukherjee, R.; Sanyal, P.; Mukhopadhyay, S.K. The post-monsoon carbon biogeochemistry of the Hooghly–Sundarbans estuarine system under different levels of anthropogenic impacts. Biogeosciences 2019, 16, 289–307. [Google Scholar] [CrossRef]
- Akhand, A.; Chanda, A.; Manna, S.; Das, S.; Hazra, S.; Roy, R.; Choudhury, S.B.; Rao, K.H.; Dadhwal, V.K.; Chakraborty, K.; et al. A comparison of CO2 dynamics and air-water fluxes in a river-dominated estuary and a mangrove-dominated marine estuary. Geophys. Res. Lett. 2016, 43, 11–726. [Google Scholar] [CrossRef]
- Dutta, M.K.; Kumar, S.; Mukherjee, R.; Sharma, N.; Bhushan, R.; Sanyal, P.; Paul, M.; Mukhopadhyay, S.K. Carbon Biogeochemistry of Two Contrasting Tropical Estuarine Ecosystems During Pre-monsoon. Estuaries Coast. 2021, 44, 1916–1930. [Google Scholar] [CrossRef]
- Akhand, A.; Chanda, A.; Watanabe, K.; Das, S.; Tokoro, T.; Hazra, S.; Kuwae, T. Drivers of inorganic carbon dynamics and air–water CO2 fluxes in two large tropical estuaries: Insights from coupled radon (222Rn) and pCO2 surveys. Limnol. Oceanogr. 2022, 67, S118–S132. [Google Scholar] [CrossRef]
- Acharya, A.; Sanyal, P.; Paul, M.; Gupta, V.K.; Bakshi, S.; Bhattacharyya, P.; Mukhopadhyay, S.K. Seasonal quantification of carbonate dissolution and CO2 emission dynamics in the Indian Sundarbans estuaries. Reg. Stud. Mar. Sci. 2022, 53, 102413. [Google Scholar] [CrossRef]
- Dutta, M.K.; Kumar, S.; Mukherjee, R.; Sharma, N.; Acharya, A.; Sanyal, P.; Bhusan, R.; Mukhopadhyay, S.K. Diurnal carbon dynamics in a mangrove-dominated tropical estuary (Sundarbans, India). Estuar. Coast. Shelf Sci. 2019, 229, 106426. [Google Scholar] [CrossRef]
- Ray, R.; Rixen, T.; Baum, A.; Malik, A.; Gleixner, G.; Jana, T.K. Distribution, sources and biogeochemistry of organic matter in a mangrove dominated estuarine system (Indian Sundarbans) during the pre-monsoon. Estuar. Coast. Shelf Sci. 2015, 167, 404–413. [Google Scholar] [CrossRef]
- Biswas, H.; Mukhopadhyay, S.K.; De, T.K.; Sen, S.; Jana, T.K. Biogenic controls on the air—Water carbon dioxide exchange in the Sundarban mangrove environment, northeast coast of Bay of Bengal, India. Limnol. Oceanogr. 2004, 49, 95–101. [Google Scholar] [CrossRef]
- Ghosh, S.; Jana, T.K.; Singh, B.N.; Choudhury, A. Comparative study of carbon dioxide system in virgin and reclaimed mangrove waters of Sundarbans. Mahasagar 1987, 20, 155–161. [Google Scholar]
- Akhand, A.; Chanda, A.; Dutta, S.; Manna, S.; Sanyal, P.; Hazra, S.; Rao, K.H.; Dadhwal, V.K. Dual character of Sundarban estuary as a source and sink of CO2 during summer: An investigation of spatial dynamics. Environ. Monit. Assess. 2013, 185, 6505–6515. [Google Scholar] [CrossRef] [PubMed]
- Biswas, H.; Mukhopadhyay, S.K.; Sen, S.; Jana, T.K. Spatial and temporal patterns of methane dynamics in the tropical mangrove dominated estuary, NE coast of Bay of Bengal, India. J. Mar. Syst. 2007, 68, 55–64. [Google Scholar] [CrossRef]
- Neetha, V. Dissolved Carbon Dioxide and Methane in Estuaries and Waters Surrounding Mangroves on the East Coast of India and the Andaman Islands. Ph.D Thesis, Anna University, Chennai, India, October 2008. Available online: http://hdl.handle.net/10603/29692 (accessed on 17 October 2022).
- Dutta, M.K.; Bianchi, T.S.; Mukhopadhyay, S.K. Mangrove methane biogeochemistry in the Indian Sundarbans: A proposed budget. Front. Mar. Sci. 2017, 4, 187. [Google Scholar] [CrossRef]
- Dutta, M.K.; Mukherjee, R.; Jana, T.K.; Mukhopadhyay, S.K. Biogeochemical dynamics of exogenous methane in an estuary associated to a mangrove biosphere; the Sundarbans, NE coast of India. Mar. Chem. 2015, 170, 1–10. [Google Scholar] [CrossRef]
- Dutta, M.K.; Ray, R.; Mukherjee, R.; Jana, T.K.; Mukhopadhyay, S.K. Atmospheric fluxes and photo-oxidation of methane in the mangrove environment of the Sundarbans, NE coast of India; A case study from Lothian Island. Agric. For. Meteorol. 2015, 213, 33–41. [Google Scholar] [CrossRef]
- Dutta, M.K.; Chowdhury, C.; Jana, T.K.; Mukhopadhyay, S.K. Dynamics and exchange fluxes of methane in the estuarine mangrove environment of the Sundarbans, NE coast of India. Atmos. Environ. 2013, 77, 631–639. [Google Scholar] [CrossRef]
- Ghosh, J.; Chakraborty, K.; Chanda, A.; Akhand, A.; Bhattacharya, T.; Das, S.; Das, I.; Hazra, S.; Choudhury, S.B.; Wells, M. Outwelling of total alkalinity and dissolved inorganic carbon from the Hooghly River to the adjacent coastal Bay of Bengal. Environ. Monit. Assess. 2021, 193, 415. [Google Scholar] [CrossRef]
- Ray, R.; Baum, A.; Rixen, T.; Gleixner, G.; Jana, T.K. Exportation of dissolved (inorganic and organic) and particulate carbon from mangroves and its implication to the carbon budget in the Indian Sundarbans. Sci. Total Environ. 2018, 621, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Dyrssen, D.; Sillén, L.G. Alkalinity and total carbonate in sea water. A plea for p-T-independent data. Tellus 1967, 19, 113–121. [Google Scholar] [CrossRef]
- Smith, S.V.; Hollibaugh, J.T. Coastal metabolism and the oceanic organic carbon balance. Rev. Geophys. 1993, 31, 75–89. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.K.; Biswas, H.D.T.K.; De, T.K.; Jana, T.K. Fluxes of nutrients from the tropical River Hooghly at the land–ocean boundary of Sundarbans, NE Coast of Bay of Bengal, India. J. Mar. Syst. 2006, 62, 9–21. [Google Scholar] [CrossRef]
- Hopkinson, C.S.; Vallino, J.J. The relationships among man’s activities in watersheds and estuaries: A model of runoff effects on patterns of estuarine community metabolism. Estuaries 1995, 18, 598–621. [Google Scholar] [CrossRef]
- Kemp, W.M.; Smith, E.M.; Marvin-DiPasquale, M.; Boynton, W.R. Organic carbon balance and net ecosystem metabolism in Chesapeake Bay. Mar. Ecol. Prog. Ser. 1997, 150, 229–248. [Google Scholar] [CrossRef]
- Cai, W.J.; Wang, Y. The chemistry, fluxes, and sources of carbon dioxide in the estuarine waters of the Satilla and Altamaha Rivers, Georgia. Limnol. Oceanogr. 1998, 43, 657–668. [Google Scholar] [CrossRef]
- Neubauer, S.C.; Anderson, I.C. Transport of dissolved inorganic carbon from a tidal freshwater marsh to the York River estuary. Limnol. Oceanogr. 2003, 48, 299–307. [Google Scholar] [CrossRef]
- Riley, G.A. Particulate organic matter in sea water. In Advances In Marine Biology; Academic Press: Cambridge, MA, USA, 1971; Volume 8, pp. 1–118. [Google Scholar] [CrossRef]
- Happ, G.; Gosselink, J.G.; Day, J.W., Jr. The seasonal distribution of organic carbon in a Louisiana estuary. Estuar. Coast. Mar. Sci. 1977, 5, 695–705. [Google Scholar] [CrossRef]
- Meybeck, M.; Helmer, R. The quality of rivers: From pristine stage to global pollution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1989, 75, 283–309. [Google Scholar] [CrossRef]
- Peterson, B.; Fry, B.; Hullar, M.; Saupe, S.; Wright, R. The distribution and stable carbon isotopic composition of dissolved organic carbon in estuaries. Estuaries 1994, 17, 111–121. [Google Scholar] [CrossRef]
- Hedges, J.I.; Keil, R.G.; Benner, R. What happens to terrestrial organic matter in the ocean? Org. Geochem. 1997, 27, 195–212. [Google Scholar] [CrossRef]
- Benner, R.; Opsahl, S.; Chin-Leo, G.; Richey, J.E.; Forsberg, B.R. Bacterial carbon metabolism in the Amazon River system. Limnol. Oceanogr. 1995, 40, 1262–1270. [Google Scholar] [CrossRef]
- Moran, M.A.; Sheldon, W.M.; Sheldon, J.E. Biodegradation of riverine dissolved organic carbon in five estuaries of the southeastern United States. Estuaries 1999, 22, 55–64. [Google Scholar] [CrossRef]
- Raymond, P.A.; Bauer, J.E. DOC cycling in a temperate estuary: A mass balance approach using natural 14C and 13C isotopes. Limnol. Oceanogr. 2001, 46, 655–667. [Google Scholar] [CrossRef]
- Gazeau, F.; Gattuso, J.P.; Middelburg, J.J.; Brion, N.; Schiettecatte, L.S.; Frankignoulle, M.; Borges, A.V. Planktonic and whole system metabolism in a nutrient-rich estuary (the Scheldt estuary). Estuaries 2005, 28, 868–883. [Google Scholar] [CrossRef]
- Raymond, P.A.; Bauer, J.E. Bacterial consumption of DOC during transport through a temperate estuary. Aquat. Microb. Ecol. 2000, 22, 1–12. [Google Scholar] [CrossRef]
- Raymond, P.A.; Bauer, J.E.; Cole, J.J. Atmospheric CO2 evasion, dissolved inorganic carbon production, and net heterotrophy in the York River estuary. Limnol. Oceanogr. 2000, 45, 1707–1717. [Google Scholar] [CrossRef]
- Harrison, J.A.; Caraco, N.; Seitzinger, S.P. Global patterns and sources of dissolved organic matter export to the coastal zone: Results from a spatially explicit, global model. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Koné, Y.J.M.; Abril, G.; Kouadio, K.N.; Delille, B.; Borges, A.V. Seasonal variability of carbon dioxide in the rivers and lagoons of Ivory Coast (West Africa). Estuaries Coast. 2009, 32, 246–260. [Google Scholar] [CrossRef]
- Van Dam, B.; Fourqurean, J.; Smyth, A. Alkalinity and CO2 fluxes in a tropical seagrass meadow. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 3–8 May 2020. [Google Scholar] [CrossRef]
- Raymond, P.A.; Cole, J.J. Increase in the export of alkalinity from North America’s largest river. Science 2003, 301, 88–91. [Google Scholar] [CrossRef] [PubMed]
- White, A.F.; Blum, A.E. Effects of climate on chemical weathering in watersheds. Geochim. Cosmochim. Acta 1995, 59, 1729–1747. [Google Scholar] [CrossRef]
- Guo, X.; Cai, W.J.; Zhai, W.; Dai, M.; Wang, Y.; Chen, B. Seasonal variations in the inorganic carbon system in the Pearl River (Zhujiang) estuary. Cont. Shelf Res. 2008, 28, 1424–1434. [Google Scholar] [CrossRef]
- White, A.F.; Buss, H.L. Natural weathering rates of silicate minerals. In Treatise on Geochemistry; Drever, J.I., Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 133–168. [Google Scholar] [CrossRef]
- Cai, W.J.; Xu, Y.Y.; Feely, R.A.; Wanninkhof, R.; Jönsson, B.; Alin, S.R.; Barbero, L.; Cross, J.N.; Azetsu-Scott, K.; Fassbender, A.J.; et al. Controls on surface water carbonate chemistry along North American ocean margins. Nat. Commun. 2020, 11, 2691. [Google Scholar] [CrossRef]
- Su, J.; Cai, W.J.; Brodeur, J.; Chen, B.; Hussain, N.; Yao, Y.; Ni, C.; Testa, J.M.; Li, M.; Xie, X.; et al. Chesapeake Bay acidification buffered by spatially decoupled carbonate mineral cycling. Nat. Geosci. 2020, 13, 441–447. [Google Scholar] [CrossRef]
- Brake, S.; Connors, K.; Romberger, S. A river runs through it: Impact of acid mine drainage on the geochemistry of West Little Sugar Creek pre-and post-reclamation at the Green Valley coal mine, Indiana, USA. Environ. Geol. 2001, 40, 1471–1481. [Google Scholar] [CrossRef]
- Oh, N.H.; Raymond, P.A. Contribution of agricultural liming to riverine bicarbonate export and CO2 sequestration in the Ohio River basin. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- Zhai, W.; Dai, M.; Cai, W.J.; Wang, Y.; Hong, H. The partial pressure of carbon dioxide and air–sea fluxes in the northern South China Sea in spring, summer and autumn. Mar. Chem. 2005, 96, 87–97. [Google Scholar] [CrossRef]
- Bouillon, S.; Middelburg, J.J.; Dehairs, F.; Borges, A.V.; Abril, G.; Flindt, M.R.; Ulomi, S.; Kristensen, E. Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania). Biogeosciences 2007, 4, 311–322. [Google Scholar] [CrossRef]
- Borges, A.V.; Djenidi, S.; Lacroix, G.; Théate, J.; Delille, B.; Frankignoulle, M. Atmospheric CO2 flux from mangrove surrounding waters. Geophys. Res. Lett. 2003, 30, 1558. [Google Scholar] [CrossRef]
- Mitra, S.; Ghosh, S.; Satpathy, K.K.; Bhattacharya, B.D.; Sarkar, S.K.; Mishra, P.; Raja, P. Water quality assessment of the ecologically stressed Hooghly River Estuary, India: A multivariate approach. Mar. Pollut. Bull. 2018, 126, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, L.C.; Maher, D.T.; Santos, I.R.; Call, M.; Reading, M.J.; Holloway, C.; Tait, D.R. The spatial and temporal drivers of pCO2, pCH4 and gas transfer velocity within a subtropical estuary. Estuar. Coast. Shelf Sci. 2018, 208, 83–95. [Google Scholar] [CrossRef]
- Tanner, E.L.; Mulhearn, P.J.; Eyre, B.D. CO2 emissions from a temperate drowned river valley estuary adjacent to an emerging megacity (Sydney Harbour). Estuar. Coast. Shelf Sci. 2017, 192, 42–56. [Google Scholar] [CrossRef]
- Lee, K.; Wanninkhof, R.; Feely, R.A.; Millero, F.J.; Peng, T.H. Global relationships of total inorganic carbon with temperature and nitrate in surface seawater. Glob. Biogeochem. Cycles 2000, 14, 979–994. [Google Scholar] [CrossRef]
- Chen, C.T.A.; Huang, T.H.; Fu, Y.H.; Bai, Y.; He, X. Strong sources of CO2 in upper estuaries become sinks of CO2 in large river plumes. Curr. Opin. Environ. Sustain. 2012, 4, 179–185. [Google Scholar] [CrossRef]
- Jayakumar, D.A.; Naqvi, S.W.A.; Narvekar, P.V.; George, M.D. Methane in coastal and offshore waters of the Arabian Sea. Mar. Chem. 2001, 74, 1–13. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Nieuwenhuize, J.; Iversen, N.; Høgh, N.; De Wilde, H.; Helder, W.; Seifert, R.; Christof, O. Methane distribution in European tidal estuaries. Biogeochemistry 2002, 59, 95–119. [Google Scholar] [CrossRef]
- Abril, G.; Iversen, N. Methane dynamics in a shallow non-tidal estuary (Randers Fjord, Denmark). Mar. Ecol. Prog. Ser. 2002, 230, 171–181. [Google Scholar] [CrossRef]
- Padhy, S.R.; Bhattacharyya, P.; Dash, P.K.; Reddy, C.S.; Chakraborty, A.; Pathak, H. Seasonal fluctuation in three mode of greenhouse gases emission in relation to soil labile carbon pools in degraded mangrove, Sundarban, India. Sci. Total Environ. 2020, 705, 135909. [Google Scholar] [CrossRef]
- Weiss, R. Carbon dioxide in water and seawater: The solubility of a non-ideal gas. Mar. Chem. 1974, 2, 203–215. [Google Scholar] [CrossRef]
- Takahashi, T. Carbon dioxide in the atmosphere and in Atlantic Ocean water. J. Geophys. Res. 1961, 66, 477–494. [Google Scholar] [CrossRef]
- Banaszak, A.T.; Neale, P.J. Ultraviolet radiation sensitivity of photosynthesis in phytoplankton from an estuarine environment. Limnol. Oceanogr. 2001, 46, 592–603. [Google Scholar] [CrossRef]
- Ganju, N.K.; Testa, J.M.; Suttles, S.E.; Aretxabaleta, A.L. Spatiotemporal variability of light attenuation and net ecosystem metabolism in a back-barrier estuary. Ocean Sci. 2020, 16, 593–614. [Google Scholar] [CrossRef]
- Domingues, R.B.; Anselmo, T.P.; Barbosa, A.B.; Sommer, U.; Galvão, H.M. Light as a driver of phytoplankton growth and production in the freshwater tidal zone of a turbid estuary. Estuar. Coast. Shelf Sci. 2011, 91, 526–535. [Google Scholar] [CrossRef]
- Sadhuram, Y.; Sarma, V.V.; Murthy, T.V.; Rao, B.P. Seasonal variability of physico-chemical characteristics of the Haldia channel of Hooghly estuary, India. J. Earth Syst. Sci. 2005, 114, 37–49. [Google Scholar] [CrossRef]
- Kumar, M.D.; Naqvi, S.W.A.; George, M.D.; Jayakumar, D.A. A sink for atmospheric carbon dioxide in the northeast Indian Ocean. J. Geophys. Res. Oceans 1996, 101, 18121–18125. [Google Scholar] [CrossRef]
- Frankignoulle, M.; Abril, G.; Borges, A.; Bourge, I.; Canon, C.; Delille, B.; Libert, E.; Théate, J.M. Carbon dioxide emission from European estuaries. Science 1998, 282, 434–436. [Google Scholar] [CrossRef]
- Liu, Q.; Charette, M.A.; Breier, C.F.; Henderson, P.B.; McCorkle, D.C.; Martin, W.; Dai, M. Carbonate system biogeochemistry in a subterranean estuary–Waquoit Bay, USA. Geochim. Cosmochim. Acta 2017, 203, 422–439. [Google Scholar] [CrossRef]
- Chen, C.T.A.; Wang, S.L.; Lu, X.X.; Zhang, S.R.; Lui, H.K.; Tseng, H.C.; Wang, B.J.; Huang, H.I. Hydrogeochemistry and greenhouse gases of the Pearl River, its estuary and beyond. Quat. Int. 2008, 186, 79–90. [Google Scholar] [CrossRef]
- Upstill-Goddard, R.C.; Salter, M.E.; Mann, P.J.; Barnes, J.; Poulsen, J.; Dinga, B.; Fiske, G.J.; Holmes, R.M. The riverine source of CH4 and N2O from the Republic of Congo, western Congo Basin. Biogeosciences 2017, 14, 2267–2281. [Google Scholar] [CrossRef]
- Krupadam, R.J.; Ahuja, R.; Wate, S.R.; Anjaneyulu, Y. Forest bound estuaries are higher methane emitters than paddy fields: A case of Godavari estuary, East Coast of India. Atmos. Environ. 2007, 41, 4819–4827. [Google Scholar] [CrossRef]
- Kristensen, E.; Flindt, M.R.; Ulomi, S.; Borges, A.V.; Abril, G.; Bouillon, S. Emission of CO2 and CH4 to the atmosphere by sediments and open waters in two Tanzanian mangrove forests. Mar. Ecol. Prog. Ser. 2008, 370, 53–67. [Google Scholar] [CrossRef]
- Das, N.; Mondal, A.; Mandal, S. Dynamics of methane and carbon dioxide emissions in the reclaimed islands of Sundarban mangrove ecosystem, India. Austral Ecol. 2022, 47, 412–427. [Google Scholar] [CrossRef]
- Moore, W.S. The effect of submarine groundwater discharge on the ocean. Annu. Rev. Mar. Sci. 2010, 2, 59–88. [Google Scholar] [CrossRef]
- Keating, E.H.; Fessenden, J.; Kanjorski, N.; Koning, D.J.; Pawar, R. The impact of CO2 on shallow groundwater chemistry: Observations at a natural analog site and implications for carbon sequestration. Environ. Earth Sci. 2010, 60, 521–536. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Cai, W.J.; Wang, Y. A comparative study of carbon dioxide degassing in river-and marine-dominated estuaries. Limnol. Oceanogr. 2008, 53, 2603–2615. [Google Scholar] [CrossRef]
- Rudra, K. Changing River courses in the western part of the Ganga-Brahmaputra delta. Geomorphology 2014, 227, 87–100. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhadury, P. Investigating monsoon and post-monsoon variabilities of bacterioplankton communities in a mangrove ecosystem. Environ. Sci. Pollut. Res. 2018, 25, 5722–5739. [Google Scholar] [CrossRef]
- Bhadury, P.; Singh, T. Analysis of marine planktonic cyanobacterial assemblages from Mooriganga Estuary, Indian Sundarbans using molecular approaches. Front. Mar. Sci. 2020, 7, 222. [Google Scholar] [CrossRef]
- Das, S.; De, M.; Ganguly, D.; Maiti, T.K.; Mukherjee, A.; Jana, T.K.; De, T.K. Depth integrated microbial community and physico-chemical properties in mangrove soil of Sundarban, India. Adv. Microbiol. 2012, 2, 234. [Google Scholar] [CrossRef]
- Das, S.; De, M.; Jana, T.K.; De, T.K. Environmental influence on cultivable microbial community in the sediment of Sundarban mangrove forest, India. Afr. J. Microbiol. Res. 2013, 7, 4655–4665. [Google Scholar]
- Mukherjee, R.; Dutta, M.K.; Sanyal, P.; Bhadury, P.; Mukhopadhyay, S.K. Bacterioplankton abundance and community structure during post-monsoon in mangrove dominated estuaries of the Indian Sundarbans; An insight to biogeochemical processes. Estuar. Coast. Shelf Sci. 2020, 243, 106895. [Google Scholar] [CrossRef]
- Dhal, P.K.; Kopprio, G.A.; Gärdes, A. Insights on aquatic microbiome of the Indian Sundarbans mangrove areas. PLoS ONE 2020, 15, 0221543. [Google Scholar] [CrossRef]
- Ciais, P.; Borges, A.V.; Abril, G.; Meybeck, M.; Folberth, G.; Hauglustaine, D.; Janssens, I.A. The impact of lateral carbon fluxes on the European carbon balance. Biogeosciences 2008, 5, 1259–1271. [Google Scholar] [CrossRef]
- Regnier, P.; Friedlingstein, P.; Ciais, P.; Mackenzie, F.T.; Gruber, N.; Janssens, I.A.; Laruelle, G.G.; Lauerwald, R.; Luyssaert, S.; Andersson, A.J.; et al. Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat. Geosci. 2013, 6, 597–607. [Google Scholar] [CrossRef]
- Santos, I.R.; Maher, D.T.; Larkin, R.; Webb, J.R.; Sanders, C.J. Carbon outwelling and outgassing vs. burial in an estuarine tidal creek surrounded by mangrove and saltmarsh wetlands. Limnol. Oceanogr. 2019, 64, 996–1013. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).