Abstract
Data available in the literature on the use of herbal products to treat inflammation-related vascular diseases were considered in this study, while also assessing the influence of gender. To this end, the articles published in PubMed over the past 10 years that described the use of plant extracts in randomized clinical trials studying the effectiveness in vascular pathologies were analyzed. The difference in efficacy of plant-derived preparations in female and male subjects was always considered when reporting. The safety profiles of the selected plants were described, reporting unwanted effects in humans and also by searching the WHO database (VigiBase®). The medicinal plants considered were Allium sativum, Campomanesia xanthocarpa, Sechium edule, Terminalia chebula. Additionally, an innovative type of preparation consisting of plant-derived nanovesicles was also reported.
1. Introduction
Inflammation is a well-known biological response of the organism to chemical and physical injuries that help the healing of tissues [1]. However, the inflammatory reaction can be harmful when it is excessively great, causing acute organ failure, or when too persistent, triggering chronic systemic inflammation [2]. In general, the inflammatory status is manifested by an increase in serum C-reactive protein (CRP) level and the release of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) [3]. Arterial and venous vessels are directly involved in inflammatory progression with alterations in endothelial permeability, causing liquid and protein leakage and tissue edema formation. TNF-α increases intracellular calcium and regulates myosin light chain kinase and RhoA, which disrupts endothelial junctions, reducing barrier function and enabling leukocyte transmigration [4,5]. Subjects with high levels of homocysteine, cholesterol, and triglycerides show a greater risk of stroke by approximately 50% than those with normal values [6]. Furthermore, the correction of hyperhomocysteinemia involves a reduction in stroke risk from 34% to 70% [7]. Among the factors that affect the amount of homocysteine present in the blood, are various physiological factors, such as age, sex, and body mass index [8,9]. In fact, women generally have lower levels than men [10]; therefore, homocysteine may be a possible factor responsible for gender differences in atherosclerosis and coronary artery disease [11].
In the blood vessel, acute and chronic inflammation results in endothelial dysfunction and vascular remodeling, affecting cardiovascular function. Several endogenous modulators play a role in vascular inflammatory processes; in particular, an increase in advanced glycation end-products (AGEs) causes activation of inflammatory pathways, oxidative stress, and procoagulant activity, leading to endothelial dysfunction [12,13]. Fishman et al. suggested that AGEs and their receptors may be useful biomarkers of the presence and severity of coronary artery disease [12]. Other inflammatory modulators are proinflammatory cytokines, such as TNF-α and interleukins (ILs) [14]. IL-1α and IL-1β are responsible for the disruption effects on the endothelial barrier, while nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and the mitogen-activated protein kinase (MAPK) signaling cause pro-atherogenic effects [15]. Moreover, the vascular adhesion protein-1 (VAP-1), also known as amine oxidase copper-containing 3 (AOC3), is a pro-inflammatory modulator with a greater expression in endothelial cells during inflammation [16,17]. VAP-1 is an ectoenzyme that catalyzes the oxidative deamination of primary amines and produces hydrogen peroxide, ammonium, and aldehydes, regulates leukocyte extravasation, and causes vascular damage and atherosclerosis [16,17]. Furthermore, endothelial dysfunction is mediated by the activation of enzymes such as heparinase and metalloproteinases (MMPs), which cleave glycoproteins anchored to the endothelial glycocalyx, which are activated by pro-inflammatory cytokines and reactive oxygen species (ROS) [18]. Furthermore, monosodium urate and cholesterol crystals, and islet amyloid polypeptides can damage the phagolysosome membrane and promote persistent activation of the NLRP3 inflammasome, also known as NALP3 or cryopyrin, causing severe inflammatory diseases such as gout, atherosclerosis, and type 2 diabetes mellitus [19,20,21]. Recently, extracellular vehicles such as platelet and endothelial microparticles have been reported to be involved in vascular regulation, including inflammatory and thrombotic homeostasis [22,23]. Therefore, vascular inflammation is clearly a complex condition that has several modulators.
The literature findings suggest that the treatment of inflammation with targeted drugs may promote the regression of vascular dysfunction [4,24,25]. However, non-steroid and steroid anti-inflammatory drugs have not shown protective effects against arterial stiffening; however, some promising results have been obtained with the use of selective inhibitors of IL-1β, IL-6, and TNF-α [4,25]. Unfortunately, the use of these inhibitors can cause the appearance of significant side effects, and their risk/benefit ratio remains to be further determined. In this context, plant-derived products with potential activity in the treatment of vascular dysfunction may be of interest. For this purpose, this study focused on the literature delineating potential plant products of utility in the treatment of cardiovascular diseases related to inflammatory damage. Particular attention was paid to the identification of differences in response to herbal medicines in women versus men.
2. Sex Differences in Vascular Function
Few studies considered the sex difference related to inflammation and metabolic and cardiovascular diseases. The general opinion is that men tend to have a worse risk factor profile than women, although this relationship changes with advancing age [26]. Differences in the prevalence of cardiovascular diseases were observed in premenopausal women compared to men of the same age; clinical data suggest that women are protected against various cardiovascular diseases primarily during the fertile period of life [27,28,29,30]. In fact, estrogens increase vascular NO• signaling, improving vasodilatation and insulin responsiveness, protecting against diabetes mellitus [30]. However, the relative risk of cardiovascular disease morbidity and mortality in subjects affected by diabetes mellitus ranges from 1 to 3 in men and from 2 to 5 in women, related to those without diabetes [31]. Furthermore, clinical studies failed to demonstrate that hormone replacement therapy (HRT) in postmenopausal women could improve cardiovascular outcomes [30]. It can also be observed that men and women have different lifestyle risk factors, such as male behavior that may include more frequently cigarette smoking, alcohol abuse, higher intake of red meat, and lower fruit and vegetable consumption [32]. Effectively, in part, behavior differences could explain why women live longer than men [33]. Focus has been on the relationship between the Western lifestyle and chronic metabolic inflammatory diseases and also on finding preventive approaches [34]. A meta-analysis that considered a total of 462,194 participants demonstrated that high ingestion of fruits and vegetables (flavonoids) was inversely associated with the risk of total cardiovascular disease mortality [35]. Recently, Parmenter et al. (2022) found that in older women, a higher intake of habitual dietary flavonoids, mainly black tea, is associated with less extensive abdominal aortic calcification [36]. Previous authors have also shown that high black tea consumption is associated with lower coronary artery and abdominal aorta calcifications [37,38,39].
3. Materials and Methods
This study considered articles identified using a PubMed search strategy up to January 2023. The terms included in the search query were ‘blood vessel’, ‘inflammation’, and ‘plant-derived compound’. The inclusion criteria for the search were as follows: 1. Published in the last 10 years, 2. Full texts, 3. English language. Of a total of 16,395 articles included, some were excluded according to other inclusion criteria, such as ‘endothelium’ and ‘female’, excluding the term ‘review’ (Figure S1). A total of 20 articles was reviewed according to their relevance to the selected topic. Additionally, other references were examined also through Google Scholar to find additional relevant articles, including in vitro and in vivo studies to facilitate the explanation of the pharmacological mechanisms. The safety profiles of the selected plants were described, along with the unwanted effects reported in humans, also by searching VigiBase®, the WHO global database of possible side effects of medicinal products [40].
4. Results
The selected medicinal plants evaluated in this review were Allium sativum, Campomanesia xanthocarpa, Sechium edule, and Terminalia chebula. Additionally, an innovative type of preparation consisting of plant-derived nanovesicles was considered. Table 1 reports a synthesis of the clinical trials that detected the efficacy of the medicinal plants considered in this review.
4.1. Allium sativum L.
4.1.1. Botanical Characteristics
Garlic (Amaryllidaceae) is a herbaceous plant whose bulbs are often used to flavor different types of food, widely known for its typical smell and taste [41]. Its alimentary use is widespread throughout the world, and its medicinal use is well-known in popular medicine as crude drug, standardized extracts, and also as a food supplement [42]. The bulbs are harvested in late spring and early summer, then dried in the shade at 40 °C, to enable storage [41]. Several traditional uses are known, such as antimicrobial, diuretic, vermifuge, adjuvant in the prevention of atherosclerosis and the relief of the common cold [43,44].
4.1.2. Phytoconstituents and Preclinical Activity
Among the most peculiar constituents, there are several sulfur compounds, including alliin, an odorless compound, which in turn is transformed by the alliinase enzyme into allicin that has the typical garlic smell [45,46,47,48]. Allicin is a diallyl thiosulfinate considered one of the most active components, although other sulfur compounds provide garlic properties, such as ajoene, allyl propyl disulfide, diallyl trisulfide, and S-allylcysteine [49]. Additionally, garlic contains saponins, flavonoids, vitamins, and minerals [41,50]. Numerous in vitro and in vivo studies suggested its efficacy in several human diseases [51,52]. Garlic powder, aged garlic, and garlic oil have shown antiplatelet and anticoagulant effects by interfering with cyclooxygenase-mediated thromboxane synthesis [49,53]. Garlic extracts showed antioxidant property, decreased expression of vascular endothelial growth factor (VEGF), hypoxia-inducible factor 1 alpha (HIF-1α), inducible nitric oxide synthase (iNOS), and metalloproteinase (MMP)-9 [54,55,56]. Furthermore, garlic prevents the expression of inflammatory cytokines such as IL-6 and monocyte chemoattractant protein-1 (MCP-1) in lipopolysaccharide (LPS)-stimulated 3T3-L1 adipocytes [57].


Table 1.
Effectiveness of herbal preparations used for the treatment of vascular diseases administered orally to human subjects.
Table 1.
Effectiveness of herbal preparations used for the treatment of vascular diseases administered orally to human subjects.
| Natural Products | Clinical Trials | Participants | Dosage | Outcomes | Refs. |
|---|---|---|---|---|---|
| Allium sativum (garlic) |
|
|
|
| [46,58,59,60,61,62] |
| Campomanesia xanthocarpa (guavirova) |
|
|
|
| [63,64,65] |
| Sechium edule (chayote) | ND | ND | ND | ND | ND |
| Terminalia chebula (black myrobalan) |
|
|
| a. and b. Improves endothelial function, increases NO•, GSH, HDL, decreases CRP, HbA1c, MDA, TG, LDL, VLDL | [66,67,68] |
| Plant-derived nanovesicles |
| 20 healthy subjects | 3 months 1000 mg day Citrus limon EVs | Decreases waist circumference in women | [69] |
CRP: C-reactive protein; DB: double-blind; EVs: extracellular vesicles; GSH: glutathione; PC: placebo-controlled; T2DM: type 2 diabetes mellitus; HbA1c: glycosylated hemoglobin A1c; HDL: high-density lipoproteins; LDL-C, low density lipoprotein cholesterol; MDA: malondialdehyde; PAI-1: plasminogen activator inhibitor 1; PC: protein carbonyls; R: randomized trial; TG, triglycerides; TAC: total antioxidant capacity; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol. ND: no documented clinical trials.
4.1.3. Therapeutic Efficacy: Clinical Trials
Garlic is a very well-known plant that is used around the world. Studies on garlic preparations have mainly tested hypocholesterolemic, antihypertensive, antimicrobial, and, also, antitumor activities [54,55,56]. A randomized placebo-controlled trial (RCT) enrolled a total of 100 pregnant women at high risk of pre-eclampsia who were treated during the third trimester of pregnancy for 8 weeks with 800 mg garlic tablets (dried garlic powder containing 1 mg allicin, and ajoene) per day or placebo [60]. The treatment prevented the increase of total cholesterol and reduced hypertension [60]. Another RCT conducted in 44 pregnant women treated with 400 mg garlic tablet (equal to 400 mg garlic and 1 mg allicin) for 9 weeks showed reduced serum CRP levels and increased plasma glutathione in the treated women compared to the untreated group [61]. Another study showed that garlic supplementation (400 mg/day, garlic extract 2% allicin) positively modifies endothelial biomarkers of cardiovascular risk, suggesting that treatment can reduce chronic inflammation in obese individuals of both sexes [58]. Furthermore, one trial also suggested that 500 mg granulated garlic powder (2–3 mg allicin) can be considered as an adjuvant treatment in patients with diabetic macular edema [62]. Recently, the acute efficacy of 180 mg fermented garlic extract enriched with 7 mg inorganic nitrite (NO2−) in healthy women and men showed a significant decrease in both systolic and diastolic pressure 30 min after ingestion of the product [70].
Systematic reviews and meta-analysis evaluating the effects of garlic supplementation have generally reported an improvement of lipid profile and insulin-resistance; however, the low quality of the trials does not permit at the moment the real effectiveness of garlic preparations to be defined [71,72]. Overall, the data available in the literature support the use of garlic extracts in the treatment of vascular diseases mainly with an atherosclerotic basis, without apparent differences between the two sexes. However, additional RCTs with standardized extracts are required to confirm the therapeutic use of garlic in cardiovascular diseases.
4.1.4. Safety
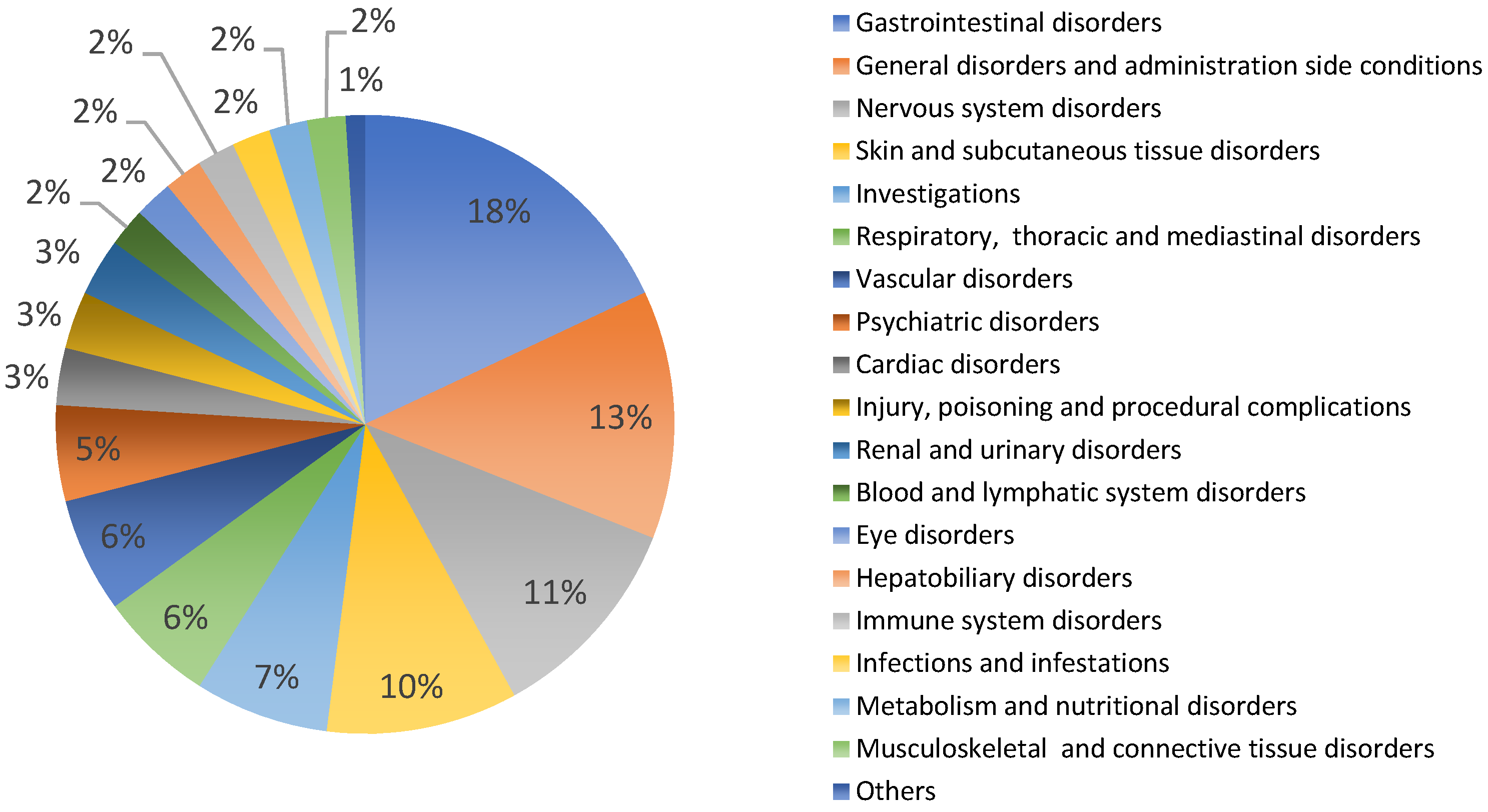
According to toxicity data from experimental studies and medicinal use, garlic preparations are generally considered safe in the usual dosage regimen. Female and male rats treated with 300 and 600 mg/kg day of an aqueous garlic bulb extract for 21 days showed changes in weight growth, biological parameters, and histological structures [73]. In humans, common side effects included odor and skin rash, and gastrointestinal upset [60,74,75]. In VigiBase® Allium sativum has 232 reports of potential side effects from all countries, mainly Europe (31%), in patients of both sexes (female 49%, male 47%, unknown 5%), in adults and older people [40]. Figure 1 shows the types and percentages of reported side effects. Among these, there are mainly gastrointestinal disorders (18%, such as vomiting, gastrointestinal pain, diarrhea, etc.), general disorders (13%, such as drug interaction, asthenis, etc.), nervous system disorders (11%, such as dizziness, hemorrhages, skin disorders, etc.) and various others (Figure 1). The large number of side effects related to garlic use may depend on the very high consumption of this plant worldwide. In general, the use of garlic even for curative purposes is considered safe in humans, avoiding use in atopic subjects [51,76,77]. The medicinal use of garlic is not recommended during pregnancy and breastfeeding due to the absence of clinical evidence showing both efficacy and non-toxicity [44]. Furthermore, it is also not recommended in patients being treated with antiplatelet and anticoagulant drugs due to the increased risk of bleeding [44,75].
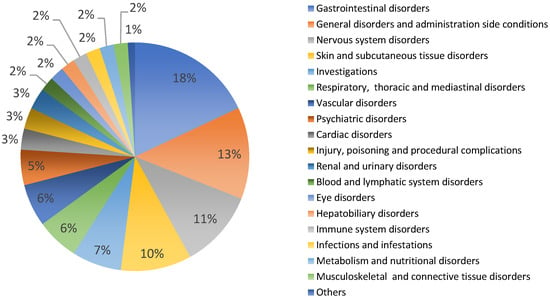
Figure 1.
Allium sativum: potential side effects reported in VigiBasis®.
4.1.5. Future Needs
The use of garlic preparations is widespread both in the diet and for healing purposes. To optimize its medical use, it is essential to define the type of formulation and the titer of sulfur derivatives, the dose, and the mode of administration, to standardize treatments and compare data from different clinical trials. Certainly, double-blind randomized clinical trials, with a sufficient number of subjects of both sexes are needed, to validate the use of garlic in therapy in the treatment of vascular diseases. Otherwise, unfortunately, it would remain only a traditional use, without valid evidence of effectiveness, the use on the basis of scientific evidence being renounced.
4.2. Campomanesia xanthocarpa Berg.
4.2.1. Botanical Characteristics
Campomanesia xanthocarpa (Myrtaceae) is a semi-deciduous tree, commonly known as “guavirova”, which grows in Brazil, Argentina, Paraguay, and Uruguay. It has edible fruits with a succulent pulp and a sweet flavor [78]. Leaves are traditionally used in herbal teas to treat inflammatory, urinary, rheumatic diseases, high blood pressure, and high cholesterol [78,79].
4.2.2. Phytoconstituents and Preclinical Activity
Leaves contain phenolic compounds, such as chlorogenic, gallic, ellagic and rosmarinic acids, glycosylated flavanols mainly of quercetin and myricetin, and pro-anthocyanidins [80,81,82,83,84]. Alkaloid theobromine (3,7-dimethyl-xanthine) was also identified in an aqueous infusion of leaves [83]. Acute administration of an aqueous extract showed a dose-dependent hypotensive effect in rats, by inhibiting the renin–angiotensin system through the block of the angiotensin II type 1 receptor (AT1R) and of calcium currents, as well as by KATP channel activation [83,84]. Furthermore, several studies reported the antioxidant activity of the fruits and leaves [80,82,85].
4.2.3. Therapeutic Efficacy: Clinical Trials
The authors studied Campomanesia xanthocarpa on inflammatory processes, oxidative stress, and lipid biomarkers of hypercholesterolemia, showing a decrease in total cholesterol and LDL levels in treated hypercholesterolemic subjects [63,64]. A small trial in 33 hypercholesterolemic subjects treated with 250 and 500 mg capsules that contained dried Campomanesia xanthocarpa leaves for 90 days revealed a significant reduction in total cholesterol and LDL levels in hypercholesterolemic subjects with total cholesterol >240 mg/dL (n = 22) [63]. In addition, another trial involving a larger number of subjects treated for 90 days with 500, 750, and 1000 mg of dried encapsulated leaves also demonstrated anticholesterolemic activity [64]. These authors also suggested that this treatment attenuates oxidative stress and pro-inflammatory reactions, improving blood flow and endothelial function [64]. Furthermore, healthy subjects were treated with 1000 mg of powdered Campomanesia xanthocarpa leaves (n = 8) and compared to those treated with 100 mg of acetylsalicylic acid (ASA, n = 7), or 500 mg of Campomanesia xanthocarpa plus 50 mg of ASA (n = 7). The authors showed that Campomanesia xanthocarpa leaves have antiplatelet activity when administered at 1000 mg for 5 days alone, or at 500 mg with low doses of ASA [65].
4.2.4. Safety
The extract of Campomanesia xanthocarpa leaf administered to male rats at 300 mg/kg iv caused cardiac depression with a dramatic drop in blood pressure and animal death [84]. In contrast, a 5000 mg/kg ethanol leaf extract administered orally to five male and five female mice did not show toxicity [81]. Genotoxic effects were observed after treatment with an aqueous leaf extract administered to male rats at 1000 mg/kg [82]; this observation should be taken into account in future studies. No adverse reactions were reported for the use of Campomanesia xanthocarpa (guavirova) in VigiBase® [40]. No information is available in the literature on its safety during pregnancy or breastfeeding in humans.
4.2.5. Future Needs
Preparations with dry leaves of Campomanesia xanthocarpa seem to have interesting hypocholesterolemic and antiplatelet activities, potentially useful in vascular diseases. However, no specific active compounds were identified in the formulations administered to the subjects, and therefore there is no reference compound for titration. Data from clinical trials are very limited, and there is certainly a need for double-blind randomized clinical trials with a sufficient number of subjects of both sexes to define the clinical usefulness of this medicinal plant.
4.3. Sechium edule (Jacq.) Sw.
4.3.1. Botanical Characteristics
Sechium edule (Cucurbitaceae) is a perennial herbaceous climbing plant cultivated mainly by Asian and Latino-American populations for food use, in particular for Chayote fruits [86,87,88]. Likewise, fruits, roots, and leaves are known in traditional medicine against kidney stones, as a diuretic and antihypertensive [89,90,91,92,93]. Furthermore, alcoholic extracts showed a very good antimicrobial efficacy against all strains of multi-resistant Staphylococci and Enterococci [94]. Moreover, various leaf and seed preparations have shown remarkable antioxidant activity [95]. Several investigations in different animal models, such as rats, mice, and dogs, defined the capacity of this plant to reduce blood pressure [86,89,91,96]. The hydroalcoholic extract and the acetone fraction obtained from the roots of Sechium edule showed antihypertensive activity by a relaxant effect on blood vessels [91,97].
4.3.2. Phytoconstituents and Preclinical Activity
Leaf, seed, stem, and also the fruit Sechium edule are rich in various bioactive components, as well as flavonoids, phenolics, vitamin C, and carotenoids [94,95,98]. The leaves contained the highest concentration of luteolin glycosides, while the most significant concentration of apigenin derivatives (C-glycosidic and O-glycosidic bonds) was found in the root extract [92]. Trans-cinnamic acid, phenylacetic acid, and α-linolenic acid were identified in the leaf extract [99]. Fruits can contain bitter principles called cucurbitacins [86,100,101].
In isolated aorta rings without endothelium, a hydro-alcoholic root extract caused a concentration-dependent vasorelaxation of angiotensin II-induced vasocontraction [91]. Furthermore, the authors reported in vivo antihypertensive effects in mice treated with angiotensin II [91]. The distinctive components of the highest active fraction were identified as cinnamic compounds, such as cinnamic acid methyl ester [91,97]. An aqueous leaf extract administered at 200 mg/kg showed nephroprotective activity against various types of chemically induced renal damage in rats [102]. The extract used at 100–200 mg/kg showed anti-inflammatory activity reducing levels of TGF-β, TNF-α, and ICAM-1 [103,104]. In rats fed with a high-fat diet, Sechium edule shoots can prevent hepatic steatosis and attenuate fatty tissue by inhibiting lipogenic enzymes and stimulating lipolysis by upregulating AMP-activating protein kinase (AMPK) [105]. The same authors also showed that the shoot extracts inhibited the expressions of fatty acid synthase and HMG-CoA reductase in rats, while also isolated caffeic acid and hesperetin, the main characteristic components of Sechium edule shoots, prevented hepatic lipid accumulation [105]. The acetone fraction of the hydro-alcoholic extract of Sechium edule roots administered to female mice at 10 mg/kg per day, orally, for 10 weeks was able to control hypertension, as well as the oxidative and inflammatory status in the kidneys, as efficiently as losartan, returning mice to normotensive levels [97,103]. Furthermore, the acetonic fraction was more effective than losartan in preventing liver and kidney damage. Therefore, the fraction was able to control endothelial dysfunction and related diseases [97,103].
4.3.3. Therapeutic Efficacy: Clinical Trials
As far as was found in the literature, no clinical studies have been conducted in patients using this plant as a single treatment. A clinical trial studied a commercial antioxidant supplement containing three components, including Sechium edule, showing an improvement of the hemorheology in alcoholics [106]. Based on available data, clinical studies are required on the use of Sechium edule in hypertension, diabetes mellitus, obesity, and, in general, in vascural-related diseases.
4.3.4. Safety
Acute toxicity was tested in rats and mice that received a single oral dose of 2000 mg/kg of an aqueous leaf extract. Treated animals showed no change in the normal behavior pattern and no evidence of toxicity and mortality [102]. Negative effects on humans were not documented. In VigiBase® there are no reports of potential side effects related to Sechium edule [40], and the literature does not provide data on the safety of medicinal use during pregnancy or breastfeeding.
4.3.5. Future Needs
Although traditional use and preclinical data suggest great interest in the use of Sechium edule in vascular diseases, the total absence of clinical studies strongly limits its use. Therefore, proper clinical trials are desirable.
4.4. Terminalia chebula Retz.
4.4.1. Botanical Characteristics
Terminalia chebula (Combretaceae), also known as black myrobalan, is a deciduous tree that grows up to 30 m, widely known in India and Southern Asia for its use in Ayurvedic medicine [107,108]. Fruits are used in traditional medicine to treat various diseases, such as used as a laxative, stomachic, tonic, and antispasmodic [107,108].
4.4.2. Phytoconsituents and Preclinical Activity
The main components of the fruit are phenolic compounds, such as hydrolysable tannins and flavonoids, saccharides, such as D-glucose, D-fructose, and saccharose [67]. The aqueous extract of Terminalia chebula fruits contains chebulagic acid, chebulinic acid, and other low molecular weight hydrolysable tannins [68]. The leaves contain polyphenols such as punicalin, punicalagin, terflavins B, C, and D [108].
The antioxidant activity of Terminalia chebula has been reported in vivo and in vitro assays [108,109,110,111]. A study described a significant decrease in glucose level in normal and alloxan-induced diabetic rats four hours after oral administration of a methanolic fruit extract (100 mg/kg) [110]. Similarly, Terminalia chebula fruit extract at a concentration of 200 mg/kg administered for 30 days significantly reduced blood glucose, glycosylated hemoglobin, urea, and creatinine levels in diabetic rats [112]. Furthermore, a cardioprotective effect of an ethanolic extract of fruits (500 mg/kg) was described in rats [113].
4.4.3. Therapeutic Efficacy: Clinical Trials
Few trials have been reported in the literature on the potential therapeutic use of Terminalia chebula. A 12-week prospective trial showed that an aqueous extract of Terminalia chebula fruits administered at 250 mg and 500 mg, twice daily, significantly improved endothelial function, systemic inflammation, and lipid profile in 60 subjects with type 2 diabetes mellitus of either gender, compared to placebo treatment [66]. Terminalia chebula extract significantly increased NO• and GSH levels, reducing oxidative stress, malondialdehyde, and CRP levels [66]. Previously, the same authors reported similar beneficial effects in 56 patients of either gender with metabolic syndrome [68]. In this study, Terminalia chebula reduced malondialdehyde levels and increased glutathione levels, improving antioxidant status. Furthermore, the treatment significantly decreased total cholesterol, triglycerides, and low-density lipoprotein cholesterol, and increased high-density lipoprotein cholesterol, while the placebo did not have a significant effect on endothelial function or any of the other clinical parameters [68].
4.4.4. Safety
Treatment with Terminalia chebula extracts was well tolerated with very few side effects; in fact, very few patients experienced dyspepsia [66]. Other authors reported increased libido, dry mouth, colic, and confusion [114]. In general, the fruit preparations are well-tolerated and do not adversely affect health [67,108]. In VigiBase®, Terminalia chebula has five reports of potential side effects, such as gastrointestinal disorders (i.e., diarrhea and gastrointestinal pain), and other general disorders [40].
4.4.5. Future Needs
The few clinical studies that have evaluated the efficacy of Terminalia chebula fruit extracts suggest a potential use in the treatment of vascular dysfunction in diabetes mellitus and/or metabolic syndrome. Unfortunately, data are limited, and other studies are certainly necessary to determine the efficacy and safety of this medicinal plant.
4.5. Plant-Derived Nanovesicles
4.5.1. General Characteristics
In recent years, various investigations have suggested that plant cells, through an exosome-like process, may release nanosized particles, which are involved in plant cell-cell communication [115,116]. Furthermore, various studies suggest that plant-derived nanovesicles may also play a role in the properties of medicinal plants in human diseases, mainly based on their biological cargo [117,118,119,120]. It is assumed that the interaction between vegetal extracellular vesicles and mammalian cells may have beneficial effects through antioxidant and anti-inflammatory activities [121,122]. Plants produce nanovesicles in response to numerous biotic and abiotic environmental stresses, including pathogen infection and attack. Plant nanovesicles carry a wide variety of molecules, including proteins, lipids, miRNAs, vitamins, and various plant metabolites [123].
4.5.2. In Vitro and In Vivo Studies
Lemon and strawberry-derived nanovesicles showed antioxidant activity in mesenchymal stem cells [124,125]. The authors verified the potential anti-osteoporotic effects of apple-derived nanovesicles using MC3T3-E1 cells, inhibiting osteoporosis by promoting osteoblastogenesis in osteoblastic MC3T3-E1 cells, by regulating the BMP2/Smad1 pathway [126]. Lemon-derived nanovesicles have been found to be rich in citric acid and vitamin C, which have a significant protective effect on oxidative stress in mesenchymal stromal cells [124].
Few studies have been carried out in animal models and humans. Oral administration of grape exosome-like nanoparticles showed beneficial effects in dextran sulfate sodium (DSS)-induced experimental colitis in mice, via induction of intestinal stem cells [120]. Similarly, broccoli-derived nanoparticles administered orally protected against various types of mice colitis, through activation of adenosine monophosphate-activated protein kinase (AMPK) in dendritic cells [127]. Additionally, ginger-derived nanoparticles protected mice from alcohol-induced liver injury, activating nuclear factor erythroid 2-related factor 2 (Nrf2) and inhibiting ROS production [128].
4.5.3. Therapeutic Efficacy: Clinical Trials
Very few trials considered the administration of plant-derived nanovesicles in the treatment of human diseases. In a prospective open-label study, 20 healthy volunteers (9 women and 11 men) were treated with a commercial preparation of extracellular vesicles from Citrus limon L., administered at 1000 mg daily for 3 months [69]. A decrease in waist circumference was found in women after 4 and 12 weeks of treatment, while no significant reduction was detected in men [69]. In the same study, the authors also observed a significant reduction in low-density lipoproteins (LDL) [69]. Significant correlations were also found in the stratified analysis between alkaline phosphatase enzymes (ALP) and glucose for women and between ALP and LDL for men [69]. A phase 1 clinical trial is currently underway studying the ability of a grape exosome preparation, administered orally for 35 days, to act as an anti-inflammatory agent against oral mucositis during radiation and chemotherapy treatment for head and neck tumors (NCT01668849) [129]. Another clinical study with ginger and aloe-derived exosomes studying the ability to mitigate insulin resistance and chronic inflammation in patients diagnosed with polycystic ovary syndrome was withdrawn because the investigator left the university before study approval (NCT03493984).
4.5.4. Safety
As reported in the literature, there are no toxicity studies or reports of undesirable effects related to human administration of these types of preparations. The use of plant-derived extracellular vesicles represents a new and very interesting approach in the treatment of diseases; however, other studies are needed to explore the advantages and, also, the disadvantages of plant-derived nanovesicles in therapy [129].
4.5.5. Future Needs
Plant-derived nanovesicles are certainly an innovative type of plant preparation, which also has considerable industrial implications. However, there are many aspects to be validated, starting from the techniques for obtaining and the definition of the constituents, up to the possible uses in the prevention or treatment of human pathologies.
5. Conclusions
Based on the data collected, it can be observed that clinical studies concerning the use of products of plant origin in the treatment of human pathologies and, in particular, in cardiovascular diseases are few and consider only small groups of subjects. Furthermore, the studies generally do not examine the differences in treatment response comparing the female or male gender. In studies in which the efficacy of the products used were reported separately, women versus men, it was not possible to obtain evidence of the difference in efficacy because the number of subjects enrolled in the trials was too small to perform any statistical estimation.
Among the plants considered, garlic has been the most studied and there are several data on its effectiveness in the treatment of vascular-related disorders. However, the available data are insufficient to validate the pharmacological use of garlic preparations for any of the conditions under consideration. Additional research that recruits more patients is desirable. Some plants, such as Campomanesia xanthocarpa, Sechium edule, and Terminalia chebula, have been proposed for their potential use in vascular problems in diabetic or hypertensive subjects. Finally, a new type of innovative preparation based on plant-derived extracellular vesicles has been suggested, but this is only an idea that still requires long investigation. Importantly, greater attention must be paid in carrying out clinical trials with the aim of obtaining a personalized use of plant products, noting the differences in the effectiveness between women and men. Improved consideration of gender-based medicine is required to improve the efficacy of therapeutic interventions and reduce adverse reactions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life13040866/s1, Figure S1: Flowchart of criteria used in PubMed research.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, C.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Maki-Petaja, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular consequences of inflammation: A position statement from the ESH working group onvascular structure and function and the ARTERY Society. J. Hypertens. 2020, 38, 1682–1698. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Rigor, R.R.; Pivetti, C.D.; Wu, M.H.; Yuan, S.Y. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc. Res. 2010, 87, 272–280. [Google Scholar] [CrossRef]
- Hao, L.; Chen, L.M.; Sai, X.Y.; Liu, Z.F.; Yang, G.; Yan, R.Z.; Wang, L.L.; Fu, C.Y.; Xu, X.; Cheng, Z.Z.; et al. Synergistic effects of elevated homocysteine level and abnormal blood lipids on the onset of stroke. Neural Regen. Res. 2013, 8, 2923–2931. [Google Scholar] [CrossRef]
- Spence, J.D. Stroke Prevention: A Lifetime of Lessons. Stroke 2020, 51, 2255–2262. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, X.; Li, M.; Neubauer, H. Elevated plasma homocysteine in obese schoolchildren with early atherosclerosis. Eur. J. Pediatr. 2006, 165, 326–331. [Google Scholar] [CrossRef]
- Brattström, L.; Lindgren, A.; Israelsson, B.; Andersson, A.; Hultberg, B. Homocysteine and cysteine: Determinants of plasma levels in middle-aged and elderly subjects. J. Intern. Med. 1994, 236, 633–641. [Google Scholar] [CrossRef]
- Cohen, E.; Margalit, I.; Shochat, T.; Goldberg, E.; Krause, I. Gender differences in homocysteine concentrations, a population-based cross-sectional study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 9–14. [Google Scholar] [CrossRef]
- Xu, R.; Huang, F.; Wang, Y.; Liu, Q.; Lv, Y.; Zhang, Q. Gender- and age-related differences in homocysteine concentration: A cross-sectional study of the general population of China. Sci. Rep. 2020, 10, 17401. [Google Scholar] [CrossRef] [PubMed]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Froldi, G.; Ragazzi, E. Selected plant-derived polyphenols as potential therapeutic agents for peripheral artery disease: Molecular mechanisms, efficacy and safety. Molecules 2022, 27, 7110. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.J.; Celermajer, D.S.; Patel, S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis 2018, 269, 262–271. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. Vascular adhesion protein-1: A cell surface amine oxidase in translation. Antioxid. Redox Signal. 2019, 30, 314–332. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Salmi, M.; Bono, P.; Hellman, J.; Leu, T.; Jalkanen, S. Cloning of vascular adhesion protein i reveals a novel multifunctional adhesion molecule. J. Exp. Med. 1998, 188, 17–27. [Google Scholar] [CrossRef]
- Dogné, S.; Flamion, B. Endothelial glycocalyx impairment in disease: Focus on hyaluronan shedding. Am. J. Pathol. 2020, 190, 768–780. [Google Scholar] [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nũez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the role of NLRP3 inflammasome in diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Puhm, F.; Boilard, E.; MacHlus, K.R. Platelet extracellular vesicles; beyond the blood. Arter. Thromb. Vasc. Biol. 2021, 41, 87–96. [Google Scholar] [CrossRef]
- Lugo-Gavidia, L.M.; Burger, D.; Matthews, V.B.; Nolde, J.M.; Galindo Kiuchi, M.; Carnagarin, R.; Kannenkeril, D.; Chan, J.; Joyson, A.; Herat, L.Y.; et al. Role of microparticles in cardiovascular disease: Implications for endothelial dysfunction, thrombosis, and inflammation. Hypertension 2021, 77, 1825–1844. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Jimenez, M.T.B.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxidative Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.X.; Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed? Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a biological variable in atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef]
- Rathod, K.S.; Kapil, V.; Velmurugan, S.; Khambata, R.S.; Siddique, U.; Khan, S.; Van Eijl, S.; Gee, L.C.; Bansal, J.; Pitrola, K.; et al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J. Clin. Investig. 2017, 127, 169–182. [Google Scholar] [CrossRef]
- Barrett-Connor, E. Sex differences in coronary heart disease why are women so superior? The 1995 Ancel keys lecture. Circulation 1997, 95, 252–264. [Google Scholar] [CrossRef]
- Murphy, E. Estrogen signaling and cardiovascular disease. Circ. Res. 2011, 109, 687–696. [Google Scholar] [CrossRef]
- Rivellese, A.A.; Riccardi, G.; Vaccaro, O. Cardiovascular risk in women with diabetes. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Sutradhar, R.; Yao, Z.; Wodchis, W.P.; Rosella, L.C. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int. J. Epidemiol. 2020, 49, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Zarulli, V.; Barthold Jones, J.A.; Oksuzyan, A.; Lindahl-Jacobsen, R.; Christensen, K.; Vaupel, J.W. Women live longer than men even during severe famines and epidemics. Proc. Natl. Acad. Sci. USA 2018, 115, E832–E840. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Latz, E. The Western lifestyle has lasting effects on metaflammation. Nat. Rev. Immunol. 2019, 19, 267–268. [Google Scholar] [CrossRef]
- Mazidi, M.; Katsiki, N.; Banach, M. A greater flavonoid intake is associated with lower total and cause-specific mortality: A meta-analysis of cohort studies. Nutrients 2020, 12, 2350. [Google Scholar] [CrossRef] [PubMed]
- Parmenter, B.H.; Bondonno, C.P.; Murray, K.; Schousboe, J.T.; Croft, K.; Prince, R.L.; Hodgson, J.M.; Bondonno, N.P.; Lewis, J.R. Higher habitual dietary flavonoid intake associates with less extensive abdominal aortic calcification in a cohort of older women. Arter. Thromb. Vasc. Biol. 2022, 42, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.; Launer, L.; Hofman, A.; Pols, H.; Witteman, J. Tea flavonoids may protect against atherosclerosis. The Rotterdam study. Arch. Intern. Med. 1999, 159, 2170–2174. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.; Zhao, D.; Frazier-Wood, A.; Michos, E.; Averill, M.; Sandfort, V.; Burke, G.; Polak, J.; Lima, J.; Post, W.; et al. Associations between coffee, tea, and caffeine intake with coronary artery calcification and cardiovascular events. Am. J. Med. 2017, 130, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.P.; Loria, C.M.; Steffen, L.M.; Zhou, X.; Van Horn, L.; Siscovick, D.S.; Jacobs, D.R.; Carr, J.J. Coffee, decaffeinated coffee, caffeine, and tea consumption in young adulthood and atherosclerosis later in life. The CARDIA study. Arter. Thromb. Vasc. Biol. 2010, 30, 2059–2066. [Google Scholar] [CrossRef]
- WHO Global Database VigiBase. Available online: https://www.vigiaccess.org/ (accessed on 14 February 2023).
- Sethi, N.; Kaura, S.; Dilbaghi, N.; Parle, M.; Pal, M. Garlic: A pungent wonder from nature. Int. Res. J. Pharm. 2014, 5, 523–529. [Google Scholar] [CrossRef]
- Song, K.; Milner, J.A. Recent advances on the nutritional effects associated with the use of garlic as a supplement. Historical perspective on the use of garlic. J. Nutr. 2001, 131, 1054S–1057S. [Google Scholar] [CrossRef]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA)/Committee on Herbal Medicinal Products (HMPC). European Union herbal monograph Allium sativum L. Eur. Med. Agency 2017, 31, 1–7. [Google Scholar]
- Weiner, L.; Shin, I.; Shimon, L.J.W.; Miron, T.; Wilchek, M.; Mirelman, D.; Frolow, F.; Rabinkov, A. Thiol-disulfide organization in alliin lyase (alliinase) from garlic (Allium sativum). Protein Sci. 2009, 18, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Beshbishy, A.; Wasef, L.; Elewa, Y.; Al-Sagan, A.; Abd El-Hack, M.; Taha, A.; Abd-Elhakim, Y. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar]
- Rahman, M.S. Allicin and other functional active components in garlic: Health benefits and bioavailability. Int. J. Food Prop. 2007, 10, 245–268. [Google Scholar] [CrossRef]
- Rana, S.V.; Pal, R.; Vaiphei, K.; Sharma, S.K.; Ola, R.P. Garlic in health and disease. Nutr. Res. Rev. 2011, 24, 60–71. [Google Scholar] [CrossRef]
- Lamponi, S. Bioactive natural compounds with antiplatelet and anticoagulant activity and their potential role in the treatment of thrombotic disorders. Life 2021, 11, 1095. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C.F.R. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1. [Google Scholar]
- Ansary, J.; Forbes-Hernández, T.Y.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Potential health benefit of garlic based on human intervention studies: A brief overview. Antioxidants 2020, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.S.; Yamato, O.; Yamasaki, M.; Maede, Y. Modulatory influence of sodium 2-propenyl thiosulfate from garlic on cyclooxygenase activity in canine platelets: Possible mechanism for the anti-aggregatory effect. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Shiju, T.M.; Rajkumar, R.; Rajesh, N.G.; Viswanathan, P. Aqueous extract of Allium sativum L bulbs offer nephroprotection by attenuating vascular endothelial growth factor and extracellular signal-regulated kinase-1 expression in diabetic rats. Indian J. Exp. Biol. 2013, 51, 139–148. [Google Scholar] [PubMed]
- Orozco-Ibarra, M.; Muñoz-Sánchez, J.; Zavala-Medina, M.E.; Pineda, B.; Magaña-Maldonado, R.; Vázquez-Contreras, E.; Maldonado, P.D.; Pedraza-Chaverri, J.; Chánez-Cárdenas, M.E. Aged garlic extract and S-allylcysteine prevent apoptotic cell death in a chemical hypoxia model. Biol. Res. 2016, 49, 7. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Hong, J.; Jeon, C.M.; Shin, N.R.; Kwon, O.K.; Kim, H.S.; Kim, J.C.; Oh, S.R.; Ahn, K.S. Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf-2/HO-1 pathway and NF-kappaB suppression. Food Chem. Toxicol. 2013, 62, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Ortuño-Sahagún, D.; Vázquez-Carrera, M.; López-Roa, R.I. Alliin, a garlic (Allium sativum) compound, prevents LPS-induced inflammation in 3T3-L1 adipocytes. Mediat. Inflamm. 2013, 2013, 381815. [Google Scholar] [CrossRef]
- Szulińska, M.; Kręgielska-Narożna, M.; Świątek, J.; Styś, P.; Kuźnar-Kamińska, B.; Jakubowski, H.; Walkowiak, J.; Bogdański, P. Garlic extract favorably modifies markers of endothelial function in obese patients—Randomized double blind placebo-controlled nutritional intervention. Biomed. Pharmacother. 2018, 102, 792–797. [Google Scholar] [CrossRef]
- Atkin, M.; Laight, D.; Cummings, M.H. The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. A pilot double blind randomized placebo controlled trial. J. Diabetes Its Complicat. 2016, 30, 723–727. [Google Scholar] [CrossRef]
- Ziaei, S.; Hantoshzadeh, S.; Rezasoltani, P.; Lamyian, M. The effect of garlic tablet on plasma lipids and platelet aggregation in nulliparous pregnants at high risk of preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 99, 201–206. [Google Scholar] [CrossRef]
- Aalami-Harandi, R.; Karamali, M.; Asemi, Z. The favorable effects of garlic intake on metabolic profiles, hs-CRP, biomarkers of oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia: Randomized, double-blind, placebo-controlled trial. J. Matern.-Fetal Neonatal Med. 2015, 28, 2020–2027. [Google Scholar] [CrossRef]
- Afarid, M.; Sadeghi, E.; Johari, M.; Namvar, E.; Sanie-Jahromi, F. Evaluation of the effect of garlic tablet as a complementary treatment for patients with diabetic retinopathy. J. Diabetes Res. 2022, 2022, 6620661. [Google Scholar] [CrossRef] [PubMed]
- Klafke, J.Z.; da Silva, M.A.; Panigas, T.F.; Belli, K.C.; de Oliveira, M.F.; Barichello, M.M.; Rigo, F.K.; Rossato, M.F.; dos Santos, A.R.S.; Pizzolatti, M.G.; et al. Effects of Campomanesia xanthocarpa on biochemical, hematological and oxidative stress parameters in hypercholesterolemic patients. J. Ethnopharmacol. 2010, 127, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Viecili, P.R.N.; Borges, D.O.; Kirsten, K.; Malheiros, J.; Viecili, E.; Melo, R.D.; Trevisan, G.; da Silva, M.A.; Bochi, G.V.; Moresco, R.N.; et al. Effects of Campomanesia xanthocarpa on inflammatory processes, oxidative stress, endothelial dysfunction and lipid biomarkers in hypercholesterolemic individuals. Atherosclerosis 2014, 234, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.S.; Hirsch, G.E.; Klafke, J.Z.; Porto, F.G.; de Almeida, A.S.; Nascimento, S.; Schmidt, A.; da Silva, B.; Pereira, R.L.D.; Jaskulski, M.; et al. Inhibitory effect of Campomanesia xanthocarpa in platelet aggregation: Comparison and synergism with acetylsalicylic acid. Thromb. Res. 2017, 154, 42–49. [Google Scholar] [CrossRef]
- Pingali, U.; Sukumaran, D.; Nutalapati, C. Effect of an aqueous extract of Terminalia chebula on endothelial dysfunction, systemic inflammation, and lipid profile in type 2 diabetes mellitus: A randomized double-blind, placebo-controlled clinical study. Phytother. Res. 2020, 34, 3226–3235. [Google Scholar] [CrossRef]
- Lopez, H.L.; Habowski, S.M.; Sandrock, J.E.; Raub, B.; Kedia, A.; Bruno, E.J.; Ziegenfuss, T.N. Effects of dietary supplementation with a standardized aqueous extract of Terminalia chebula fruit (AyuFlex®) on joint mobility, comfort, and functional capacity in healthy overweight subjects: A randomized placebo-controlled clinical trial. BMC Complement. Altern. Med. 2017, 17, 475. [Google Scholar] [CrossRef]
- Kishore, K.K.; Kishan, P.V.; Ramakanth, G.S.H.; Chandrasekhar, N.; Pinhali, U. A Study of Terminalia chebula extract on endothelial dysfunction and biomarkers of oxidative stress in patients with metabolic syndrome. Eur. J. Biomed. Pharm. Sci. 2016, 3, 181–188. [Google Scholar]
- Raimondo, S.; Nikolic, D.; Conigliaro, A.; Giavaresi, G.; Lo Sasso, B.; Giglio, R.V.; Chianetta, R.; Manno, M.; Raccosta, S.; Corleone, V.; et al. Preliminary results of citravesTM effects on low density lipoprotein cholesterol and waist circumference in healthy subjects after 12 weeks: A pilot open-label study. Metabolites 2021, 11, 276. [Google Scholar] [CrossRef]
- Baik, J.S.; Min, J.H.; Ju, S.M.; Ahn, J.H.; Ko, S.H.; Chon, H.S.; Kim, M.S.; Shin, Y. Il Effects of fermented garlic extract containing nitric oxide metabolites on blood flow in healthy participants: A randomized controlled trial. Nutrients 2022, 14, 5238. [Google Scholar] [CrossRef]
- Osadnik, T.; Goławski, M.; Lewandowski, P.; Morze, J.; Osadnik, K.; Pawlas, N.; Lejawa, M.; Jakubiak, G.K.; Mazur, A.; Schwingschackl, L.; et al. A network meta-analysis on the comparative effect of nutraceuticals on lipid profile in adults. Pharmacol. Res. 2022, 183, 106402. [Google Scholar] [CrossRef]
- Gyawali, D.; Vohra, R.; Orme-Johnson, D.; Ramaratnam, S.; Schneider, R.H. A systematic review and meta-analysis of Ayurvedic herbal preparations for hypercholesterolemia. Medicina 2021, 57, 546. [Google Scholar] [CrossRef] [PubMed]
- Fehri, B.; Aiache, J.M.; Korbi, S.; Monkni, M.; Ben Said, M.; Memmi, A.; Hizaoui, B.; Boukef, K. Toxic effects induced by the repeat administration of Allium sativum L. J. Pharm. Belg. 1991, 46, 363–374. [Google Scholar]
- Mulrow, C.; Lawrence, V.; Ackermann, R.; Ramirez, G.; Morbidoni, L.; Aguilar, C.; Arterburn, J.; Block, E.; Chiquette, E.; Gardener, C.; et al. Garlic: Effects on Cardiovascular Risks and Disease, Protective Effects against Cancer, and Clinical Adverse Effects: Summary. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11910/ (accessed on 16 January 2023).
- Tattelman, E. Health effects of garlic. Am. Fam. Physician 2005, 72, 103–106. [Google Scholar] [PubMed]
- Borrelli, F.; Capasso, R.; Izzo, A.A. Garlic (Allium sativum L.): Adverse effects and drug interactions in humans. Mol. Nutr. Food Res. 2007, 51, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Quintero-Fabián, S.; Lopez-Roa, R.I.; Flores-Gutierrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuno-Sahagun, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef]
- Lorenzi, H. Arvores Brasileiras. In Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil; Editora Plantarum Ltda: São Paulo, Brazil, 1992; p. 256. [Google Scholar]
- Bunchen, S. Conhecimento etnobotânico sobre as plantas medicinais utilizadas pela comunidade do Bairro Cidade Alta, município de Videira, Santa Catarina, Brasil. Unoesc Ciência–ACBS Joaçaba 2011, 2, 129–140. [Google Scholar]
- de Oliveira Raphaelli, C.; dos Santos Pereira, E.; Camargo, T.M.; Ribeiro, J.A.; Pereira, M.C.; Vinholes, J.; Dalmazo, G.O.; Vizzotto, M.; Nora, L. Biological activity and chemical composition of fruits, seeds and leaves of guabirobeira (Campomanesia xanthocarpa O. Berg–Myrtaceae): A review. Food Biosci. 2021, 40, 100899. [Google Scholar] [CrossRef]
- Markman, B.E.O.; Bacchi, E.M.; Kato, E.T.M. Antiulcerogenic effects of Campomanesia xanthocarpa. J. Ethnopharmacol. 2004, 94, 55–57. [Google Scholar] [CrossRef]
- De Sousa, J.A.; da Prado, L.S.; Alderete, B.L.; Boaretto, F.B.M.; Allgayer, M.C.; Miguel, F.M.; De Sousa, J.T.; Marroni, N.P.; Lemes, M.L.B.; Corrêa, D.S.; et al. Toxicological aspects of Campomanesia xanthocarpa Berg. associated with its phytochemical profile. J. Toxicol. Environ. Health—Part A Curr. Issues 2019, 82, 62–74. [Google Scholar] [CrossRef]
- Sant’anna, L.S.; Merlugo, L.; Ehle, C.S.; Limberger, J.; Fernandes, M.B.; Santos, M.C.; Mendez, A.S.L.; Paula, F.R.; Moreira, C.M. Chemical composition and hypotensive effect of Campomanesia xanthocarpa. Evid.-Based Complement. Altern. Med. 2017, 2017, 1591762. [Google Scholar] [CrossRef] [PubMed]
- de Morais, I.B.M.; Silva, D.B.; Carollo, C.A.; Ferreira-Neto, M.L.; Fidelis-de-Oliveira, P.; Bispo-da-Silva, L.B. Hypotensive activity of Campomanesia xanthocarpa leaf extract: Beyond angiotensin II type 1 receptor blockage. Nat. Prod. Res. 2021, 35, 4798–4802. [Google Scholar] [CrossRef] [PubMed]
- Klafke, J.Z.; Pereira, R.L.D.; Hirsch, G.E.; Parisi, M.M.; Porto, F.G.; de Almeida, A.S.; Rubin, F.H.; Schmidt, A.; Beutler, H.; Nascimento, S.; et al. Study of oxidative and inflammatory parameters in LDLr-KO mice treated with a hypercholesterolemic diet: Comparison between the use of Campomanesia xanthocarpa and acetylsalicylic acid. Phytomedicine 2016, 23, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Iñiguez, J.; Arévalo-Galarza, L.; Avendaño-Arrazate, C.H.; Soto-Hernández, M.; del Ruiz-Posadas, L.M.; Santiago-Osorio, E.; Acosta-Ramos, M.; Cisneros-Solano, V.M.; Aguirre-Medina, J.F.; Ochoa-Martínez, D. Production, genetics, postharvest management and pharmacological characteristics of Sechium edule (Jacq.) Sw. Fresh Prod. 2007, 1, 41–53. [Google Scholar]
- Booth, S.; Bressani, R.; Johns, T. Nutrient content of selected indigenous leafy vegetables consumed by the Kekchi people of Alta Verapaz, Guatemala. J. Food Compos. Anal. 1992, 5, 25–34. [Google Scholar] [CrossRef]
- Cook, O.F. The Chayote: A Tropical Vegetable; US Department of Agriculture, Division of Botany: Washington, DC, USA, 1901; Volume 18, pp. 1–31.
- Ibarra-Alvarado, C.; Rojas, A.; Mendoza, S.; Bah, M.; Gutiérrez, D.M.; Hernández-Sandoval, L.; Martínez, M. Vasoactive and antioxidant activities of plants used in Mexican traditional medicine for the treatment of cardiovascular diseases. Pharm. Biol. 2010, 48, 732–739. [Google Scholar] [CrossRef]
- Nunes, M.G.S.; Bernardino, A.; Martins, R.D. Use of medicinal plants by people with hypertension. Rev. Rede Enferm. Nordeste 2015, 16, 775. [Google Scholar] [CrossRef]
- Lombardo-Earl, G.; Roman-Ramos, R.; Zamilpa, A.; Herrera-Ruiz, M.; Rosas-Salgado, G.; Tortoriello, J.; Jiménez-Ferrer, E. Extracts and fractions from edible roots of Sechium edule (Jacq.) Sw. with antihypertensive activity. Evid.-Based Complement. Altern. Med. 2014, 2014, 594326. [Google Scholar] [CrossRef]
- Siciliano, T.; De Tommasi, N.; Morelli, I.; Braca, A. Study of flavonoids of Sechium edule (Jacq) Swartz (Cucurbitaceae) different edible organs by liquid chromatography photodiode array mass spectrometry. J. Agric. Food Chem. 2004, 52, 6510–6515. [Google Scholar] [CrossRef]
- de A. Ribeiro, R.; de Barros, F.; de Melo, M.M.R.F.; Muniz, C.; Chieia, S.; das Graças Wanderley, M.; Gomes, C.; Trolin, G. Acute diuretic effects in conscious rats produced by some medicinal plants used in the state of São Paulo, Brasil. J. Ethnopharmacol. 1988, 24, 19–29. [Google Scholar] [CrossRef]
- Ordoñez, A.A.L.; Gomez, J.D.; Cudmani, N.M.; Vattuone, M.A.; Isla, M.I. Antimicrobial activity of nine extracts of Sechium edule (Jacq.) Swartz. Microb. Ecol. Health Dis. 2003, 15, 33–39. [Google Scholar] [CrossRef]
- Ordoñez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Isla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Gordon, E.A.; Guppy, L.J.; Nelson, M. The antihypertensive effects of the Jamaican Cho-Cho (Sechium edule). West Indian Med. J. 2000, 49, 27–31. [Google Scholar] [PubMed]
- Trejo-Moreno, C.; Castro-Martínez, G.; Méndez-Martínez, M.; Jiménez-Ferrer, J.E.; Pedraza-Chaverri, J.; Arrellín, G.; Zamilpa, A.; Medina-Campos, O.N.; Lombardo-Earl, G.; Barrita-Cruz, G.J.; et al. Acetone fraction from Sechium edule (Jacq.) S.w. edible roots exhibits anti-endothelial dysfunction activity. J. Ethnopharmacol. 2018, 220, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Pinho, O.; Ferreira, I.M.P.L.V.O.; Delerue-Matos, C. Chayote (Sechium edule): A review of nutritional composition, bioactivities and potential applications. Food Chem. 2019, 275, 557–568. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Biona, K.; Shen, C.C. Chemical constituents of Sechium edule (Jacq.) Swartz. Der Pharma Chem. 2014, 6, 251–255. [Google Scholar]
- Cadena-Iñiguez, J.; de la Luz Riviello-Flores, M.; Marcos Soto-Hernández, R.; del Mar Ruiz-Posadas, L.; Gómez-Merino, F.C.; Aguiñiga Sanchez, I.; Arévalo-Galarza, L. Functionally active metabolites in two cultivars of chayote (Sechium edule (Jacq.) Swartz). Acta Hortic. 2019, 1256, 231–237. [Google Scholar] [CrossRef]
- Huerta-Reyes, M.; Tavera-Hernández, R.; Alvarado-Sansininea, J.J.; Jiménez-Estrada, M. Selected species of the Cucurbitaceae family used in Mexico for the treatment of diabetes mellitus. Molecules 2022, 27, 3440. [Google Scholar] [CrossRef]
- Firdous Mumtaz, S.M.; Paul, S.; Bag, A.K. Effect of Sechium edule on chemical induced kidney damage in experimental animals. Bangladesh J. Pharmacol. 2013, 8, 28–35. [Google Scholar] [CrossRef]
- Trejo-Moreno, C.; Castro-Martínez, G.; Méndez-Martínez, M.; Jiménez-Ferrer, J.E.; Pedraza-Chaverri, J.; Arrellín, G.; Zamilpa-Álvarez, A.; Medina-Campos, O.N.; Lombardo-Earl, G.; Barrita-Cruz, G.J.; et al. Data of the effects of acetone fraction from Sechium edule (Jacq.) S.w. edible roots in the kidney of endothelial dysfunction induced mice. Data Brief 2018, 18, 448–453. [Google Scholar] [CrossRef]
- Castañeda, R.; Cáceres, A.; Cruz, S.M.; Aceituno, J.A.; Marroquín, E.S.; Barrios Sosa, A.C.; Strangman, W.K.; Williamson, R.T. Nephroprotective plant species used in traditional Mayan Medicine for renal-associated diseases. J. Ethnopharmacol. 2023, 301, 115755. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chan, K.C.; Lee, Y.J.; Chang, X.Z.; Wu, C.H.; Wang, C.J. Sechium edule shoot extracts and active components improve obesity and a fatty liver that involved reducing hepatic lipogenesis and adipogenesis in high-fat-diet-fed rats. J. Agric. Food Chem. 2015, 63, 4587–4596. [Google Scholar] [CrossRef]
- Marotta, F.; Safran, P.; Tajiri, H.; Princess, G.; Anzulovic, H.; Idéo, G.M.; Rouge, A.; Seal, M.G.; Idéo, G.M. Improvement of hemorheological abnormalities in alcoholics by an oral antioxidant. Hepato-Gastroenterol. 2001, 48, 511–517. [Google Scholar]
- Bulbul, M.R.H.; Chowdhury, M.N.U.; Naima, T.A.; Sami, S.A.; Imtiaj, M.S.; Huda, N.; Uddin, M.G. A comprehensive review on the diverse pharmacological perspectives of Terminalia chebula Retz. Heliyon 2022, 8, e10220. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Suchalatha, S.; Srinivasulu, C.; Devi, S. Antioxidant activity of ethanolic extract of Terminalia chebula fruit against isoproterenol-induced oxidative stress in rats. Indian J. Biochem. Biophys. 2005, 42, 246–249. [Google Scholar] [PubMed]
- Sabu, M.C.; Kuttan, R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J. Ethnopharmacol. 2002, 81, 155–160. [Google Scholar] [CrossRef]
- Lee, H.S.; Nam, H.W.; Kyoung, H.K.; Lee, H.; Jun, W.; Lee, K.W. Antioxidant effects of aqueous extract of Terminalia chebula in vivo and in vitro. Biol. Pharm. Bull. 2005, 28, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, G.P.; Subramanian, S.P. Biochemical studies on the effect of Terminalia chebula on the levels of glycoproteins in streptozotocin-induced experimental diabetes in rats. J. Appl. Biomed. 2008, 6, 105–115. [Google Scholar] [CrossRef]
- Suchalatha, S.; Devi, C.S.S. Protective effect of Terminalia chebula against experimental myocardial injury induced by isoproterenol. Indian J. Exp. Biol. 2004, 42, 174–178. [Google Scholar]
- Banazadeh, M.; Mehrabani, M.; Banazadeh, N.; Dabaghzadeh, F.; Shahabi, F. Evaluating the effect of black myrobalan on cognitive, positive, and negative symptoms in patients with chronic schizophrenia: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2022, 36, 543–550. [Google Scholar] [CrossRef]
- Yamada, Y.; Benichou, G.; Cosimi, A.B.; Kawai, T.; Cosimi, B.A.; Kawai, T. Apoplastic exosome-like vesicles? A new way of protein secretion in plants. Plant Signal. Behav. 2012, 7, 544–546. [Google Scholar] [CrossRef]
- An, Q.; Hückelhoven, R.; Kogel, K.H.; van Bel, A.J.E. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell. Microbiol. 2006, 8, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T.; Tsouknidas, A. Plant-derived extracellular vesicles as therapeutic nanocarriers. Int. J. Mol. Sci. 2022, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Vitorino, C.; Salgueiro, L.; Girão, H. Plant nanovesicles for essential oil delivery. Pharmaceutics 2022, 14, 2581. [Google Scholar] [CrossRef]
- Kim, S.Q.; Kim, K.H. Emergence of edible plant-derived nanovesicles as functional food components and nanocarriers for therapeutics delivery: Potentials in human health and disease. Cells 2022, 11, 2232. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ma, J.; Zhou, Y.; Lu, R. Focusing on future applications and current challenges of plant derived extracellular vesicles. Pharmaceuticals 2022, 15, 708. [Google Scholar] [CrossRef]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like nanovesicles isolated from Citrus limon L. exert antioxidative effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Sim, Y.; Seo, H.-J.; Kim, D.-H.; Lee, S.-H.; Kwon, J.; Kwun, I.-S.; Jung, C.; Kim, J.-I.; Lim, J.-H.; Kim, D.-K.; et al. The effect of apple-derived nanovesicles on the osteoblastogenesis of osteoblastic MC3T3-E1 cells. J. Med. Food 2023, 26, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).