Current Update on Role of Hesperidin in Inflammatory Lung Diseases: Chemistry, Pharmacology, and Drug Delivery Approaches
Abstract
:1. Introduction
2. Search Strategy
3. Sources of Hesperidin
4. Chemistry
5. Physical Properties
6. Pharmacokinetics
7. Toxicity of Hesperidin
8. Role of Hesperidin in Inflammatory Lung Diseases
8.1. Asthma
8.2. COPD
8.3. Pulmonary Fibrosis
8.4. Lung Cancer
8.5. Acute Lung Injury or ARDS
8.6. Pneumonia
8.7. COVID-19
9. Drug Delivery in Respiratory Diseases
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aboussouan, L.S. Respiratory disorders in neurologic diseases. Clevel. Clin. J. Med. 2005, 72, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Benditt, J.O. Pathophysiology of Neuromuscular Respiratory Diseases. Clin. Chest Med. 2018, 39, 297–308. [Google Scholar] [CrossRef]
- Birnkrant, D.J. The assessment and management of the respiratory complications of pediatric neuromuscular diseases. Clin. Pediatr. 2002, 41, 301–308. [Google Scholar] [CrossRef]
- Boentert, M.; Wenninger, S.; Sansone, V.A. Respiratory involvement in neuromuscular disorders. Curr. Opin. Neurol. 2017, 30, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Aksu, E.H.; Kandemir, F.M.; Küçükler, S. Ameliorative effect of hesperidin on streptozotocin-diabetes mellitus-induced testicular DNA damage and sperm quality degradation in Sprague-Dawley rats. J. Food Biochem. 2021, 45, e13938. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Oliveira, J.M.P.; Santos, C.; Fernandes, E. Therapeutic potential of hesperidin and its aglycone hesperetin: Cell cycle regulation and apoptosis induction in cancer models. Phytomed. Int. J. Phytother. Phytopharm. 2020, 73, 152887. [Google Scholar] [CrossRef]
- Garg, A.; Garg, S.; Zaneveld, L.J.; Singla, A.K. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res. PTR 2001, 15, 655–669. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Gabrieli, C.; Kokkalou, E.; Georgarakis, M.; Niopas, I. Simultaneous reversed-phase high-performance liquid chromatographic method for the determination of diosmin, hesperidin and naringin in different citrus fruit juices and pharmaceutical formulations. J. Pharm. Biomed. Anal. 2003, 33, 243–249. [Google Scholar] [CrossRef]
- Inoue, T.; Tsubaki, S.; Ogawa, K.; Onishi, K.; Azuma, J.-I. Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chem. 2010, 123, 542–547. [Google Scholar] [CrossRef]
- Rouseff, R.L.; Martin, S.F.; Youtsey, C.O. Quantitative survey of narirutin, naringin, hesperidin, and neohesperidin in citrus. J. Agric. Food Chem. 1987, 35, 1027–1030. [Google Scholar] [CrossRef]
- Suzuki, H.; Asakawa, A.; Kawamura, N.; Yagi, T.; Inui, A. Hesperidin potentiates ghrelin signaling. Recent Pat. Food Nutr. Agric. 2014, 6, 60–63. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent for Obesity. Drug Des. Dev. Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef] [Green Version]
- Tadros, F.J.; Andrade, J.M. Impact of hesperidin in 100% orange juice on chronic disease biomarkers: A narrative systematic review and gap analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 8335–8354. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Sroka, Z.; Fecka, I.; Cisowski, W. Antiradical and anti-H2O2 properties of polyphenolic compounds from an aqueous peppermint extract. Z. Naturforsch. C J. Biosci. 2005, 60, 826–832. [Google Scholar] [CrossRef]
- Ćirić, A.; Prosen, H.; Jelikić-Stankov, M.; Đurđević, P. Evaluation of matrix effect in determination of some bioflavonoids in food samples by LC-MS/MS method. Talanta 2012, 99, 780–790. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Majumdar, S.; Srirangam, R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: A natural bioflavonoid. Pharm. Res. 2009, 26, 1217–1225. [Google Scholar] [CrossRef] [Green Version]
- Ameer, B.; Weintraub, R.A.; Johnson, J.V.; Yost, R.A.; Rouseff, R.L. Flavanone absorption after naringin, hesperidin, and citrus administration. Clin. Pharmacol. Ther. 1996, 60, 34–40. [Google Scholar] [CrossRef]
- Kim, M.; Kometani, T.; Okada, S.; Shimuzu, M. Permeation of Hesperidin Glycosides across Caco-2 Cell Monolayers Via the Paracellular Pathway. Biosci. Biotechnol. Biochem. 1999, 63, 2183–2188. [Google Scholar] [CrossRef]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma Kinetics and Urinary Excretion of the Flavanones Naringenin and Hesperetin in Humans after Ingestion of Orange Juice and Grapefruit Juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanal, J.L.; Cousse, H.; Sicart, M.T.; Bonnaud, B.; Marignan, R. Absorption and elimination of (14C) hesperidin methylchalcone in the rat. Eur. J. Drug Metab. Pharmacokinet. 1981, 6, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Rajavel, T.; Nabavi, S.F.; Setzer, W.N.; Ahmadi, A.; Mansouri, K.; Nabavi, S.M. Hesperidin: A promising anticancer agent from nature. Ind. Crops Prod. 2015, 76, 582–589. [Google Scholar] [CrossRef]
- Granner, D.K. The role of glucocorticoid hormones as biological amplifiers. Monogr. Endocrinol. 1979, 12, 593–611. [Google Scholar] [CrossRef] [PubMed]
- Birnhuber, A.; Biasin, V.; Schnoegl, D.; Marsh, L.M.; Kwapiszewska, G. Transcription factor Fra-2 and its emerging role in matrix deposition, proliferation and inflammation in chronic lung diseases. Cell. Signal. 2019, 64, 109408. [Google Scholar] [CrossRef]
- Wei, D.; Ci, X.; Chu, X.; Wei, M.; Hua, S.; Deng, X. Hesperidin Suppresses Ovalbumin-Induced Airway Inflammation in a Mouse Allergic Asthma Model. Inflammation 2012, 35, 114–121. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Hsu, H.-T.; Wang, K.-H.; Wang, C.-S.; Chen, C.-M.; Ko, W.-C. Hesperidin-3′-O-Methylether Is More Potent than Hesperidin in Phosphodiesterase Inhibition and Suppression of Ovalbumin-Induced Airway Hyperresponsiveness. Evid.-Based Complement. Altern. Med. 2012, 2012, 908562. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.S.; Wynn, T.A. Pulmonary fibrosis: Pathogenesis, etiology and regulation. Mucosal Immunol. 2009, 2, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Kandhare, A.D.; Kandhare, A.A.; Bodhankar, S.L. Hesperidin ameliorates bleomycin-induced experimental pulmonary fibrosis via inhibition of TGF-β1/Smad3/AMPK and IκBalpha/NF-κB pathways. Excli J. 2019, 18, 723–745. [Google Scholar] [CrossRef]
- Guo, J.; Fang, Y.; Jiang, F.; Li, L.; Zhou, H.; Xu, X.; Ning, W. Neohesperidin inhibits TGF-β1/Smad3 signaling and alleviates bleomycin-induced pulmonary fibrosis in mice. Eur. J. Pharmacol. 2019, 864, 172712. [Google Scholar] [CrossRef]
- Haddadi, G.H.; Rezaeyan, A.; Mosleh-Shirazi, M.A.; Hosseinzadeh, M.; Fardid, R.; Najafi, M.; Salajegheh, A. Hesperidin as Radioprotector against Radiation-Induced Lung Damage in Rat: A Histopathological Study. J. Med. Phys. 2017, 42, 25–32. [Google Scholar] [CrossRef]
- Gormeli, C.; Saraç, K.; Ciftci, O.; Timurkaan, N.; Malkoç, S. The effects of hesperidin on idiopathic pulmonary fibrosis evaluated by histopathologial-biochemical and micro-computed tomography examinations in a bleomycin-rat model. Biomed. Res. 2016, 27, 737–742. [Google Scholar]
- Waters, D.W.; Blokland, K.E.C.; Pathinayake, P.S.; Wei, L.; Schuliga, M.; Jaffar, J.; Westall, G.P.; Hansbro, P.M.; Prele, C.M.; Mutsaers, S.E.; et al. STAT3 Regulates the Onset of Oxidant-Induced Senescence in Lung Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2019, 61, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Rezaeyan, A.; Fardid, R.; Haddadi, G.H.; Takhshid, M.A.; Hosseinzadeh, M.; Najafi, M.; Salajegheh, A. Evaluating Radioprotective Effect of Hesperidin on Acute Radiation Damage in the Lung Tissue of Rats. J. Biomed. Phys. Eng. 2016, 6, 165–174. [Google Scholar]
- Yao, Y.; Lin, M.; Liu, Z.; Liu, M.; Zhang, S.; Zhang, Y. Hesperidin Inhibits Lung Cancer In Vitro and In Vivo through PinX1. Front. Pharm. 2022, 13, 918665. [Google Scholar] [CrossRef]
- Tanaka, T.; Tanaka, T.; Tanaka, M.; Kuno, T. Cancer Chemoprevention by Citrus Pulp and Juices Containing High Amounts of β-Cryptoxanthin and Hesperidin. J. Biomed. Biotechnol. 2012, 2012, 516981. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-X.; Yu, D.-D.; Chen, M.-J.; Sun, T.; Li, G.; Huang, W.-J.; Nie, H.; Wang, C.; Zhang, Y.-X.; Gong, Q.; et al. Hesperidin ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting HMGB1 release. Int. Immunopharmacol. 2015, 25, 370–376. [Google Scholar] [CrossRef]
- Kamaraj, S.; Ramakrishnan, G.; Anandakumar, P.; Jagan, S.; Devaki, T. Antioxidant and anticancer efficacy of hesperidin in benzo(a)pyrene induced lung carcinogenesis in mice. Investig. New Drugs 2009, 27, 214–222. [Google Scholar] [CrossRef]
- Matuschak, G.M.; Lechner, A.J. Acute lung injury and the acute respiratory distress syndrome: Pathophysiology and treatment. Mo. Med. 2010, 107, 252–258. [Google Scholar]
- Yeh, C.-C.; Kao, S.-J.; Lin, C.-C.; Wang, S.-D.; Liu, C.-J.; Kao, S.-T. The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro. Life Sci. 2007, 80, 1821–1831. [Google Scholar] [CrossRef]
- de Souza, A.B.F.; de Matos, N.A.; de Castro, T.F.; de Costa, G.P.; Oliveira, L.A.M.; de Nogueira, K.O.P.C.; Ribeiro, I.M.L.; Talvani, A.; Cangussú, S.D.; de Menezes, R.C.A.; et al. Effects in vitro and in vivo of hesperidin administration in an experimental model of acute lung inflammation. Free Radic. Biol. Med. 2022, 180, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ahmad Farouk, I.; Lal, S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses 2021, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Mani, C.S. Acute Pneumonia and Its Complications. Princ. Pract. Pediatr. Infect. Dis. 2018, 18, 238. [Google Scholar]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, B.-K.; Lee, Y.-C. Antiasthmatic Effects of Hesperidin, a Potential Th2 Cytokine Antagonist, in a Mouse Model of Allergic Asthma. Mediat. Inflamm. 2011, 2011, 485402. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; He, N.; Xing, H.; Sun, Y.; Ding, J.; Liu, L. Function of hesperidin alleviating inflammation and oxidative stress responses in COPD mice might be related to SIRT1/PGC-1α/NF-κB signaling axis. J. Recept. Signal Transduct. 2020, 40, 388–394. [Google Scholar] [CrossRef]
- Kohno, H.; Taima, M.; Sumida, T.; Azuma, Y.; Ogawa, H.; Tanaka, T. Inhibitory effect of mandarin juice rich in β-cryptoxanthin and hesperidin on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced pulmonary tumorigenesis in mice. Cancer Lett. 2001, 174, 141–150. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, Z.; Lan, Q.; Zhou, H.; Mai, Z.; Wang, Y.; Ding, X.; Zhang, W.; Pi, J.; Evans, C.E.; et al. Nasal Delivery of Hesperidin/Chitosan Nanoparticles Suppresses Cytokine Storm Syndrome in a Mouse Model of Acute Lung Injury. Front. Pharm. 2020, 11, 592238. [Google Scholar] [CrossRef]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef] [Green Version]
- Attia, G.H.; Moemen, Y.S.; Youns, M.; Ibrahim, A.M.; Abdou, R.; El Raey, M.A. Antiviral zinc oxide nanoparticles mediated by hesperidin and in silico comparison study between antiviral phenolics as anti-SARS-CoV-2. Colloids Surf. B Biointerfaces 2021, 203, 111724. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef]
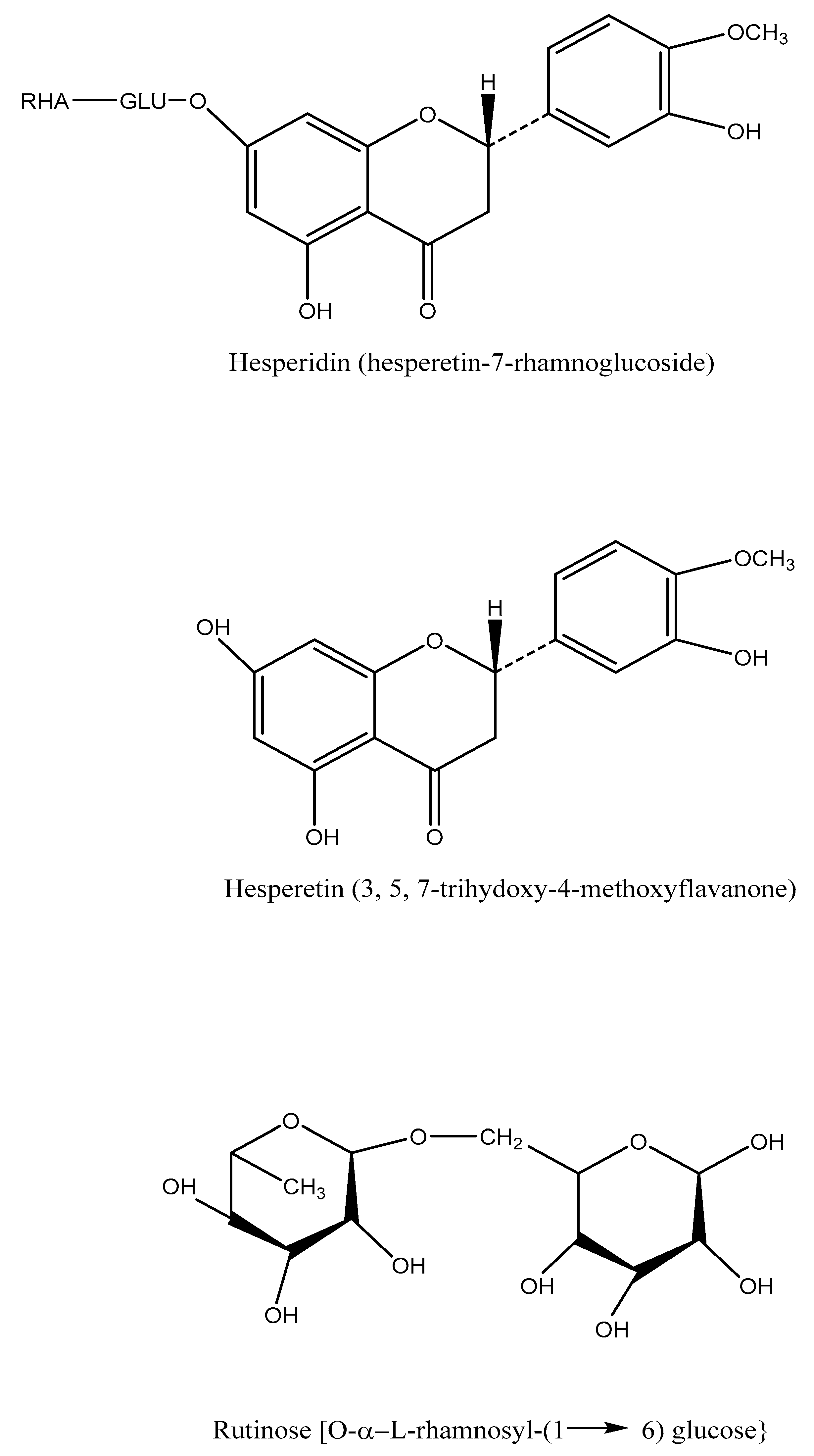

| Citrus Fruit Juices | Quantity | References |
|---|---|---|
| Grapefruit concentrate juice | 1.55 mg/100 mL | [11] |
| Pure grapefruit juice | 0.65 mg/100 mL | [11] |
| Juice from the concentrate of lemon | 24.99 mg/100 mL | [12] |
| Lemon juice, pure | 17.81 mg/100 mL | [12] |
| Pure juice, lime | 13.41 mg/100 mL | [12] |
| Orange [Blond], concentrate juice | 52.68 mg/100 mL | [13] |
| Pure orange [Blond] juice | 25.85 mg/100 mL | [13] |
| Orange [Blond], concentrate juice | 51.30 mg/100 mL | [13] |
| Orange [Blond] juice, undiluted | 43.61 mg/100 mL | [13] |
| Tangerine, concentrate juice | 36.11 mg/100 mL | [14] |
| Herbs | Quantity | References |
| Dried, peppermint | 480.65 mg/100 g FW | [15] |
| Fresh welsh onion | 0.02 mg/100 g FW | [16] |
| Respiratory Diseases | Model | Aim | Findings | References |
|---|---|---|---|---|
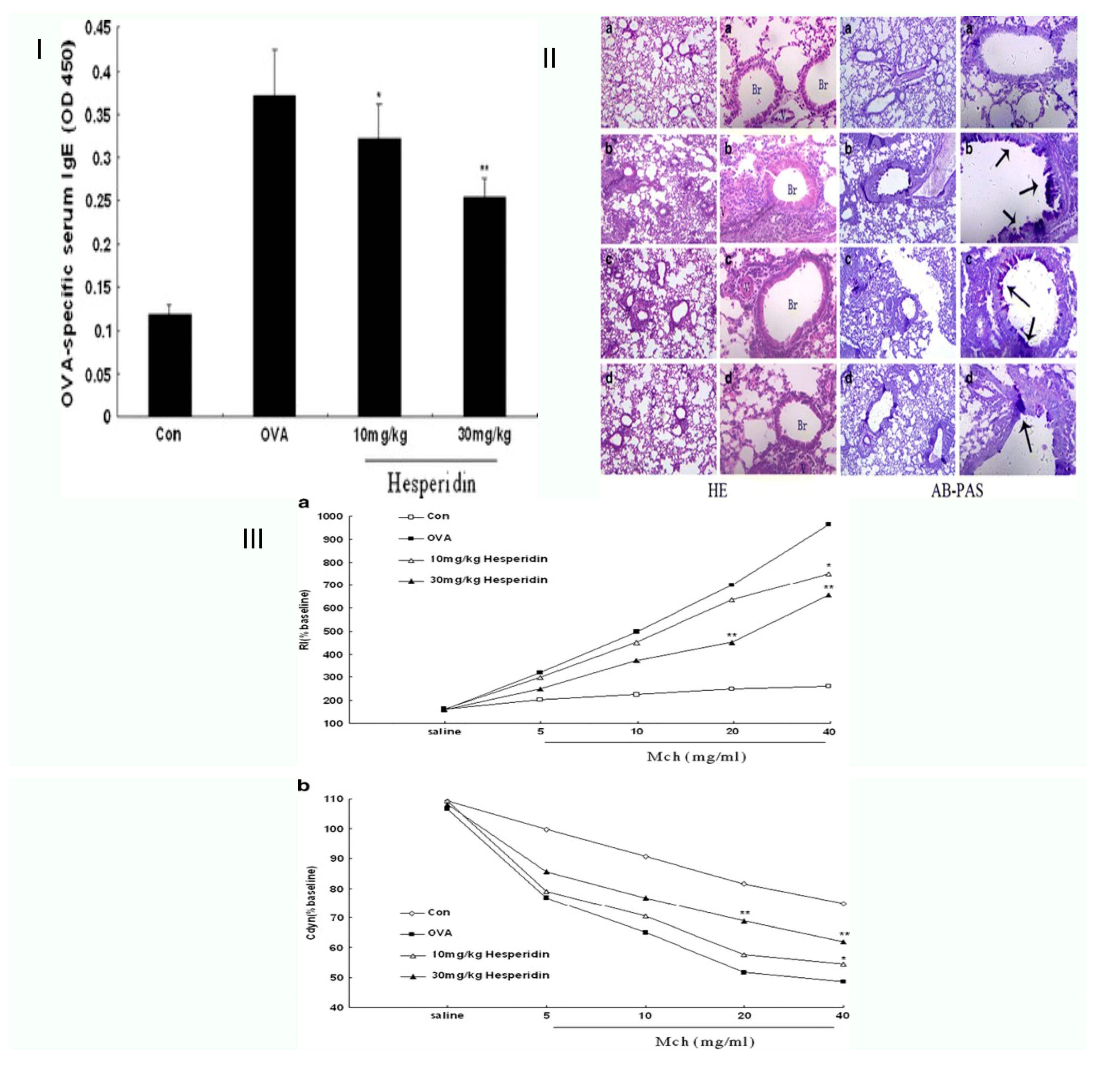
| Asthma | OVA-challenged mice | Allergic asthma model of the mouse. Hesperidin downregulated ovalbumin-induced inflammation of the airways. | Hesperidin suppressed inflammatory cell infiltration and mucus hypersecretion, decreasing OVA-specific IgE levels. | [26] |
| OVA-induced lung eosinophilia and mucus hypersecretion in a mouse model of asthma | Hesperidin-3′-O-Methylether inhibits phosphodiesterase more effectively than hesperidin airway hyperresponsiveness brought on by ovalbumin: inhibition and suppression | It decreased the blood levels of OVA-specific immunoglobulin (Ig)E and the total number of macrophages, macrophages, neutrophils, lymphocytes, and eosinophils. | [27] | |
| Eotaxin, IL-17, and OVA-specific IgE in vivo asthma model mice | Hesperidin, a prospective Th2 cytokine antagonist, has anti-asthmatic effects in a mouse model of asthma. | HPN efficiently cures asthma by inhibiting the transcription factor GATA-3, which lowers the development of the eotaxin and Th2 cytokines (IL-5). | [46] | |
| Asthmatic mouse model induced by ovalbum | Hesperidin’s anti-inflammatory properties and its underlying mechanisms in a mouse model of asthma induced by ovalbumin | Decreased serum OVA-specific immunoglobulin (Ig)E levels, total inflammatory cell counts, macrophages, lymphocytes, neutrophils, and eosinophils, considerably reducing all asthmatic symptoms. | [26] | |
| COPD | Cigarette smoke-exposed mice | Hesperidin’s ability to reduce oxidative stress and inflammatory reactions in mice having COPD may be connected to the SIRT1/PGC-1/NF-B signalling axis. | In mice with COPD caused by CES, hesperidin reduced the inflammatory and oxidative stress responses. | [47] |
| Pulmonary Fibrosis | Sprague–Dawley rats | By inhibiting the TGF-beta1/Smad3/AMPK, kappa alpha/NF-kappa B, and bleomycin-induced experimental lung fibrosis pathways, hesperidin reduces the severity of the condition. | Hesperidin reduces the effects of BLM-induced IPF by inhibiting the IB/NF-B, TGF-1/AMPK/Smad3 and oxide-inflammatory marker (HO-1and Nrf2) pro-inflammatory marker (TNF-, IL-6 and IL-1,) pathways. | [29] |
| Mice | Neohesperidin reduces bleomycin-induced lung fibrosis in rats and suppresses TGF-1/Smad3 signalling. | Lowered the formation of extracellular matrix, fibroblast migration, and myofibroblast differentiation caused by TGF-1. | [30] | |
| Tissue damage in the lung of male rats by induced radiation | Analyzing hesperidin’s radioprotective effect on acute radiation damage in rat lung tissue | In rats, oral treatment of HES was observed to protect against irradiation-induced pulmonary damage. This protective action against inflammatory diseases is likely a result of HES’s capacity to scavenge free radicals and stabilise membranes. | [34] | |
| Radiation-induced lung injury of male rats | Hesperidin as a radioprotector regarding radiation-induced lung damage in rat | HES dramatically reduced lung tissue fibrosis, inflammation, and mast cell proliferation. It also reduced radiation fibrosis and radiation pneumonitis. | [31] | |
| Bleomycin-rat model. | Hesperidin’s effects on pulmonary fibrosis were assessed using micro-computed tomography, histopathology, and a bleomycin-rat model. | Due to its anti-inflammatory, chemical, and antioxidant capabilities, HP therapy causes lung fibrosis while decreasing lipid peroxidation and raising antioxidant status. | [29] | |
| Bleomycin-induced pulmonary fibrosis in mice. | Hesperidin reduces pulmonary fibrosis by inhibiting lung fibroblast senescence through the IL-6/STAT3 signaling pathway. | Hesperidin decreased senescence-associated-galactosidase-positive cells both in vivo and in vitro, and it can block the IL6/STAT3 signalling pathway. | [33] | |
| Lung cancer | Inhibit cancer cell viability in vitro | Through pinx1, hesperidin prevents lung cancer both in vitro and in vivo. | Hesperidin dramatically enhanced pinx1 protein levels, and blocking pinx1 with a targeted siRNA prevented hesperidin’s protective effects. | [35] |
| Rodent model of lung cancer | Citrus juices and pulp with high levels of hesperidin and cryptoxanthin prevent cancer | HPs are a promising cancer chemopreventive agent against the formation of tongue, colon, and lung cancer because they inhibit chemically induced carcinogenesis by detoxifying enzymes, controlling the proliferation and mRNA expression of various cytokines and inflammatory enzymes. | [36] | |
| Lung initiated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in male A/J mice | Cryptoxanthin and hesperidin-rich mandarin juice’s inhibitory effects on 4-(methylnitrosamino)-1-(3-pyridyl) mouse pulmonary tumorigenesis brought on by 1-butanone | HP decreased the PCNA-positive index in lung cancers while not affecting the PCNA index in lesions with hyperplastic alveolar cells. | [48] | |
| Swiss albino mice | Hesperidin’s ability to fight cancer and benzo(a)pyrene-induced lung cancer in mice | Lipid peroxides (LPO), carcinoembryonic antigen (CEA), a lung-specific tumour marker, and the serum marker aryl hydrocarbon hydroxylase (AHH) and lactate dehydrogenase were all enhanced by HP. Conversely, these modifications exhibiting a strong anticancer impact in lung cancer were dramatically decreased by hesperidin. | [38] | |
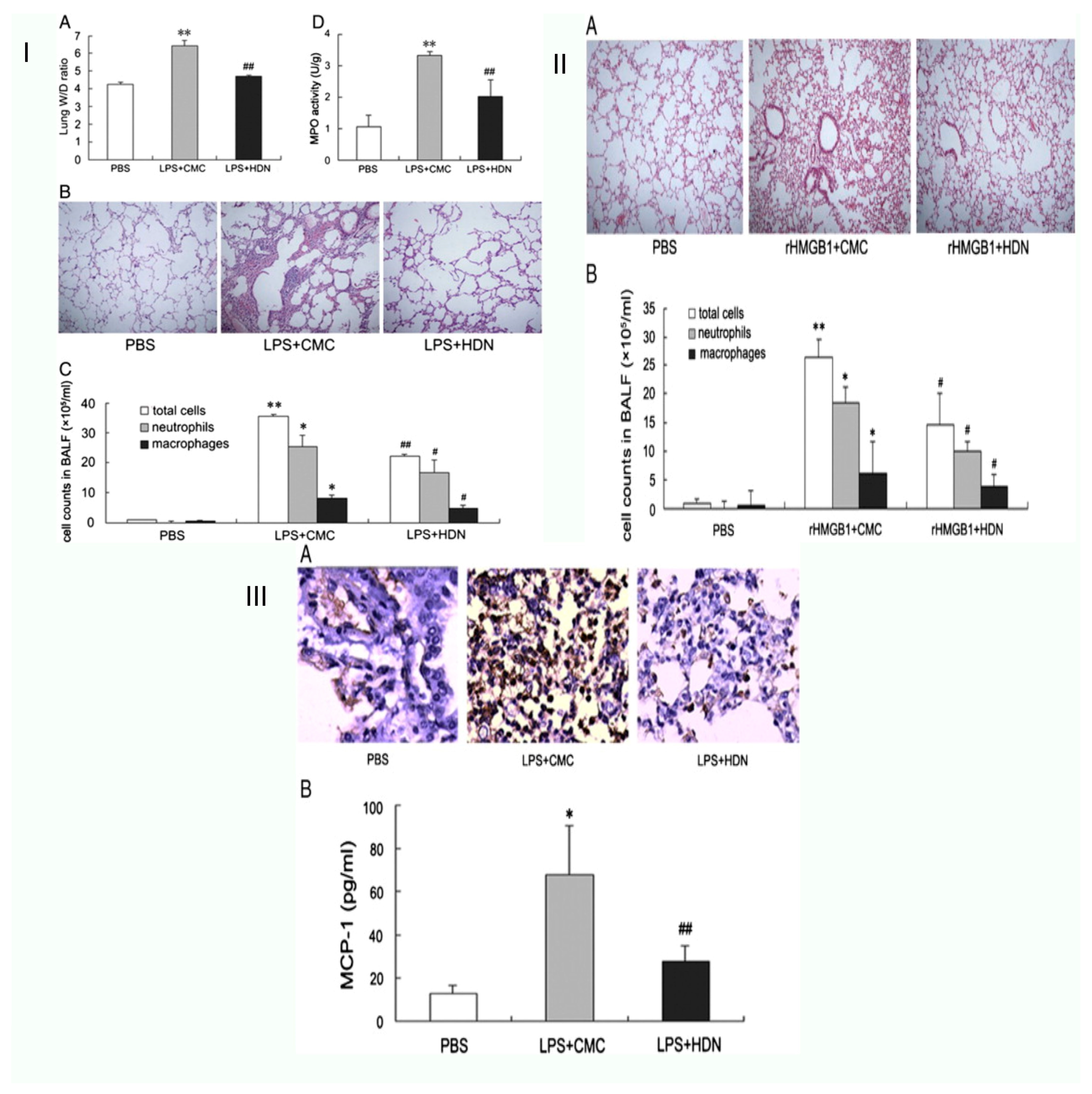
| Acute lung injury | Male BALB/c mice | By preventing the release of HMGB1, hesperidin reduces the acute lung damage brought on by lipopolysaccharide in mice. | HP inhibits the invasion of neutrophils and the synthesis of MCP-1, preventing the transcription and release of HMGB1. Along with neutrophils, macrophages, and myeloperoxidase (MPO) activity, HP cells lowered the elevation of the lung wet-to-dry weight ratio, and therefore lessened lung histological damage | [37] |
| In vivo and in vitro model of acute lung inflammation | Effects of hesperidin treatment in a model system of acute respiratory inflammation both in vitro and in vivo. | By regulating the chronic inflammation and redox imbalance, hesperidin may protect the lungs of mice exposed to mechanical ventilation and may even work to stop MV harm. | [41] | |
| LPS mice in vivo and cell line in vitro | Hesperidin’s in vivo and in vitro innate immunity of endotoxin-induced severe lung damage. | HES inhibits the inflammatory pathways and suppresses the production of ICAM-1, IL-8, IL-1, TNF, IL-6, IL-12, and VCAM-1 in THP-1 and A549 cells. | [40] | |
| Mouse model of acute lung injury | In a mouse model of acute lung injury, nasal administration of chitosan/ nanoparticles of hesperidin suppresses the cytokine storm syndrome. | In a mouse model of chronic lung illness, nasal administration of HPD/NPS lowers CSS and ALI/ARDS, indicating that anti-inflammatory nanoparticle-based therapeutic approaches may be employed to lessen CSS and ALI in people with inflammatory lung damage. | [49] | |
| Pneumonia | Radiation-induced lung injury male rats | A histopathological study on hesperidin as a radioprotector against radiation-induced lung damage in rats. | HES dramatically reduced lung tissue fibrosis, inflammation, and mast cell proliferation. It also reduced radiation fibrosis and radiation pneumonitis. | [31] |
| COVID-19 | Computational models | Using computational approaches, the medical targets for SARS-CoV-2 are analyzed, and promising medications are found. | One group of andrographolide compounds, hesperidin, showed a strong affinity. | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosawi, S. Current Update on Role of Hesperidin in Inflammatory Lung Diseases: Chemistry, Pharmacology, and Drug Delivery Approaches. Life 2023, 13, 937. https://doi.org/10.3390/life13040937
Hosawi S. Current Update on Role of Hesperidin in Inflammatory Lung Diseases: Chemistry, Pharmacology, and Drug Delivery Approaches. Life. 2023; 13(4):937. https://doi.org/10.3390/life13040937
Chicago/Turabian StyleHosawi, Salman. 2023. "Current Update on Role of Hesperidin in Inflammatory Lung Diseases: Chemistry, Pharmacology, and Drug Delivery Approaches" Life 13, no. 4: 937. https://doi.org/10.3390/life13040937
APA StyleHosawi, S. (2023). Current Update on Role of Hesperidin in Inflammatory Lung Diseases: Chemistry, Pharmacology, and Drug Delivery Approaches. Life, 13(4), 937. https://doi.org/10.3390/life13040937






