Expression of Glucose Transporters 1 and 3 in the Placenta of Pregnant Women with Gestational Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Collection of Placentas
2.3. Gene Expression Estimation
2.4. Protein Expression
2.5. Antibodies
2.6. Immunohistochemical (IHC) Staining
2.7. Apoptosis
2.8. Statistical Analysis
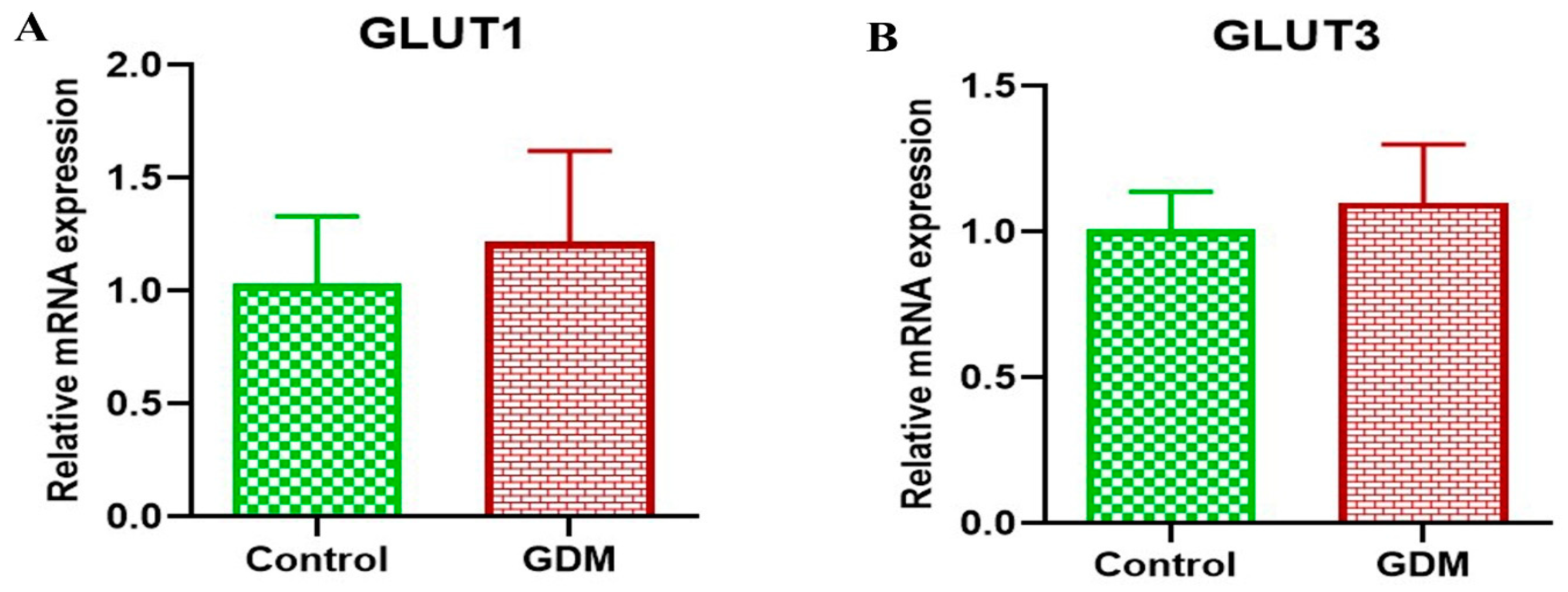
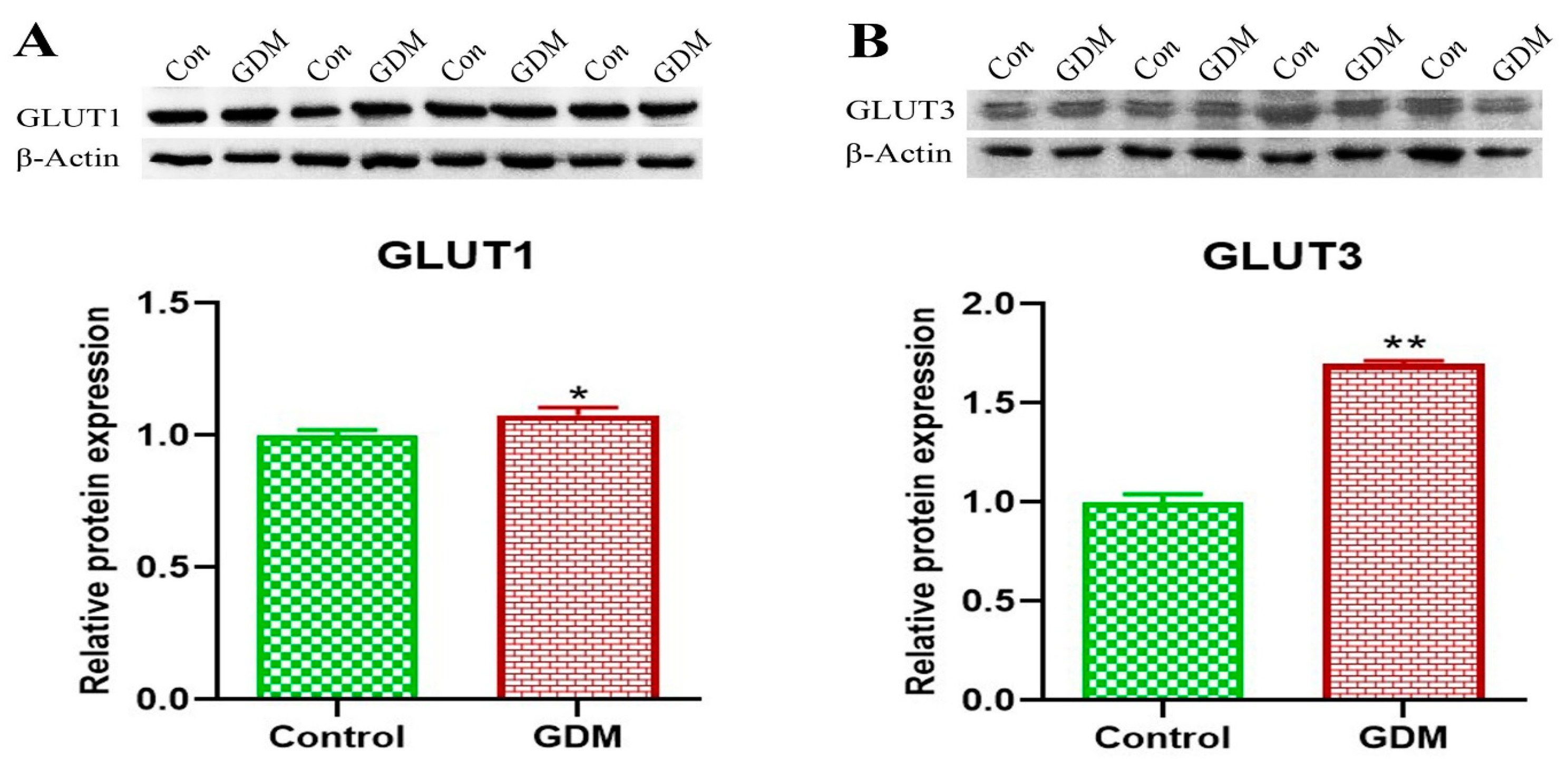
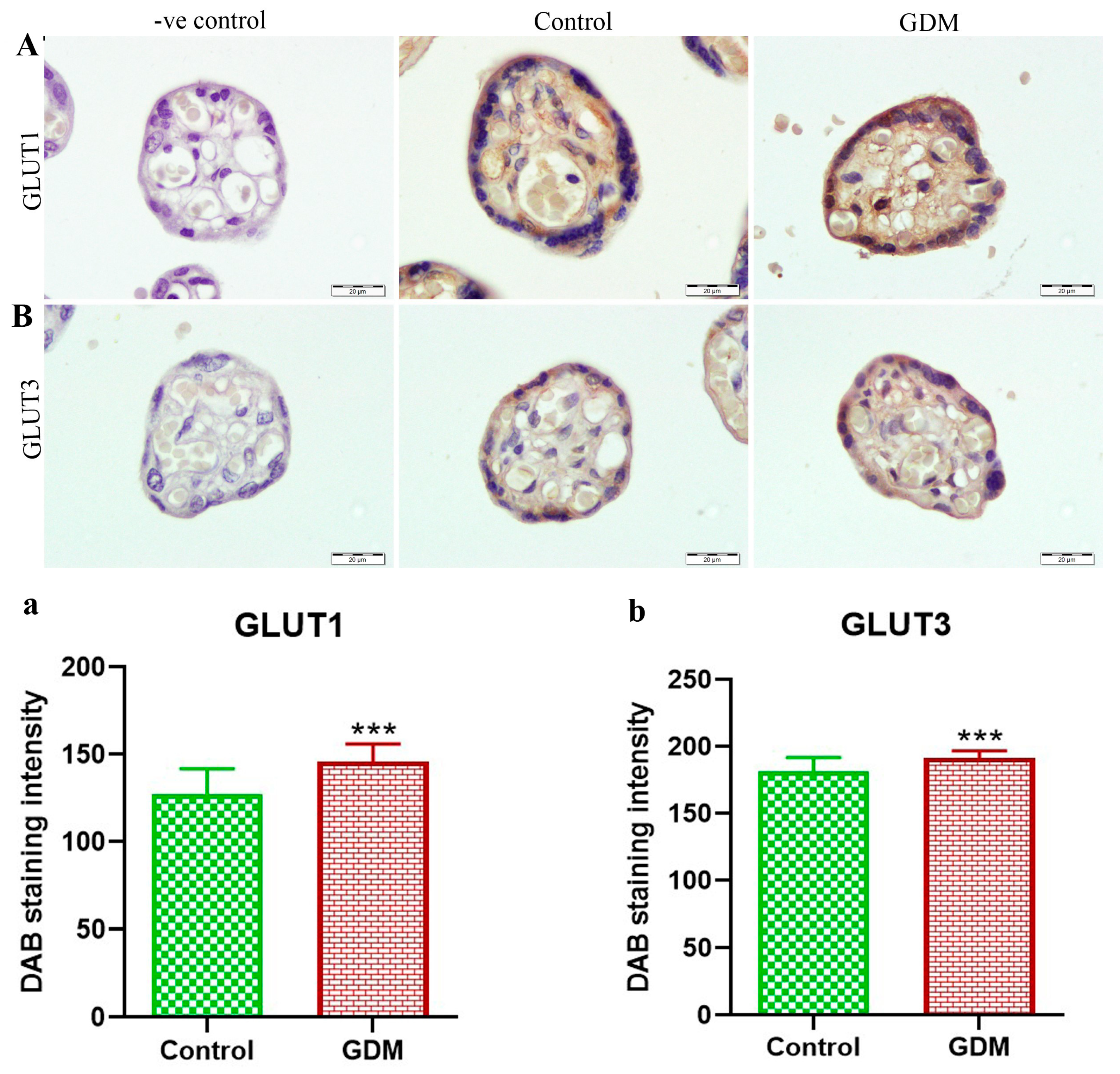
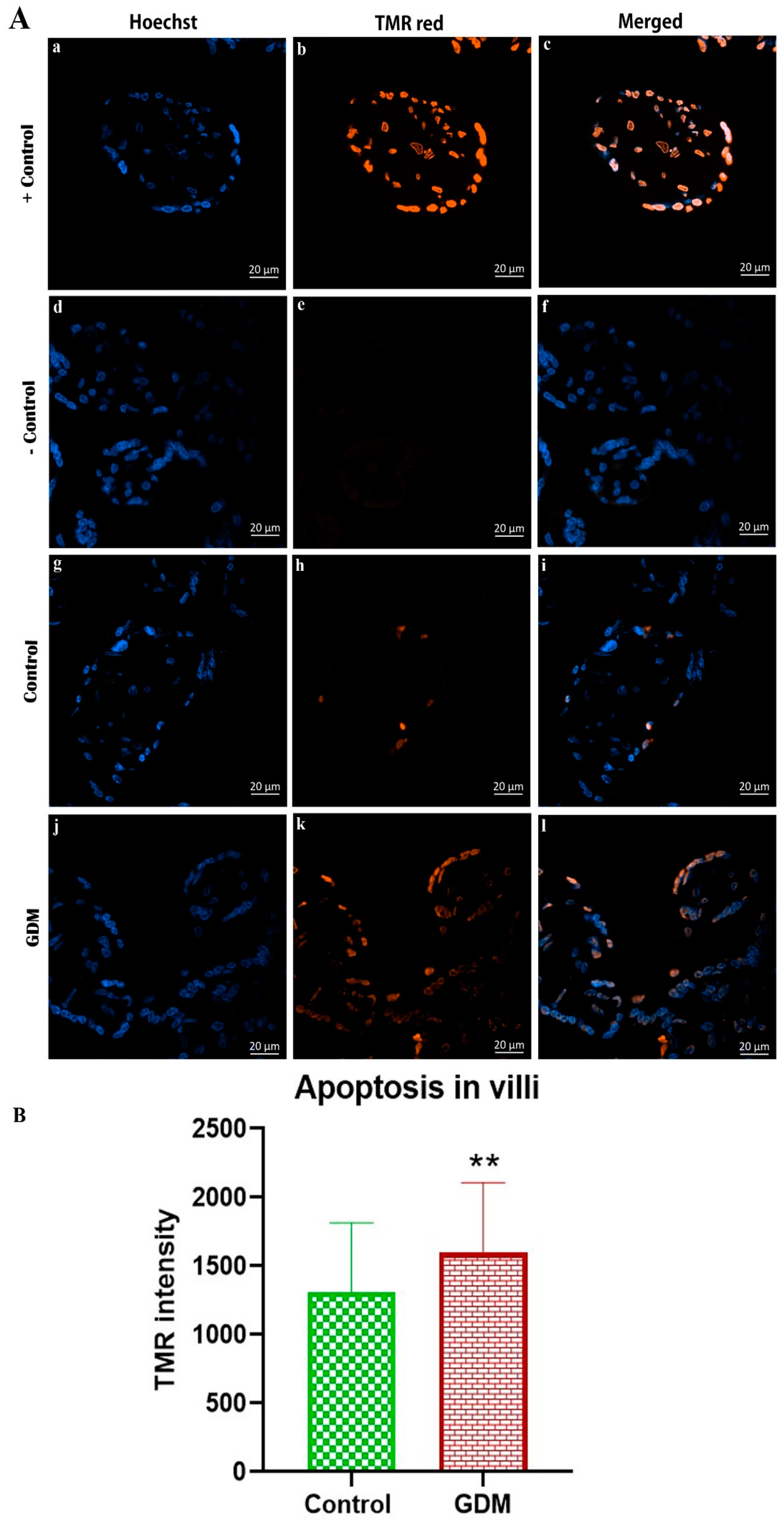
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAB | 3:3′-diaminobenzidine |
| ECL | Enhanced chemiluminescent |
| GDM | Gestational diabetes mellitus |
| GLUT | Glucose transporter |
| GLUT1 | Glucose transporter 1 |
| GLUT3 | Glucose transporter 3 |
| IADPSG | International Association of Diabetes and Pregnancy Study Groups |
| IHC | Immunohistochemical |
| KSUMC | King Saud University Medical City |
| NBF | Neutral buffered formalin |
| OGTT | Oral glucose tolerance test |
| PVDF | Polyvinylidene difluoride |
| TdT | Terminal deoxynucleotidyl transferase |
| TE | Tris-EDTA (Ethylenediaminetetraacetic acid) |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36, S67–S74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homko, C.; Sivan, E.; Chen, X.; Reece, E.A.; Boden, G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2001, 86, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Whalen, K.L.; Taylor, J.R. Gestational diabetes mellitus. In Pharmacotherapy Self-Assessment Program: Book 1, Endocrine/Nephrology; ACCP: Lenexa, KS, USA, 2017; pp. 7–23. [Google Scholar]
- Ferrara, A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care 2007, 30, S141–S146. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Du, S.; Sun, D.; Li, X.; Heianza, Y.; Hu, G.; Sun, L.; Pei, X.; Shang, X.; Qi, L. Prevalence and Trends in Gestational Diabetes Mellitus Among Women in the United States, 2006–2017: A Population-Based Study. Front. Endocrinol. 2022, 13, 868094. [Google Scholar] [CrossRef] [PubMed]
- Melchior, H.; Kurch-Bek, D.; Mund, M. The Prevalence of Gestational Diabetes. Dtsch. Arztebl. Int. 2017, 114, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Powe, C.E.; Immanuel, J.; Karuranga, S. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2021, 183, 109050. [Google Scholar] [CrossRef]
- Al-Rifai, R.H.; Abdo, N.M.; Paulo, M.S.; Saha, S.; Ahmed, L.A. Prevalence of Gestational Diabetes Mellitus in the Middle East and North Africa, 2000–2019: A Systematic Review, Meta-Analysis, and Meta-Regression. Front. Endocrinol. 2021, 12, 668447. [Google Scholar] [CrossRef]
- Dodd, J.M.; Crowther, C.A.; Antoniou, G.; Baghurst, P.; Robinson, J.S. Screening for gestational diabetes: The effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Aust. N. Z. J. Obs. Gynaecol. 2007, 47, 307–312. [Google Scholar] [CrossRef]
- Ozbasli, E.; Takmaz, O.; Karabuk, E.; Gungor, M. Comparison of factor XII levels in gestational diabetes, fetal macrosomia, and healthy pregnancies. BMC Pregnancy Childbirth 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Sermer, M.; Naylor, C.D.; Gare, D.J.; Kenshole, A.B.; Ritchie, J.W.K.; Farine, D.; Cohen, H.R.; McArthur, K.; Holzapfel, S.; Biringer, A.; et al. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes: The Toronto tri-hospital gestational diabetes project. Am. J. Obstet. Gynecol. 1995, 173, 146–156. [Google Scholar] [CrossRef]
- Group, H.S.C.R. The hyperglycemia and adverse pregnancy outcome (HAPO) study. Int. J. Gynecol. Obstet. 2002, 78, 69–77. [Google Scholar]
- Catalano, P.M.; Ehrenberg, H.M. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.; González, M.; Acurio, J.; Valenzuela, F.; Sobrevia, L. The role of placenta in the fetal programming associated to gestational diabetes. Gestation. Diabetes-Causes Diagn. Treat. 2013, 1, 139–141. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Guo, F.; Peng, Y.; Chen, R.; Zhou, W.; Wang, H.; OuYang, J.; Yu, B.; Xu, Z. Transcriptomic Profiling of Human Placenta in Gestational Diabetes Mellitus at the Single-Cell Level. Front. Endocrinol. 2021, 12, 679582. [Google Scholar] [CrossRef] [PubMed]
- Silverman, B.L.; Rizzo, T.; Green, O.C.; Cho, N.H.; Winter, R.J.; Ogata, E.S.; Richards, G.E.; Metzger, B.E. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes 1991, 40, 121–125. [Google Scholar] [CrossRef]
- Holder, T.; Giannini, C.; Santoro, N.; Pierpont, B.; Shaw, M.; Duran, E.; Caprio, S.; Weiss, R. A low disposition index in adolescent offspring of mothers with gestational diabetes: A risk marker for the development of impaired glucose tolerance in youth. Diabetologia 2014, 57, 2413–2420. [Google Scholar] [CrossRef] [Green Version]
- Sheiner, E. Gestational Diabetes Mellitus: Long-Term Consequences for the Mother and Child Grand Challenge: How to Move on Towards Secondary Prevention? Front. Clin. Diabetes Healthc. 2020, 1, 546256. [Google Scholar] [CrossRef]
- Kalhan, S.; Parimi, P. Gluconeogenesis in the fetus and neonate. Semin. Perinatol. 2000, 24, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Brett, K.E.; Ferraro, Z.M.; Yockell-Lelievre, J.; Gruslin, A.; Adamo, K.B. Maternal–fetal nutrient transport in pregnancy pathologies: The role of the placenta. Int. J. Mol. Sci. 2014, 15, 16153–16185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Biswas, S.; Mitra, D.; Adhikari, A.; Saha, C. Histologic and morphometric study of human placenta in gestational diabetes mellitus. Ital. J. Anat. Embryol. 2014, 119, 1–9. [Google Scholar]
- Treesh, S.A.; Khair, N.S. Histological changes of the human placenta in pregnancies complicated with diabetes. J. Cytol. Histol. 2015, 6, 1. [Google Scholar] [CrossRef]
- El Sawy, N.A.; Iqbal, M.S.; Alkushi, A.G.; EL Sawy, N.; Iqbal, M.; Alkushi, A. Histomorphological study of placenta in gestational diabetes mellitus. Int. J. Morphol. 2018, 36, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Aldahmash, W.M.; Alwasel, S.H.; Aljerian, K. Gestational diabetes mellitus induces placental vasculopathies. Environ. Sci. Pollut. Res. 2022, 29, 19860–19868. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Lee, K.E.; Byeon, E.J.; Choi, J.; Kim, S.J.; Shin, J.E. Maternal Gestational Diabetes Influences DNA Methylation in the Serotonin System in the Human Placenta. Life 2022, 12, 1869. [Google Scholar] [CrossRef] [PubMed]
- Stanirowski, P.J.; Szukiewicz, D.; Pyzlak, M.; Abdalla, N.; Sawicki, W.; Cendrowski, K. Impact of pre-gestational and gestational diabetes mellitus on the expression of glucose transporters GLUT-1, GLUT-4 and GLUT-9 in human term placenta. Endocrine 2017, 55, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Baumann, M.U.; Deborde, S.; Illsley, N.P. Placental glucose transfer and fetal growth. Endocrine 2002, 19, 13–22. [Google Scholar] [CrossRef]
- Illsley, N. Current topic: Glucose transporters in the human placenta. Placenta 2000, 21, 14–22. [Google Scholar] [CrossRef]
- Brown, K.; Heller, D.S.; Zamudio, S.; Illsley, N.P. Glucose transporter 3 (GLUT3) protein expression in human placenta across gestation. Placenta 2011, 32, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Jansson, T.; Wennergren, M.; Illsley, N.P. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J. Clin. Endocrinol. Metab. 1993, 77, 1554–1562. [Google Scholar] [CrossRef]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Addo, V.N. Body Mass Index, Weight Gain during Pregnancy and Obstetric Outcomes. Ghana. Med. J. 2010, 44, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Taricco, E.; Radaelli, T.; De Santis, M.N.; Cetin, I. Foetal and placental weights in relation to maternal characteristics in gestational diabetes. Placenta 2003, 24, 343–347. [Google Scholar] [CrossRef]
- Alfadhli, E. Gestational diabetes in Saudi women identified by the International Association of Diabetes and Pregnancy Study Group versus the former American Diabetes Association criteria: A prospective cohort study. Ann. Saudi. Med. 2015, 35, 428–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsaedi, S.A.; Altalhi, A.A.; Nabrawi, M.F.; Aldainy, A.A.; Wali, R.M. Prevalence and risk factors of gestational diabetes mellitus among pregnant patients visiting National Guard primary health care centers in Saudi Arabia. Saudi. Med. J. 2020, 41, 144. [Google Scholar] [CrossRef]
- Kadivar, M.; Khamseh, M.E.; Malek, M.; Khajavi, A.; Noohi, A.H.; Najafi, L. Histomorphological changes of the placenta and umbilical cord in pregnancies complicated by gestational diabetes mellitus. Placenta 2020, 97, 71–78. [Google Scholar] [CrossRef]
- Mondestin, M.A.; Ananth, C.V.; Smulian, J.C.; Vintzileos, A.M. Birth weight and fetal death in the United States: The effect of maternal diabetes during pregnancy. Am. J. Obs. Gynecol. 2002, 187, 922–926. [Google Scholar] [CrossRef]
- Daskalakis, G.; Marinopoulos, S.; Krielesi, V.; Papapanagiotou, A.; Papantoniou, N.; Mesogitis, S.; Antsaklis, A. Placental pathology in women with gestational diabetes. Acta. Obstet. Et. Gynecol. Scand. 2008, 87, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; El-Sayed, A.A.; Khoja, T.; Alshamsan, R.; Millett, C.; Rawaf, S. Diabetes in the Middle-East and North Africa: An update. Diabetes Res. Clin. Pract. 2014, 103, 218–222. [Google Scholar] [CrossRef]
- Augustine, G.; Pulikkathodi, M.; Renjith, S.; Jithesh, T. A study of placental histological changes in gestational diabetes mellitus on account of fetal hypoxia. Int. J. Med. Sci. Public Health 2016, 5, 2457–2460. [Google Scholar] [CrossRef] [Green Version]
- Barrosa, L.; Yudilevich, D.; Jarvis, S.M.; Beaumont, N.; Baldwin, S. Quantitation and immunolocalization of glucose transporters in the human placenta. Placenta 1995, 16, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, A.; Hamark, B.; Powell, T.; Jansson, T. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum. Reprod. 2005, 20, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Takata, K.; Kasahara, T.; Kasahara, M.; Ezaki, O.; Hirano, H. Localization of erythrocyte/HepG2-type glucose transporter (GLUT1) in human placental villi. Cell. Tissue. Res. 1992, 267, 407–412. [Google Scholar] [CrossRef]
- Jansson, T.; Wennergren, M.; Powell, T.L. Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes. Am. J. Obstet. Gynecol. 1999, 180, 163–168. [Google Scholar] [CrossRef]
- Jansson, T.; Ekstrand, Y.; Wennergren, M.; Powell, T.L. Placental glucose transport in gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2001, 184, 111–116. [Google Scholar] [CrossRef]
- Gaither, K.; Quraishi, A.N.; Illsley, N.P. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J. Clin. Endocrinol. Metab. 1999, 84, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Muralimanoharan, S.; Maloyan, A.; Myatt, L. Mitochondrial function and glucose metabolism in the placenta with gestational diabetes mellitus: Role of miR-143. Clin. Sci. 2016, 130, 931–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, P.; Dimasuay, K.G.; Koele-Schmidt, L.; Jang, B.; Barbour, L.A.; Jansson, T.; Powell, T.L. Glyburide treatment in gestational diabetes is associated with increased placental glucose transporter 1 expression and higher birth weight. Placenta 2017, 57, 52–59. [Google Scholar] [CrossRef]
- Borges, M.H.; Pullockaran, J.; Catalano, P.M.; Baumann, M.U.; Zamudio, S.; Illsley, N.P. Human placental GLUT1 glucose transporter expression and the fetal insulin-like growth factor axis in pregnancies complicated by diabetes. Biochim. Et. Biophys. Acta. (BBA)-Mol. Basis. Dis. 2019, 1865, 2411–2419. [Google Scholar] [CrossRef]
- Illsley, N.P.; Baumann, M.U. Human placental glucose transport in fetoplacental growth and metabolism. Biochim. Et. Biophys. Acta. (BBA)-Mol. Basis. Dis. 2020, 1866, 165359. [Google Scholar] [CrossRef]
- Desoye, G.; Shafrir, E. Placental metabolism and its regulation in health and diabetes. Mol. Asp. Med. 1994, 15, 505–682. [Google Scholar] [CrossRef] [PubMed]
- Colomiere, M.; Permezel, M.; Riley, C.; Desoye, G.; Lappas, M. Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur. J. Endocrinol. 2009, 160, 567–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, P.; Powell, T.L.; Jansson, T. The role of placental nutrient sensing in maternal-fetal resource allocation. Biol. Reprod. 2014, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.W. The placenta: Not just a conduit for maternal fuels. Diabetes 1991, 40, 44–50. [Google Scholar] [CrossRef]
- Song, T.-R.; Su, G.-D.; Chi, Y.-L.; Wu, T.; Xu, Y.; Chen, C.-C. Dysregulated miRNAs contribute to altered placental glucose metabolism in patients with gestational diabetes via targeting GLUT1 and HK2. Placenta 2021, 105, 14–22. [Google Scholar] [CrossRef]
- Osmond, D.; Nolan, C.; King, R.G.; Brennecke, S.P.; Gude, N.M. Effects of gestational diabetes on human placental glucose uptake, transfer, and utilisation. Diabetologia 2000, 43, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.P.; Islam, Z. Gestational diabetes: Effect on gross morphology of human placenta and birth weight. Pak. J. Med. Health Sci. 2013, 7, 1077–1081. [Google Scholar]
- Bhanu, S.; Sankar, D.; Swetha, M.; Kiran, S.; Devi, S. Morphological and micrometrical changes of the placental terminal villi in normal and pregnancies complicated with gestational diabetes mellitus. J. Evid. Based Med. Health 2016, 3, 3676–3680. [Google Scholar] [CrossRef]
- Araújo, J.R.; Keating, E.; Martel, F. Impact of gestational diabetes mellitus in the maternal-to-fetal transport of nutrients. Curr. Diab. Rep. 2015, 15, 569. [Google Scholar] [CrossRef]
- Griffin, M.E.; Hamilton, B.J.; Roy, K.M.; Du, M.; Willson, A.M.; Keenan, B.J.; Wang, X.W.; Nichols, R.C. Post-transcriptional regulation of glucose transporter-1 by an AU-rich element in the 3’UTR and by hnRNP A2. Biochem. Biophys. Res. Commun. 2004, 318, 977–982. [Google Scholar] [CrossRef]
- Sgarbosa, F.; Barbisan, L.F.; Brasil, M.A.; Costa, E.; Calderon, I.M.; Gonçalves, C.R.; Bevilacqua, E.; Rudge, M.V. Changes in apoptosis and Bcl-2 expression in human hyperglycemic, term placental trophoblast. Diabetes Res. Clin. Pract. 2006, 73, 143–149. [Google Scholar] [CrossRef]
- Akarsu, S.; Bagirzade, M.; Omeroglu, S.; Büke, B. Placental vascularization and apoptosis in Type-1 and gestational DM. J. Matern. -Fetal. Neonatal. Med. 2017, 30, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Li, D.F.; Feng, Z.Q.; Du, J.; Zhao, W.H.; Huang, N.; Jia, J.C.; Wu, Z.Y.; Wang, Y.Y.; Ji, X.L.; Yu, L. Mechanism of placenta damage in gestational diabetes mellitus by investigating TXNIP of patient samples and gene functional research in cell line. Diabetes Ther. 2019, 10, 2265–2288. [Google Scholar] [CrossRef] [Green Version]
- Gül, M.; Bayat, N.; Çetin, A.; Kepekçi, R.A.; Şimşek, Y.; Kayhan, B.; Turhan, U.; Otlu, A. Histopathological, Ultrastructural and Apoptotic Changes in Diabetic Rat Placenta. Balk. Med. J. 2015, 32, 296–302. [Google Scholar] [CrossRef]
- Ji, L.; Chen, Z.; Xu, Y.; Xiong, G.; Liu, R.; Wu, C.; Hu, H.; Wang, L. Systematic Characterization of Autophagy in Gestational Diabetes Mellitus. Endocrinology 2017, 158, 2522–2532. [Google Scholar] [CrossRef]
- Nelissen, E.C.; van Montfoort, A.P.; Dumoulin, J.C.; Evers, J.L. Epigenetics and the placenta. Hum. Reprod. Update 2011, 17, 397–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sailasree, S.P.; Srivastava, S.; Mishra, R.K. The placental gateway of maternal transgenerational epigenetic inheritance. J. Genet. 2017, 96, 465–482. [Google Scholar] [CrossRef]
- Januar, V.; Desoye, G.; Novakovic, B.; Cvitic, S.; Saffery, R. Epigenetic regulation of human placental function and pregnancy outcome: Considerations for causal inference. Am. J. Obstet. Gynecol. 2015, 213, S182–S196. [Google Scholar] [CrossRef]
- Lesseur, C.; Chen, J. Adverse maternal metabolic intrauterine environment and placental epigenetics: Implications for fetal metabolic programming. Curr. Environ. Health Rep. 2018, 5, 531–543. [Google Scholar] [CrossRef]
- Filardi, T.; Catanzaro, G.; Mardente, S.; Zicari, A.; Santangelo, C.; Lenzi, A.; Morano, S.; Ferretti, E. Non-coding RNA: Role in gestational diabetes pathophysiology and complications. Int. J. Mol. Sci. 2020, 21, 4020. [Google Scholar] [CrossRef]
| Control (n = 34) | GDM (n = 31) | p Value | ||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Mothers | Age (year) | 28.2 ± 5.9 | 32.4 ± 5.3 | <0.01 |
| Height (cm) | 158 ± 4.3 | 157.3 ± 4.3 | 0.51 | |
| Weight (kg) | 73.4 ± 14.5 | 84.3 ± 14.3 | <0.01 | |
| BMI (kg/m2) | 29.2 ± 5.4 | 34.1 ± 5.4 | <0.01 | |
| OGTT | Fasting | 4.5 ± 0.8 | 5.2 ± 1.2 | 0.02 |
| (mmol/L) | 1-h | 7.2 ± 1.8 | 9.9 ± 1.8 | <0.01 |
| 2-h | 5.9 ± 1.6 | 8.9 ± 1.9 | <0.01 | |
| Newborns | Gestational age (week) | 39.3 ± 0.9 | 38.5 ± 1.6 | 0.02 |
| Birth weight (g) | 3110 ± 344 | 3338 ± 377 | 0.02 | |
| Thigh circumference (cm) | 15.8 ± 1.2 | 16.6 ± 1.7 | 0.04 | |
| Placentas | Weight (g) | 400 ± 58 | 451.2 ± 87 | <0.01 |
| Length (cm) | 18.4 ± 2 | 19.6 ± 2.3 | 0.04 | |
| Width (cm) | 16.2 ± 1.6 | 16.2 ± 1.8 | 0.86 | |
| Umbilical Cords | Length (cm) | 53 ± 9.8 | 56.1 ± 13 | 0.19 |
| Diameter (cm) | 1.05 ± 0.18 | 1.2 ± 0.18 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldahmash, W.; Harrath, A.H.; Aljerian, K.; Sabr, Y.; Alwasel, S. Expression of Glucose Transporters 1 and 3 in the Placenta of Pregnant Women with Gestational Diabetes Mellitus. Life 2023, 13, 993. https://doi.org/10.3390/life13040993
Aldahmash W, Harrath AH, Aljerian K, Sabr Y, Alwasel S. Expression of Glucose Transporters 1 and 3 in the Placenta of Pregnant Women with Gestational Diabetes Mellitus. Life. 2023; 13(4):993. https://doi.org/10.3390/life13040993
Chicago/Turabian StyleAldahmash, Waleed, Abdel Halim Harrath, Khaldoon Aljerian, Yasser Sabr, and Saleh Alwasel. 2023. "Expression of Glucose Transporters 1 and 3 in the Placenta of Pregnant Women with Gestational Diabetes Mellitus" Life 13, no. 4: 993. https://doi.org/10.3390/life13040993







