Substance P Alleviates Retinal Pigment Epithelium Dysfunction Caused by High Glucose-Induced Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. SP Administration
2.4. MTT Assay
2.5. Western Blotting
2.6. Glutathione Peroxidase (GPx) Activity Assay
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Transwell Migration Assay
2.9. Statistical Analysis
3. Results
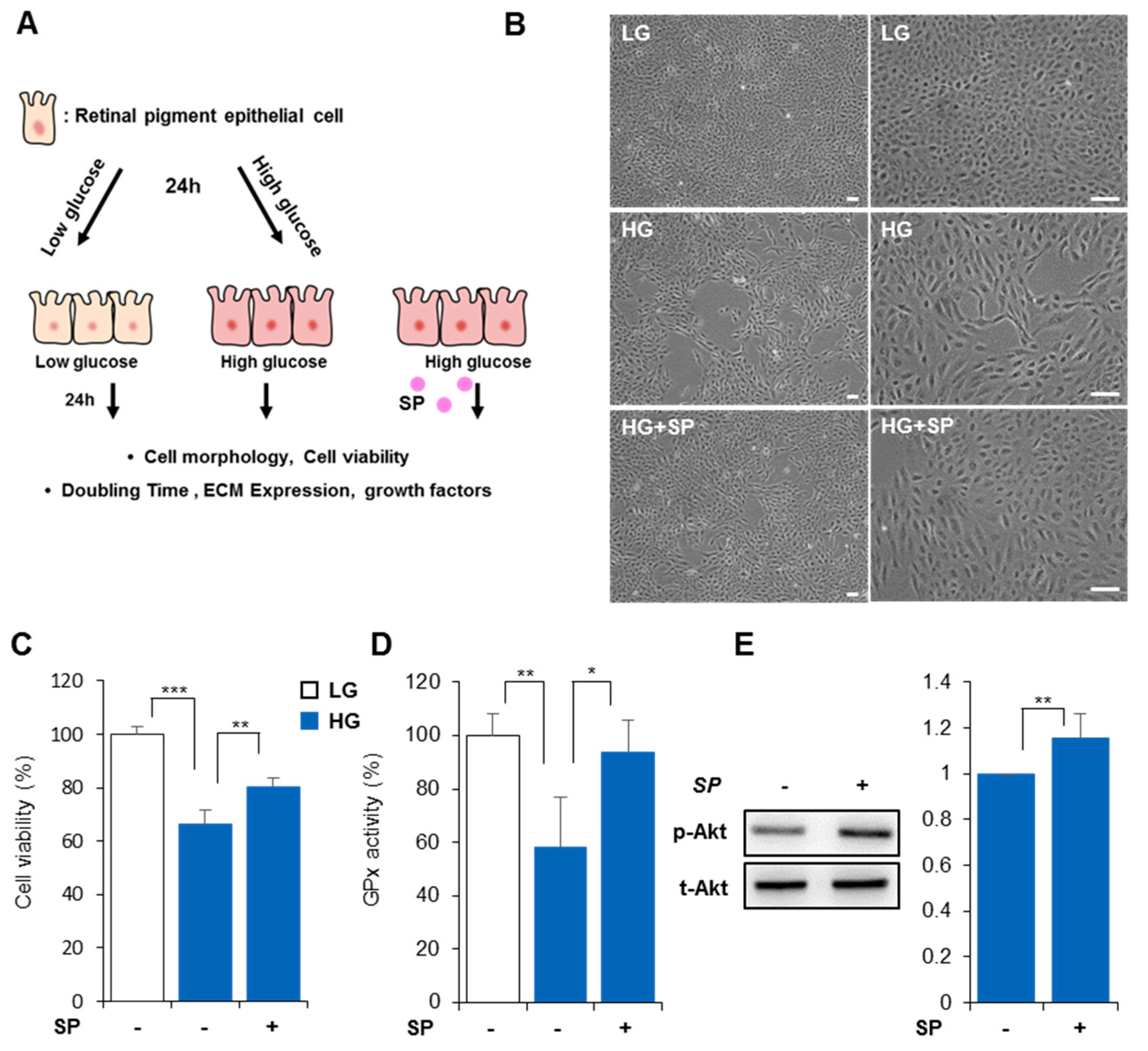
3.1. High Glucose Reduces Cellular Activity and Proliferation Rate of RPE
3.2. High Glucose Levels Alter RPE Cellular Characteristics
3.3. SP Prevents Reduction of RPE Activity Due to High Glucose Levels
3.4. SP Mitigates Functional/Structural Deficiency of RPE from High Glucose-Induced Oxidative Stress
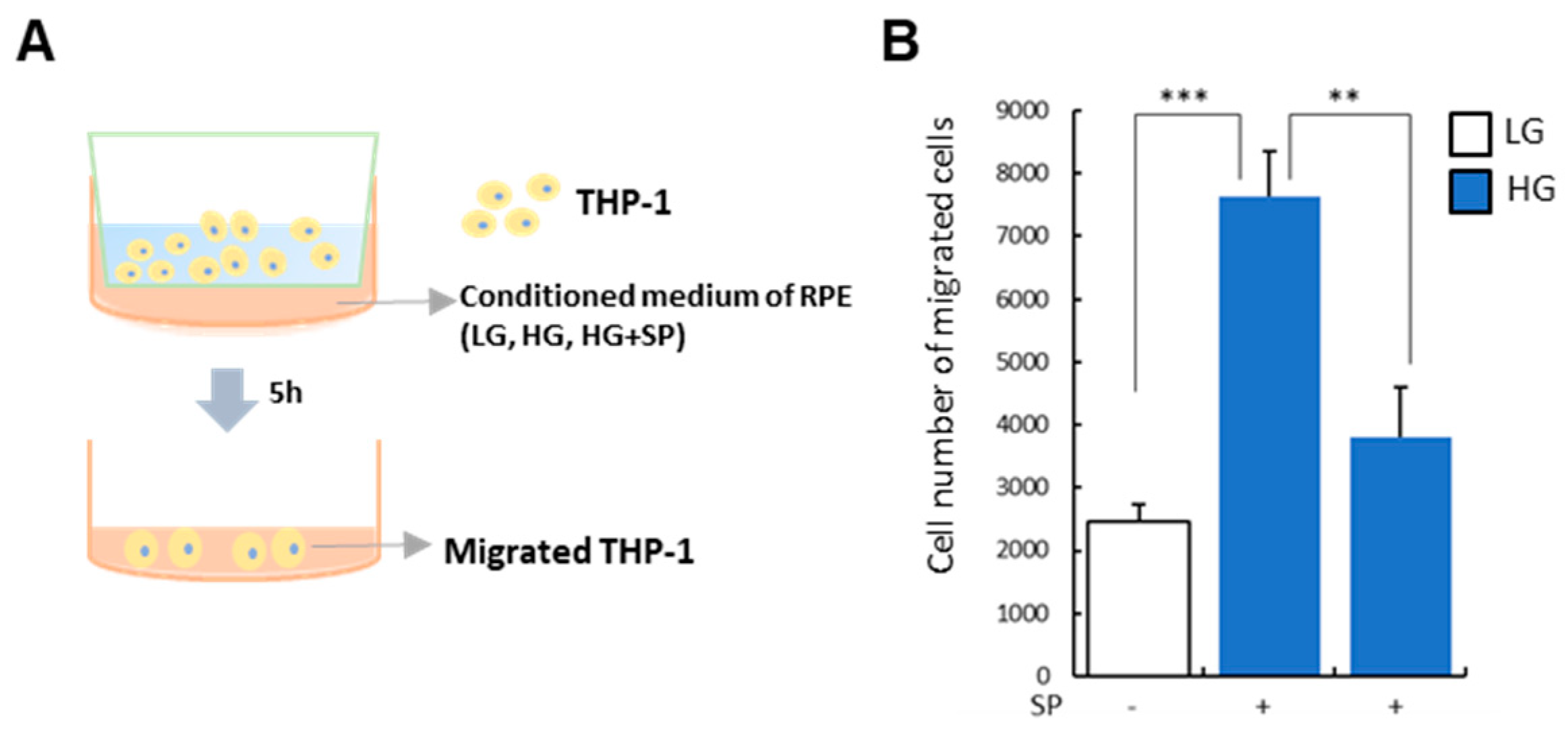
3.5. SP Treatment Modulates Paracrine Action of RPE under HG Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, Q.; Zhao, Y.; Xu, J.; Li, W.J.; Chen, Y.; Sun, H.J. NFE2/miR-423-5p/TFF1 axis regulates high glucose-induced apoptosis in retinal pigment epithelial cells. BMC Mol. Cell Biol. 2019, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasad, S.; Pearce, E. The unmet need for better risk stratification of non-proliferative diabetic retinopathy. Diabet. Med. 2019, 36, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Murugeswari, P.; Subramani, M.; Jayadev, C.; Shetty, R.; Das, D. Retinal pigment epithelium-secretome: A diabetic retinopathy perspective. Cytokine 2017, 95, 126–135. [Google Scholar]
- Yang, S.; Zhou, J.; Li, D. Functions and diseases of the retinal pigment epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef]
- Murakami, T.; Yoshimura, N. Structural changes in individual retinal layers in diabetic macular edema. J. Diabetes Res. 2013, 2013, 920713. [Google Scholar] [CrossRef]
- Pavan, B.; Dalpiaz, A. Retinal pigment epithelial cells as a therapeutic tool and target against retinopathies. Drug Discov. Today 2018, 23, 1672–1679. [Google Scholar] [CrossRef]
- Plafker, S.M.; O’Mealey, G.B.; Szweda, L.I. Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. Int. Rev. Cell Mol. Biol. 2012, 298, 135–177. [Google Scholar] [CrossRef]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Xu, H.; Song, Z.; Fu, S.; Zhu, M.; Le, Y. RPE barrier breakdown in diabetic retinopathy: Seeing is believing. J. Ocul. Biol. Dis. Inform. 2011, 4, 83–92. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Farnoodian, M.; Halbach, C.; Slinger, C.; Pattnaik, B.R.; Sorenson, C.M.; Sheibani, N. High glucose promotes the migration of retinal pigment epithelial cells through increased oxidative stress and PEDF expression. Am. J. Physiol. Cell Physiol. 2016, 311, C418–C436. [Google Scholar] [CrossRef] [PubMed]
- Korte, G.E.; Reppucci, V.; Henkind, P. RPE destruction causes choriocapillary atrophy. Invest. Ophthalmol. Vis. Sci. 1984, 25, 1135–1145. [Google Scholar] [PubMed]
- Klettner, A. Oxidative stress induced cellular signaling in RPE cells. Front. Biosci. (Schol. Ed.) 2012, 4, 392–411. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Peairs, J.J.; Tano, R.; Jaffe, G.J. Oxidant-mediated Akt activation in human RPE cells. Invest. Ophthalmol. Vis. Sci. 2016, 47, 4598–4606. [Google Scholar] [CrossRef]
- Vinores, S.A.; Gadegbeku, C.; Campochiaro, P.A.; Green, W.R. Immunohistochemical localization of blood-retinal barrier breakdown in human diabetics. Am. J. Pathol. 1989, 134, 231–235. [Google Scholar]
- Scarinci, F.; Jampol, L.M.; Linsenmeier, R.A.; Fawzi, A.A. Association of diabetic macular nonperfusion with outer retinal disruption on optical coherence tomography. JAMA Ophthalmol. 2015, 133, 1036–1044. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Murugeswari, P.; Shukla, D.; Rajendran, A.; Kim, R.; Namperumalsamy, P.; Muthukkaruppan, V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and Eales’ disease. Retina 2008, 28, 817–824. [Google Scholar] [CrossRef]
- Maugeri, G.; Bucolo, C.; Drago, F.; Rossi, S.; di Rosa, M.; Imbesi, R.; d’Agata, V.; Giunta, S. Attenuation of high glucose-induced damage in RPE cells through p38 MAPK signaling pathway inhibition. Front. Pharmacol. 2021, 12, 684680. [Google Scholar] [CrossRef]
- Rajala, R.V.; Anderson, R.E. Rhodopsin-regulated insulin receptor signaling pathway in rod photoreceptor neurons. Mol. Neurobiol. 2010, 42, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Sun, H.; Zhang, C.; Li, C.; Wang, J.; Shen, J.; Yu, D.; Kong, L. Protective function of pyridoxamine on retinal photoreceptor cells via activation of the p Erk1/2/Nrf2/Trx/ASK1 signalling pathway in diabetic mice. Mol. Med. Rep. 2016, 14, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wang, Z.; Liu, C.H.; Gong, Y.; Cakir, B.; Liegl, R.; Sun, Y.; Meng, S.S.; Burnim, S.B.; Arellano, I.; et al. Fibroblast growth factor 21 protects photoreceptor function in type 1 diabetic mice. Diabetes 2018, 67, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Atlasz, T.; Kiss, P.; Reglodi, D.; Szabo, A.; Kovacs, K.; Szalontai, B.; Setalo, G.; Banki, E.; Csanaky, K.; et al. Protective effects of the neuropeptide PACAP in diabetic retinopathy. Cell Tissue Res. 2012, 348, 37–46. [Google Scholar] [CrossRef]
- Arimura, N.; Otsuka, H.; Yamakiri, K.; Sonoda, Y.; Nakao, S.; Noda, Y.; Hashiguchi, T.; Maruyama, I.; Sakamoto, T. Vitreous mediators after intravitreal bevacizumab or triamcinolone acetonide in eyes with proliferative diabetic retinopathy. Ophthalmology 2009, 116, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, W.; Tzekov, R.; Jiang, F.; Mao, S.; Tong, Y. Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema: A meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e115797. [Google Scholar] [CrossRef]
- Radtke, N.D.; Aramant, R.B.; Petry, H.M.; Green, P.T.; Pidwell, D.J.; Seiler, M.J. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am. J. Ophthalmol. 2008, 146, 172–182. [Google Scholar] [CrossRef]
- Binder, S.; Krebs, I.; Hilgers, R.D.; Abri, A.; Stolba, U.; Assadoulina, A.; Kellner, L.; Stanzel, B.V.; Jahn, C.; Feichtinger, H.; et al. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: A prospective trial. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4151–4160. [Google Scholar] [CrossRef]
- Smit-McBride, Z.; Morse, L.S. MicroRNA and diabetic retinopathy-biomarkers and novel therapeutics. Ann. Transl. Med. 2021, 9, 1280. [Google Scholar] [CrossRef]
- Gaddam, S.; Periasamy, R.; Gangaraju, R. Adult stem cell therapeutics in diabetic retinopathy. Int. J. Mol. Sci. 2019, 20, 4876. [Google Scholar] [CrossRef]
- Hong, H.; Lee, J.; Lee, E.; Kwon, Y.; Lee, E.; Ahn, W.; Jiang, M.; Kim, J.; Son, Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29 (+) stromal-like cells. Nat. Med. 2009, 15, 425–435. [Google Scholar] [CrossRef]
- Lim, J.; Chung, E.; Son, Y. A neuropeptide, Substance-P, directly induces tissue-repairing M2 like macrophages by activating the PI3K/Akt/mTOR pathway even in the presence of IFNγ. Sci. Rep. 2017, 7, 9417. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Hong, H.S.; Son, Y. Substance P ameliorates tumor necrosis factor-alpha-induced endothelial cell dysfunction by regulating eNOS expression in vitro. Microcirculation 2018, 25, e12443. [Google Scholar] [CrossRef]
- Jin, Y.; Hong, H.S.; Son, Y. Substance P enhances mesenchymal stem cells-mediated immune modulation. Cytokine 2015, 71, 145–153. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.S.; Kim, D.; Hong, H.S. Substance P hinders bile acid-induced hepatocellular injury by modulating oxidative stress and inflammation. Antioxidants 2022, 11, 920. [Google Scholar] [CrossRef]
- Kim, D.Y.; Piao, J.; Park, J.S.; Lee, D.; Hong, H.S. Substance P ameliorates TNF-α-mediated impairment of human aortic vascular cells in vitro. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1288–1297. [Google Scholar] [CrossRef]
- Baek, S.M.; Yu, S.Y.; Son, Y.; Hong, H.S. Substance P promotes the recovery of oxidative stress-damaged retinal pigmented epithelial cells by modulating Akt/GSK-3β signaling. Mol. Vis. 2016, 22, 1015–1023. [Google Scholar] [PubMed]
- Yoo, K.; Son, B.K.; Kim, S.; Son, Y.; Yu, S.Y.; Hong, H.S. Substance P prevents development of proliferative vitreoretinopathy in mice by modulating TNF-α. Mol. Vis. 2017, 23, 933–943. [Google Scholar] [PubMed]
- Arden, G.B.; Sivaprasad, S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr. Diabetes Rev. 2011, 7, 291–304. [Google Scholar] [CrossRef]
- Frimmel, S.; Zandi, S.; Sun, D.; Zhang, Z.; Schering, A.; Melhorn, M.I.; Nakao, S.; Hafezi-Moghadam, A. Molecular imaging of retinal endothelial injury in diabetic animals. J. Ophthalmic. Vis. Res. 2017, 12, 175–182. [Google Scholar] [CrossRef]
- Chen, P.; Miao, Y.; Yan, P.; Wang, X.J.; Jiang, C.; Lei, Y. MiR-455-5p ameliorates HG-induced apoptosis, oxidative stress and inflammatory via targeting SOCS3 in retinal pigment epithelial cells. J. Cell. Physiol. 2019, 234, 21915–21924. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hu, A.; Luo, Y.; Sun, W.; Hu, X.; Tang, S. Interleukin-4 and melatonin ameliorate high glucose and interleukin-1β stimulated inflammatory reaction in human retinal endothelial cells and retinal pigment epithelial cells. Mol. Vis. 2014, 20, 921. [Google Scholar] [PubMed]
- Xu, H.Z.; Le, Y.Z. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2160–2164. [Google Scholar] [CrossRef] [PubMed]
- Calderon, G.D.; Juarez, O.H.; Hernandez, G.E.; Punzo, S.M.; de la Cruz, Z.D. Oxidative stress and diabetic retinopathy: Development and treatment. Eye 2017, 31, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; de Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef]
- Aikawa, R.; Nawano, M.; Gu, Y.; Katagiri, H.; Asano, T.; Zhu, W.; Nagai, R.; Komuro, I. Insulin prevents cardiomyocytes from oxidative stress–induced apoptosis through activation of PI3 kinase/Akt. Circulation 2000, 102, 2873–2879. [Google Scholar] [CrossRef]
- Wimmers, S.; Karl, M.O.; Strauss, O. Ion channels in the RPE. Prog. Retin. Eye Res. 2007, 26, 263–301. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, X.; Mei, Y.; Zhao, X.; Zhou, L.; Ma, M.; Liu, S.; Zha, X.; Yang, Y. A mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ROS/PINK1/Parkin signal pathway. Biomed. Pharmacother. 2019, 111, 1315–1325. [Google Scholar] [CrossRef]
- Liu, J.; Hou, Y.; Lin, L.; Yu, N.; Zhang, Y. MicroRNA-5195-3p alleviates high glucose-induced injury in human ARPE-19 cells by targeting GMFB. PLoS ONE 2021, 16, e0260071. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, L.; Xin, G.; Li, S.; Ma, L.; Xu, Y.; Zhuang, M.; Xiong, Q.; Wei, Z.; Xing, Z.; et al. Oxidative stress mediated by lipid metabolism contributes to high glucose-induced senescence in retinal pigment epithelium. Free Radic. Biol. Med. 2019, 130, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Ran, Z.; Zhang, Y.; Wen, X.; Ma, J. Curcumin inhibits high glucose induced inflammatory injury in human retinal pigment epithelial cells through the ROS PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 2019, 19, 1024–1031. [Google Scholar] [CrossRef]
- Arumugam, B.; Palanisamy, U.D.; Chua, K.H.; Kuppusamy, U.R. Amelioration of hyperglycemia-induced oxidative damage in ARPE-19 cells by myricetin derivatives isolated from Syzygium malaccense. J. Funct. Foods 2020, 67, 103844. [Google Scholar] [CrossRef]
- Li, X.; Cai, Y.; Wang, Y.S.; Shi, Y.Y.; Hou, W.; Xu, C.S.; Wang, H.Y.; Ye, Z.; Yao, L.B.; Zhang, J. Hyperglycaemia exacerbates choroidal neovascularisation in mice via the oxidative stress-induced activation of STAT3 signalling in RPE cells. PLoS ONE 2012, 7, e47600. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; del-Rio-Vellosillo, M. Update on the effects of antioxidants on diabetic retinopathy: In vitro experiments, animal studies and clinical trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Carrasco, E.; García-Ramírez, M.; Hernández, C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr. Diabetes Rev. 2006, 2, 71–98. [Google Scholar] [CrossRef]
- Kim, D.Y.; Piao, J.; Hong, H.S. Substance-P inhibits cardiac microvascular endothelial dysfunction caused by high glucose-induced oxidative stress. Antioxidants 2021, 10, 1084. [Google Scholar] [CrossRef]
- Hong, H.S.; Lim, S.; Son, Y. Genotoxicity studies of substance-P by using short-term assay. Mol. Cell. Toxicol. 2016, 12, 447–452. [Google Scholar] [CrossRef]
- Hong, H.S.; Lim, S.; Son, Y. Evaluation of substance-P toxicity with single dose and repeated dose in rats. Mol. Cell. Toxicol. 2015, 11, 201–211. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Hong, H.S. Substance P Alleviates Retinal Pigment Epithelium Dysfunction Caused by High Glucose-Induced Stress. Life 2023, 13, 1070. https://doi.org/10.3390/life13051070
Lee D, Hong HS. Substance P Alleviates Retinal Pigment Epithelium Dysfunction Caused by High Glucose-Induced Stress. Life. 2023; 13(5):1070. https://doi.org/10.3390/life13051070
Chicago/Turabian StyleLee, Dahyeon, and Hyun Sook Hong. 2023. "Substance P Alleviates Retinal Pigment Epithelium Dysfunction Caused by High Glucose-Induced Stress" Life 13, no. 5: 1070. https://doi.org/10.3390/life13051070





