Abstract
Microbial degradation of aromatic hydrocarbons is an emerging technology, and it is well recognized for its economic methods, efficiency, and safety; however, its exploration is still scarce and greater emphasis on cyanobacteria–bacterial mutualistic interactions is needed. We evaluated and characterized the phenanthrene biodegradation capacity of consortium dominated by Fischerella sp. under holoxenic conditions with aerobic heterotrophic bacteria and their molecular identification through 16S rRNA Illumina sequencing. Results indicated that our microbial consortium can degrade up to 92% of phenanthrene in five days. Bioinformatic analyses revealed that consortium was dominated by Fischerella sp., however different members of Nostocaceae and Weeksellaceae, as well as several other bacteria, such as Chryseobacterium, and Porphyrobacter, were found to be putatively involved in the biological degradation of phenanthrene. This work contributes to a better understanding of biodegradation of phenanthrene by cyanobacteria and identify the microbial diversity related.
1. Introduction
As the world population grows, crude oil demand and its derived products increase proportionally, so do oil spills and the inadequate disposal of industrial wastes, thus contributing to the ever-increasing environmental pollution [1,2,3]. Among the crude oil pollutant components, Polycyclic Aromatic Hydrocarbons (PAHs) are a ubiquitous class of environmental contaminants of great concern due to their toxicity, carcinogenicity, teratogenicity, and mutagenicity [4,5]. PAHs are highly recalcitrant molecules, and they are not easy to remove due to their hydrophobicity and low water solubility. Thus, it is possible to find them in crude oil, asphalt, creosote, tars, and waste products associated with wood preservatives [6,7]. The simplest PAH is naphthalene, because it has only two benzene rings and narrowly speaking is not polycyclic. Therefore, the increase in hydrophobicity and recalcitrance of these molecules is directly proportional to the increase in the number of rings [8] (Table 1).

Table 1.
Properties of the 16 PAHs included in the USEPA list of priority pollutants [8,9,10].
Diverse PAHs have “bay/k” regions and it is well established that epoxides produced by these regions have high biological and chemical reactivity. PAHs’ environmental behavior and persistence vary according to their degree of solubility, the number of aromatic rings, molecular weight, concentration, and pollutant bioavailability, among others [10,11]. However, environmental factors, such as soil type, pH, temperature, pollutant time exposure, dissolved oxygen, salinity, nutrients, and water, can affect microbial activity which plays an important role in the persistence time of PAHs in the environment [12,13]. In addition, another factor that prolongs the persistence of PAHs in the environment is their association with heavy metals, where it is possible that the presence of heavy metals in the soil inhibits the cellular development of microbial communities [10,12].
Phenanthrene is a tricyclic aromatic hydrocarbon that can be found on sediments, terrestrial surfaces, and aquatic ecosystems [12,14,15,16]. It does not represent a hazard to human health because it has not shown genotoxic or carcinogenic effects, nevertheless, alterations in chromatin and inhibition of intracellular communication in Gap unions have been demonstrated [17]. The noncarcinogen phenanthrene is due to the simplest hydrocarbon that contains a bay region [18,19]. However, several studies have demonstrated phenanthrene toxicity in fish, algae, and superior organisms [18,19,20]. Phenanthrene is the simplest PAH that contains two regions, where epoxides are formed by the “k-region” which are highly carcinogenic [21,22]. It has been listed as 1 of the 16 PAHs of priority pollutants (Table 1) by USEPA [23]. So far, it is known that PAHs present in the environment could be removed by chemical and mechanical processes including evaporation by rotary tubular kiln, extraction by fluidized beds, removal of volatile fractions by rotary drum, chemical oxidation in reactors, direct pumping of the aquifer, elevation of hydrocarbons to the surface by flooding, adsorption by pumping on activated carbon and in situ leaching by surfactants [16]. However, for over two decades it has been demonstrated that the main and most useful process for PAH removal is through microbial processing and degradation [24]. It is well known that sediments with PAH contamination can be degraded by indigenous microorganisms that reside there [24,25].
Currently, in addition to the physicochemical methods of the decontaminating hydrocarbon-polluted matrices, hydrocarbon biodegradation is an emergent technology, and it is well recognized for its economical, efficiency, and security methods. Yet, the complex interaction between hydrocarbons, environment, and microbial community composition can make it relatively slow under normal conditions [2].
Cyanobacteria are the oldest phototrophic microorganisms and very versatile microorganisms, which, from natural ecosystems are able to develop microcosms under synergistic relationships with aerobic heterotrophic bacteria, where these interactions tend to be mutualistic [26,27]. They can thrive in diverse habitats, such as hot springs, Antarctic lakes and soils, and extreme euryhaline and eurythermal environments [28,29,30,31,32]. Due to this, previous studies indicate that holoxenic cultures have produced higher and more efficient biodegradation yields compared to axenic cultures [26,33,34], but despite the numerous studies mentioning the bacterial biodegradation of phenanthrene, virtually little is known about cyanobacteria and their enzymes involved in PAHs mineralization. These microorganisms can produce a wide variety of secondary metabolites. These compounds have been shown to be of industrial interest for their biological activity with pharmaceutical, nutraceutical, and agricultural potential as antitumor, anticancer, antiviral, antibiotic, and antifungal agents, among others [31,35,36]. Moreover, cyanobacteria have kindled great industrial interest, as their global product market is estimated grow from US 1547.23 million in 2020 to around US 2811.10 million in 2028; it is expected to grow at a CAGR of 7.9% from 2021 to 2028 [37].
Several ecological studies of microbial consortia have shown relevant improvements over the last 60 years, which were enhanced by the development of the polymerase chain reaction for the amplification of specific genes without the requirement of microbiological cultures. Thus, two decades ago, the proposed next-generation sequencing (NGS) opened the possibility to avoid traditional mutation detection methods to sequence multiple genes and unveil millions of variants simultaneously, as well as the minimal requirement of genomic material and consequently, the generation of several billions of nucleotides in a very short time and low cost [38]. Therefore, molecular characterization by massive sequencing of the 16S rRNA gene has shown great potential by introducing amplicons into next generation sequencing (NGS) to classify individual reads to specific taxa for the purpose of characterizing microbial consortiums [39,40,41,42].
As a matter of clarity, in this study we determined the biomass and biodegradation kinetics of phenanthrene using the cyanobacterium Fischerella sp. in consortium with aerobic heterotrophic bacteria and molecular characterization of the microbial community using the 16S rRNA gene sequencing by synthesis, which provided us with a deeper insight into the composition of the microbial consortium that is dominated by Fischerella sp. and that is capable of biodegrading phenanthrene as a model molecule.
2. Materials and Methods
2.1. Enrichment Culture, Incubation Conditions, and Photobioreaction System for Fischerella sp.
The cyanobacteria Fischerella sp. and its microbial consortia were collected and isolated from Cacahoatan, Chiapas region, Mexico. Moreover, the sampling point location (14°58′03.20″ N, 92°03′46.96″ W) was chosen with the presence of photosynthetic microorganisms developing over the sweetwater pond surface, on a site with no historical hydrocarbon pollution records. It is worth noting that samples were collected in triplicate using 250 mL sterilized Duran flasks (Schott, Mainz, Germany). The samples were transported in a cooler to the laboratory and stored at 25 °C at room temperature.
Taking a fresh sample from the isolation site, 1 mL of wet biomass was inoculated to the center of the 60 × 15 mm Petri dish (SYM laboratorios, Puebla, Mexico) in 0.7% w/v semi-solid BG-11 medium. Next, the cyanobacterial stock cultures on holoxenic conditions were inoculated with 1% v/v in 200 mL BG-11 liquid medium contained in 250 mL Duran flask (Schott, Germany) [43,44,45], with the final composition per liter being: C6H11FeNO7 6 g, HNO3 6 g, NaNO3 1.5 g, K2HPO4∙3H2O 40 g, MgSO4∙7H2O 75 g, CaCl2∙2H2O 36 g, Na2CO3 20 g, MgNa2EDTA∙H2O 1 g, and 1 mL of trace metal solution. The trace metal solution was composed of H3BO3 2.86 g, MnCl2∙4H2O 1.81 g, ZnSO4∙7H2O 0.22 g, CuSO4∙5H2O 0.079 g, Na2MoO4∙2H2O 0.391 g, and Co(NO3)2∙6H2O 0.049 g. The pH value of the culture was kept at 7.1 ± 0.2 after autoclave (15 min at 121 °C under 100 kPa) and before use. The trace metal solution was added after sterilization of the macroelements to avoid precipitation of the components.
Cultures were incubated at 28 ± 1 °C with a photoperiod of 12:12 h (Light:Dark) employing cool-white fluorescent lamps (Electro Mag S.A. de C.V., Cuauhtémoc, Mexico) as artificial fluorescent illumination, with a continuous irradiance of 73 µmol/m2/s. Atmospheric air was supplied using an air compressor (Hagen, Mansfield, MA, USA) through a silicone hose (Cole-Parmer, Vernon Hills, IL, USA) with 1.5 vvm constant flux. The air was sterilized using Midisart 2000 (Sartorius, Göttingen, Germany) 0.20 m air filters.
A scanning electron microscope (SEM) with an accelerating voltage of 25 kV (JEOL Ltd. Model JSM7600F, Tokyo, Japan) was used to visualize the morphology and cell structure of the cyanobacteria in greater detail. Samples were coated with gold to increase electrical conductivity.
2.2. Cell Growth Quantifications and Biomass Dry Weight Measurements
Cell growth quantifications were obatined through drying a membrane (Millipore, Darmstadt, Germany) of 0.22 m, with a humidity balance PMB (Adam Equipment, Milton Keynes, UK) at 140 °C for 5 min. Next, excessive humidity on the membrane was removed by employing a desiccator (Bel-Art, Warminster, PA, USA) for up to 24 h and then weighted in an analytical balance (RADWAG Wagi Elektroniczne, Radom, Poland). The biomass was filtered via the KG 47 GLASS SUPPORT (Advantec MFS, Tokyo, Japan) with 300 mL and a Kitasato 1000 mL flask (KIMAX, Vineland, NJ, USA), using a vacuum pump (WACO, Nowon-gu, Seoul, Republic of Korea). The membrane with living biomass was dried out using the humidity balance PMB at 140 °C for 5 min and again excessive humidity was removed using a desiccator for 24 h, and weighed with an analytical balance. All samples were measured in triplicate.
The specific growth rate parameter () associated with biomass development was determined considering the exponential phase through Equations (1) and (2) [46,47].
where, dX/dt represents the rate of biomass production in the culture at a given time and is the specific growth rate (1/day). Moreover, the duplication time (td) can be expressed by Equation (3).
2.3. Phenanthrene Biodegradation Monitoring
In order, to determine the PAH mineralization rate, phenanthrene concentrations in the culture media were analyzed using a UV-Vis-NIR spectrophotometer (Agilent, Santa Clara, CA, USA). The concentration of phenanthrene was calculated from a standard curve based on peak area at 293 nm. The biodegradation process was determined using experiments carried out in triplicate, in a 250 mL Duran flask (Schott, Germany) with 200 mL of BG-11 liquid medium (pH 7.1 ± 0.2) employing the photobioreaction systems previously described in Section 2.1 of Materials and Methods.
The experimental photobioreactors set up were treated with phenanthrene concentrations of 100, 50, and 10 mg/L, while the control experiments were left uninoculated and analyzed using the UV-Vis-NIR spectrophotometer described previously. For 5 days, the phenanthrene biodegradation rate was monitored every 24 h. All samples were measured in triplicate. The phenanthrene consumption was determined by Equation (4).
where, −dS/dt describes the rate of substrate consumption due to microbial activity at a given time, therefore, qs is the specific consumption rate of the substrate (1/day).
2.4. Genomic DNA Extraction and PCR Amplification of Variable Regions 3–4 of the 16S rRNA Gene
At the end of the experiment, samples were collected for further molecular analysis. DNA extraction was performed in duplicate using the DNeasy PowerSoil Kit (Qiagen, Venlo, The Netherlands) following the manufacturer’s protocol and a TissueLyser LT (Qiagen, The Netherlands) was used for cell disruption. DNA quality was evaluated by 1% agarose electrophoresis. The 16S rRNA gene amplicons were obtained using the universal bacterial primers SD-Bact-0341-bS-17 y SD-Bact-0785-aA-21 [48], spanning the V3 and V4 regions. PCR products were performed in duplicate and mixed in equal amounts for sequencing. Thermal cycling consisted of 3 min at 95 °C and 25 cycles of 30 s at 95 °C, 30 s at 55 °C, and 30 s at 72 °C, followed by 5 min at 72 °C. Amplicons were purified with the Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA). PCR products were indexed using the Nextera XT Index, in accordance with Illumina’s protocol. PCR barcoded amplicons were again purified as described and then quantified using a Qubit 3.0 fluorometer (Life Technologies, Carlsbad, CA, USA). The correct size of the amplicons was verified using an Advanced QIAxcel (Qiagen, The Netherlands). Libraries were diluted with 10 mM Tris (pH 8.5) and pooled in equimolar concentrations (9 pM).
2.5. Illumina High-Throughput Sequencing and Bioinformatic Analysis
The final library was loaded onto the flow cell of the V3 MiSeq Reagent Kit (600 cycles), proceeding with a paired-end sequencing on the Illumina MiSeq platform (Illumina, San Diego, CA, USA) in CINVESTAV Merida. The demultiplexed resulting data were analyzed using the open-source bioinformatics pipeline QIIME2 (2017.11) [49]. The sequencing errors and their correction to properly resolve the amplicon sequence variants (ASV) were addressed via the DADA2 plugin, and chimeras were removed using the “consensus” method [50]. The taxonomic assignment of the representative’s ASVs was performed using the VSEARCH plugin [51], against the SILVA gene database (v. 132). A phylogeny was constructed from the resolved ASVs using the FastTree algorithm [52]. For a better understanding, in the next sections, we will refer to the determined ASVs as Operational Taxonomic Units (OTUs), but the ASV concept is a more precise alternative to the classical clustering based on a fixed percentage identity threshold [53]. Microbial community analysis and graphical outputs were performed in R (R Core-team, Vienna, Austria) using phyloseq [54], ggplot2 [55], and vegan [56] libraries.
2.6. Nucleotide Sequence Accession Number
The obtained 16S rRNA gene sequences were deposited in the NCBI archive under bioproject PRJNA562544.
2.7. Statistical Analysis
Statistical analysis was performed by means of a one-way analysis of variance (ANOVA). Tukey’s test was used in order to determine significant differences between all screening tests related to biomass growth and phenanthrene biodegradation (p < 0.0001), using the commercial software InfoStat.
3. Results and Discussion
3.1. Biomass Growth and Phenanthrene Biodegradation Monitoring
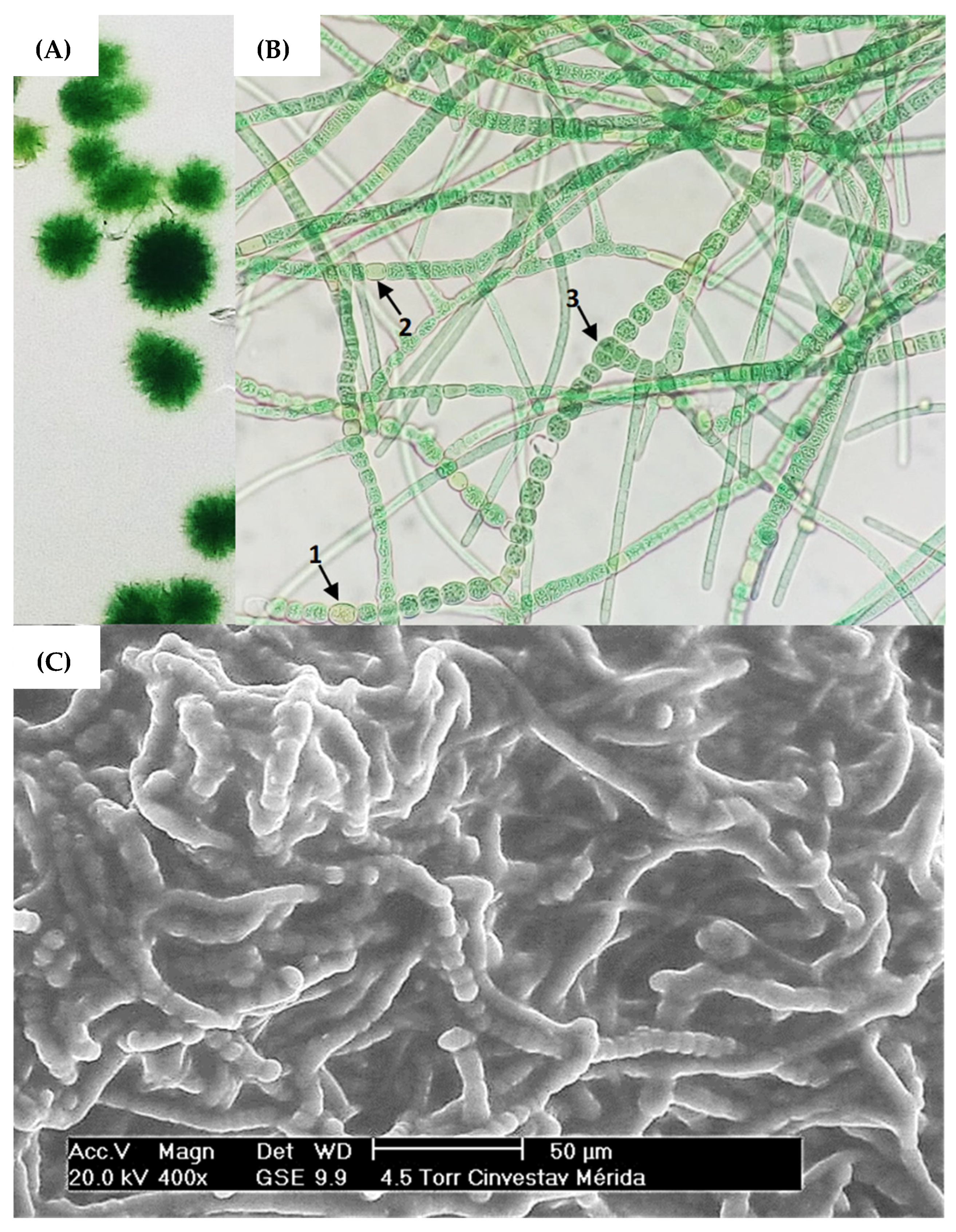
The cyanobacteria strain was isolated from the sweetwater pond surface (Section 2.1) via enrichment in a semi-solid BG-11 medium (Figure 1A), therefore SEM microscopy showed that the strain had a filamentous structure. It was noticed that its characteristic filamentous structure is developed through communication channels between vegetative cells (hormogonium), heterocysts, and acinetes (Figure 1B).
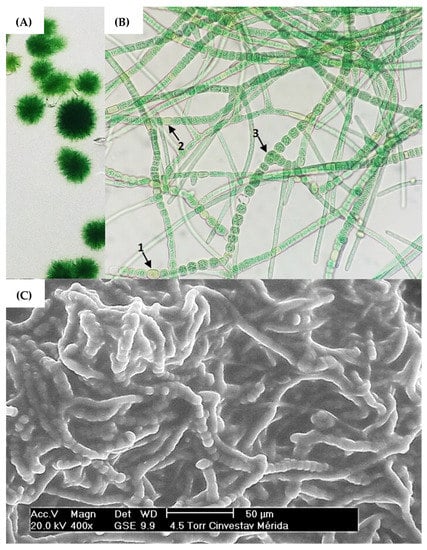
Figure 1.
(A) Cyanobacteria colonies of strain Fischerella sp. on semi-solid agar cultures. (B) Brightfield microscopy with 40× objective, highlighting the morphological structures (1) acinetes, (2) heterocyst and (3) vegetative cell. (C) SEM microscopy of strain Fischerella sp.
Vegetative cells can divide in more than one plane; i.e., they produce lateral branching to produce a mature trichome. Heterocysts can be found in terminal or lateral regions and hormogonia are composed of small cylindrical cells that enlarge and round into vegetative cells [32,35,57]. Based on this, ovoid vegetative cells approximately 7 m in diameter were observed producing mature trichomes coiled amongst themselves (Figure 1B). The presence of mucilage, forming a secretion matrix coating the filaments, was also observed (Figure 1C), which has been described in research as a region that provides a phytosphere for bacteria in consortium with the cyanobacterial host [58]. Nonetheless, molecular identification via 16S RNA of the cyanobacterium Fischerella sp. and the microbial consortium was subsequently performed.
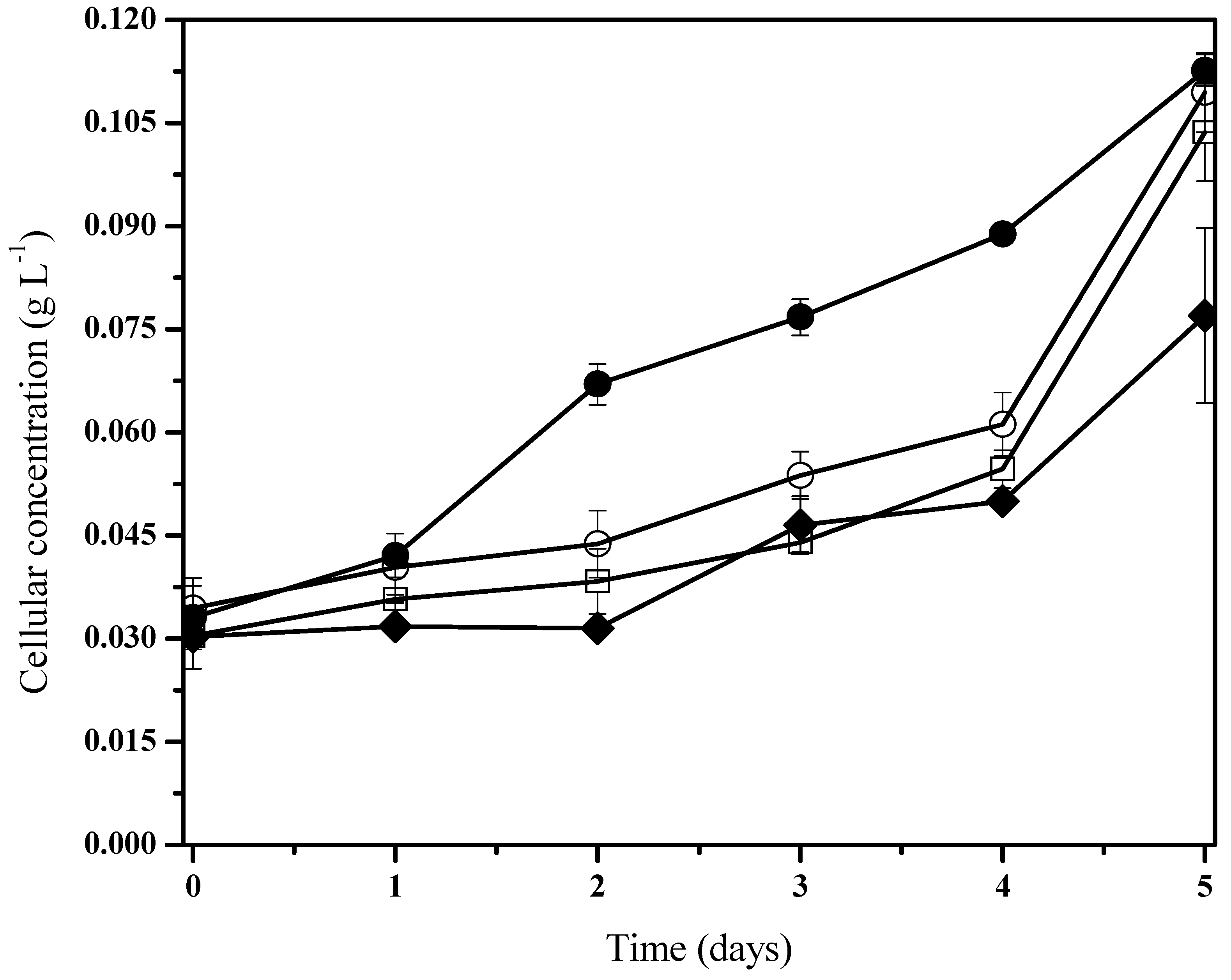
To demonstrate the biodegradation capacity of phenanthrene, the cyanobacteria consortium growth was measured for five days. Three different concentrations of pollutant (100, 50, and 10 mg/L) were employed in order to screen the experiment, and control tests were left uninoculated. It was observed that biomass growth was related to phenanthrene degradation as supplied on the medium as an additional source of carbon (Figure 2). By the fifth day, the microcosm behavior with phenanthrene showed a homologous tendency (Figure 2), which can be described as a low pollutant concentration detected by the chemotaxis mechanism from the bacterial consortium [59].
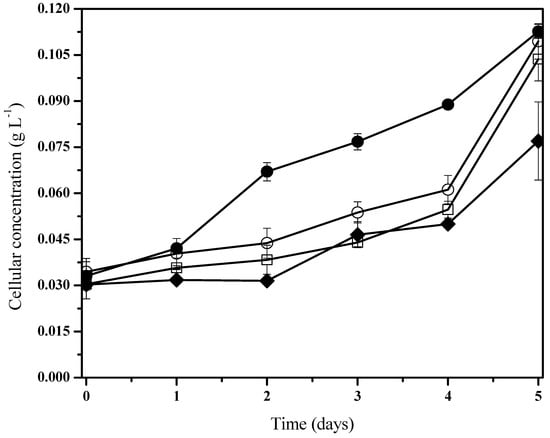
Figure 2.
Fischerella sp. biomass behavior under holoxenic consortium during phenanthrene biodegradation. Pollutant concentrations are 100 mg/L (●), 50 mg/L (□), 10 mg/L (○), and control experiments (♦). Data shown as average and ± SD of triplicate determinations, significant differences between the groups (control group and phenanthrene concentrations were determined using one-way analysis of variance (ANOVA), significant difference existed between these experiments (p < 0.0001).
According to previous works [60,61] these results indicate that biomass growth kinetics from the presence of hydrocarbons can be fitted to the typical Monod’s kinetic, where adaptation phases are virtually nonexistent or reduced with relatively accelerated logarithmic phases. Moreover, it was noted in the cohort cells from control systems that there is an initial adaptation phase when compared to the group under phenanthrene treatment (Figure 2). The bacterial consortia presented a slight increase in their biomass development with pollutant exposition (Figure 2). Nevertheless, under the 50 mg/L phenanthrene concentration, there was a significantly higher specific growth rate when compared to the rest of the cultures with the presence and non-presence of phenanthrene (Table 2). It is to be noted that we characterized a mixed consortium of aerobic heterotrophic bacteria.

Table 2.
Cellular growth kinetic parameters from phenanthrene biodegradation.
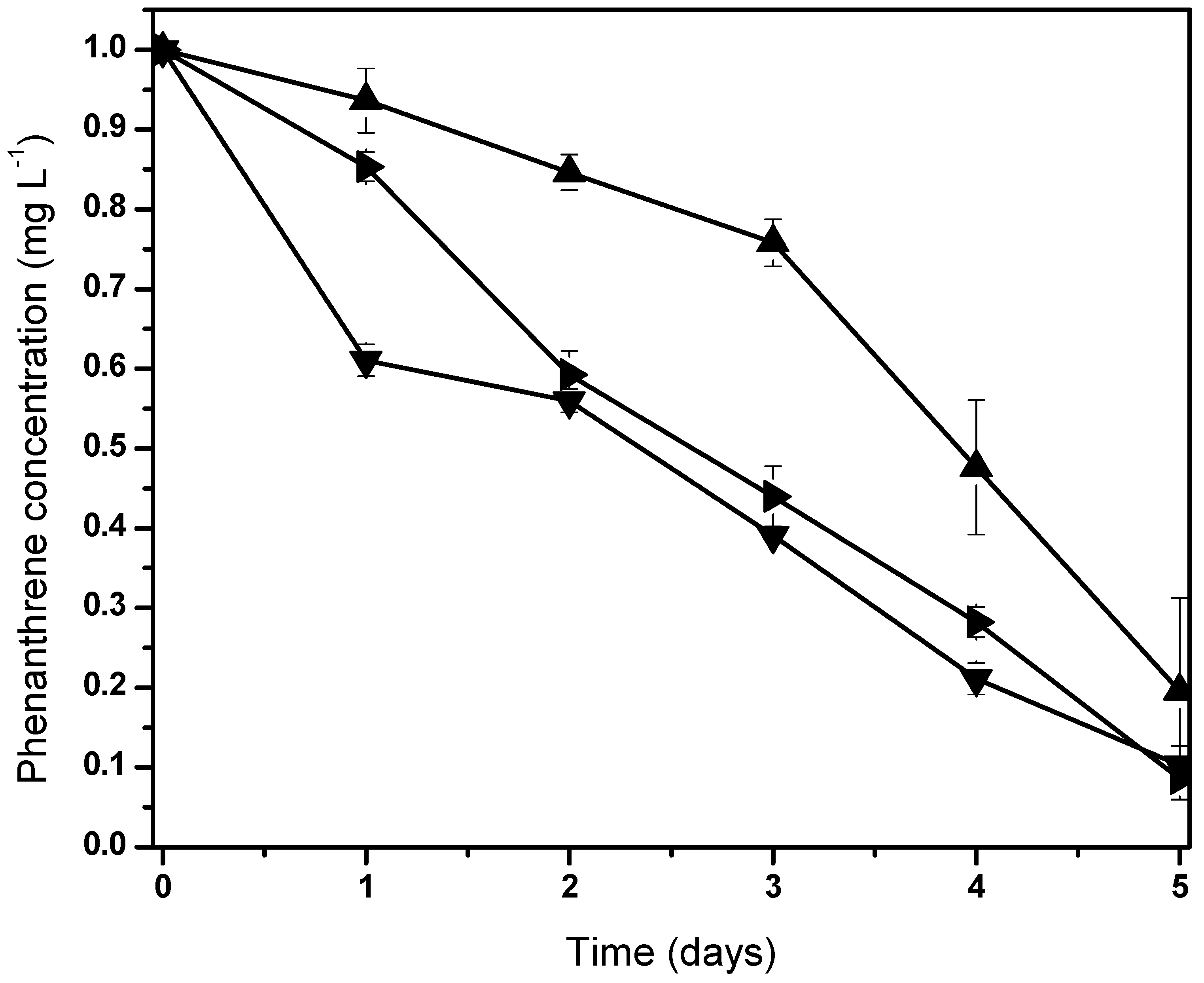
We hypothesized that these microorganisms possibly are related to Fischerella sp. in order to metabolize carbon compounds produced by the cyanobacteria [62]. The phenanthrene mineralization by the consortium was achieved almost totally after 5 days (Figure 3). Initial phenanthrene concentrations (100, 50, and 10 ) were degraded to 89.7%, 91.3%, and 80.4%, respectively. All screening tests showed fluctuations with phenanthrene concentrations, where the highest PAH biodegradation was reached at 50 mg/L.
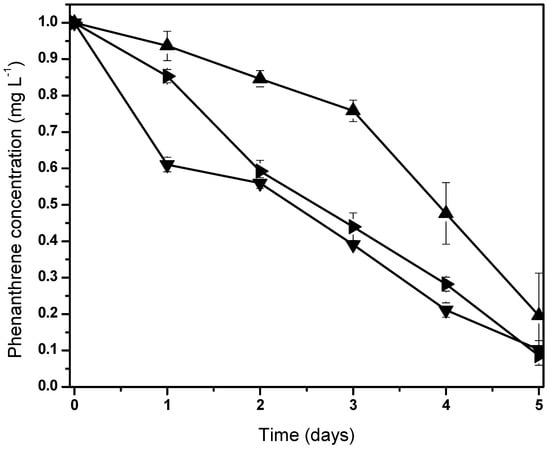
Figure 3.
Normalized behaviors from phenanthrene biodegradation by Fischerella sp. under holoxenic consortium. Pollutant concentrations are 100 (▼), 50 (►) and 10 (▲). Data shown as average ± S.D. in triplicate, significant difference existed between these experiments (p < 0.0001). Control screen test data are not shown due to null variation in the phenanthrene concentration.
It is possible to associate several routes of PAHs consumption by microbial metabolism, as shown is various other studies related to bacterial, fungal, and algal biodegradation pathways [2,4,6,8,12,15]; however, the present research work focuses on the aerobic biodegradation of phenanthrene by bacteria. In general, reports have described that the basic mechanism of PAH biodegradation starts with the oxidation of the aromatic ring, followed by its scission, and ending in its transformation into biomass or molecules of linear structure, such as carbon dioxide and water [63]. Particularly, the biodegradation pathway of phenanthrene under aerobic conditions by bacteria starts with the enzymatic attack of phenanthrene dioxygenase at the 1,2- and 3,4-carbon atom positions to form cis-1,2-dihydroxy-1,2-dihydrophenanthrene and cis-3,4-dihydroxy-3,4-dihydrophenanthrene, where the main isomer is cis-3,4-dihydroxy-3,4-dihydrophenanthrene. Next, cis-3,4-dihydroxy-3,4-dihydrophenanthrene is oxidized to form 3,4-dihydroxyphenanthrene, which is subsequently cleaved to form 1-hydroxy-2-naphthoic acid, the main metabolite of the phenanthrene oxidative pathway which can be bifurcated depending on the enzymatic machinery present [64]. One catabolic pathway involves the hydroxylation of 1-hydroxy-2-naphthoic acid with the formation of 1,2-dihydroxynaphthalene, a compound that enters the naphthalene degradation pathway and subsequently metabolized to catechol, where the ortho- and meta-fission metabolites are transformed to succinate, acetyl-CoA, pyruvate, and acetaldehyde, which pass directly to the central pathways of cellular metabolism (Krebs cycle). On the other hand, the aromatic ring of 1-hydroxy-2-naphthoic acid is oxidized by the enzyme 1-hydroxy-2-naphthoate dioxygenase and forms another catabolic pathway, in which enzymatic reactions produce phthalic acid (phthalate) to form protocatechuic acid via the protocatechuate metabolic pathway and enter the Krebs cycle again [6,19,63,65].
Therefore, PAHs biodegradation can be performed from the whole-cell or biocatalytic approach. The use of the whole-cell approach involves the direct application of active microorganisms that use hydrocarbons as a carbon source, which means that the microorganisms secrete extracellular enzymes or degrade PAHs through their intracellular biocatalysts, however, this process is relatively slow due to predatory activity by other microorganisms and also due to the conditions of the contaminated site [66]. On the other hand, the biocatalytic approach has shown promising results in cases where low PAH concentrations are used and has the versatility of employing free or immobilized biocatalysts on surface cross-linked supports [67]. However, despite numerous studies on bacterial biodegradation of phenanthrene, currently little is known about cyanobacteria and the enzymes which catalyze the oxidation of PAHs. In addition, with the average kinetic parameters associated with phenanthrene biodegradation, it was possible to determine the specific substrate consumption rate and apparent biomass substrate yield (Table 2). It is evident through the fitting of the quantified data that the experiments with a PAH at 50 , achieved greater assimilation of phenanthrene.
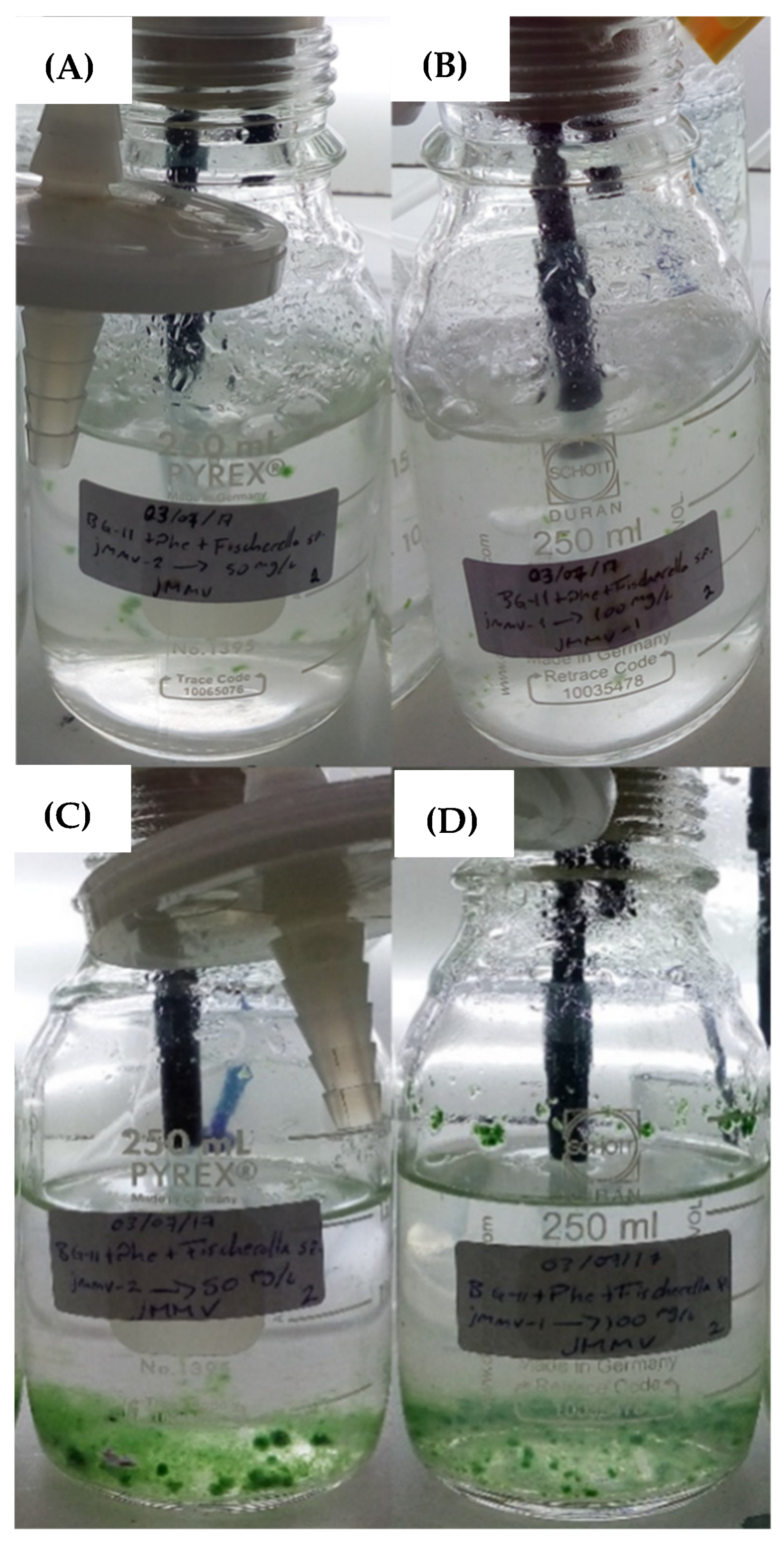
On the other hand, during the preparation of the cultures, it was noticeable that the addition of phenanthrene generated turbidity in the liquid medium; however, after 4 days of culture, not only the cellular development of Fischerella sp. was visually noticeable, but also a clarification was visible in the culture medium. This provides a relatively clear evidence of PAH bioassimilation by the cyanobacterium Fischerella sp. And its microbial consortium (Figure 4). Cultures with higher turbidity due to phenanthrene concentration are displayed.

Figure 4.
Fischerella sp. Cultures in photobioreactors. Cultures supplied with phenanthrene at 50 mg/L (A) and 100 mg/L (B) during the first day of growth and, cultures with phenanthrene at 50 mg/L (C) and 100 mg/ (D) after 4 days of operation.
Interestingly, an alternative to exploring the degradation capabilities of the consortium presented in this work is the addition of amphiphilic molecules, either of the synthetic or biological source. It has been described that the use of surfactants in biodegradation studies of polycyclic aromatic hydrocarbons has generated wide interest [68,69]. Reports in the literature [70] have shown that even with the remarkable capacity of phenanthrene, and other PAHs in the synergism of the consortia, when applying biosurfactants into in vitro tests it was possible to reach up to 97% biodegradation in almost 30 days with the strain Microbacterium esteraromaticum; however, the rest of the isolated strains showed similar patterns in the enhancement of biodegradation. This alternative could be in contrast with the tests developed with the Fischerella sp. dominated consortium, taking into consideration that nearly 92% of phenanthrene biodegradation was achieved in five days.
Likewise, Table 2 shows average kinetic parameters associated with phenanthrene biodegradation, such as the specific rate of substrate consumption (qs) and the apparent biomass-substrate yield (Yxs) in the photobioreaction systems with phenanthrene concentrations. It is evident from the fit of the quantified data that cultures supplied with 50 achieved higher assimilation of phenanthrene to their metabolism. On the other hand, it is argued that cyanobacteria possess the capacity to oxidize hydrocarbons, so it is suggested to pursue further studies of Fischerella sp. to evaluate biodegradation activities under axenic conditions. However, based on several authors [71], it is mentioned that some microorganisms can metabolize polycyclic aromatic hydrocarbons as a carbon source, but due to the hydrophobicity of these compounds, they cross through the liquid interface where the active zone of the microorganisms is located at a slow rate.
3.2. Molecular Characterization of the Bacterial Consortium
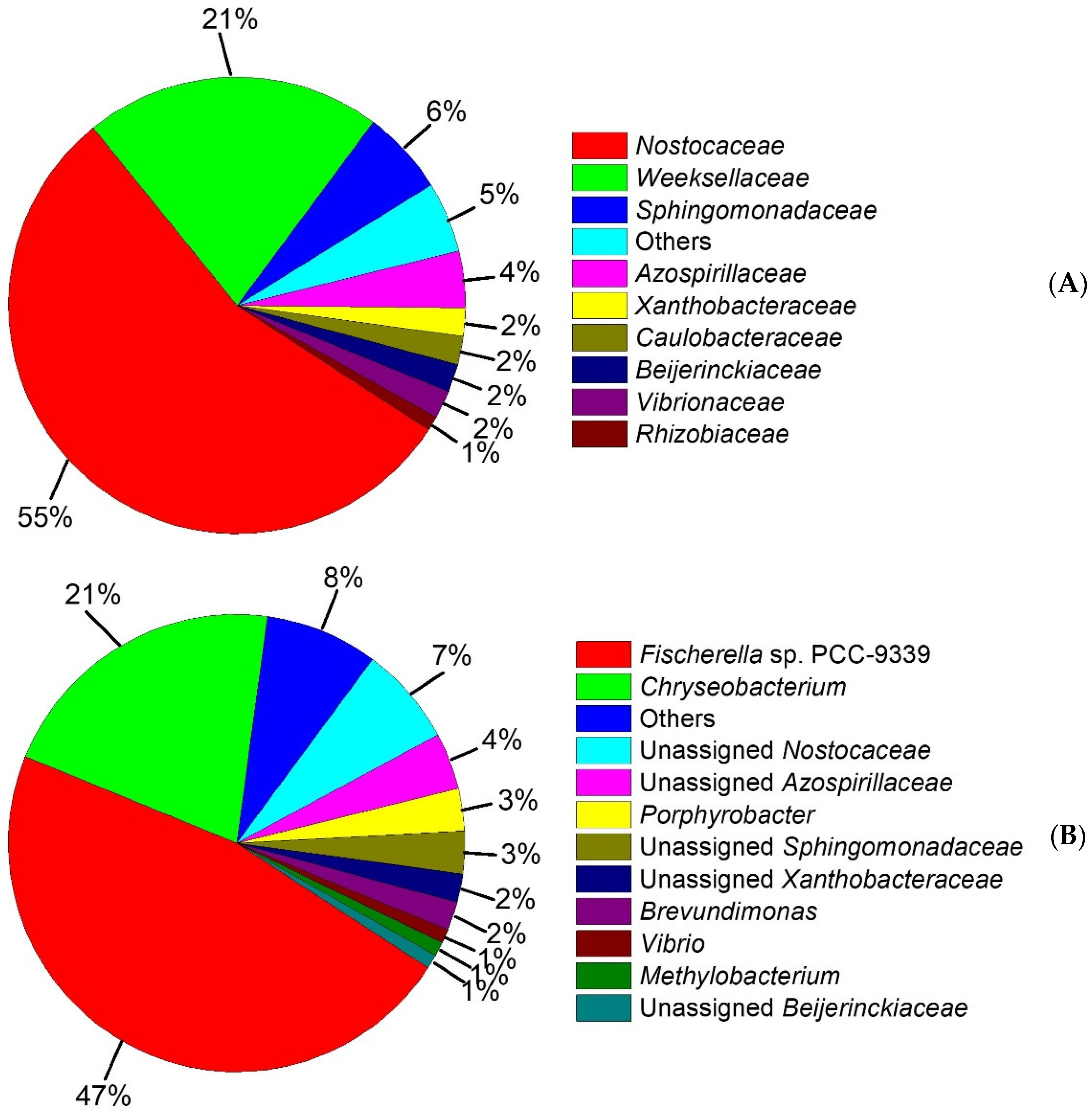
In this study to further examine the taxonomic composition of the microbial consortium, the Illumina sequencing of the 16S rRNA gene was performed and the results are shown in Figure 5. The bacterial community was distributed among 8 different families (Figure 5A) including Nostocaceae (55%), Weeksellaceae (21%), Sphingomonadaceae (6%), Azospirillaceae (4%), Xanthobacteraceae (2%), Caulobacteraceae (2%), Beijerinckiaceae (2%), Vibrionaceae (2%), Rhizobiaceae (1%) and others (5%). However, the most dominant families were Nostocaceae and Weeksellaceae. This result is in agreement with those reported in the literature [8,72,73,74,75], which suggested that Weeksellaceae, and other microbial families that we found, such as Sphingomonadaceae, Xanthobacteraceae, Caulobacteraceae, and Rhizobiaceae, are microbial families capable of degrading low molecular weight PAHs.
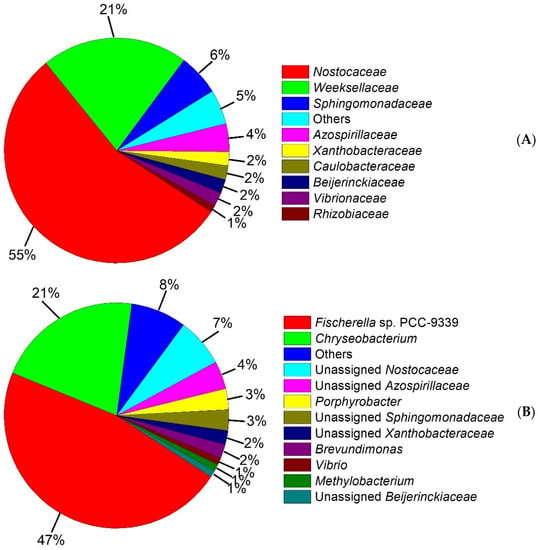
Figure 5.
Taxonomic classification of the bacterial community. (A) Relative abundance of ≥1% from dominant families is shown, (B) Relative abundance of ≥1% from dominant genus is shown.
In this sense, the OTUs’ molecular characterization showed that the consortium was composed of 11 genera (Figure 5B) including Fischerella sp. PCC-9339 (47%), Chryseobacterium (21%), unassigned Nostocaceae (7%), unassigned Azospirillaceae (4%), Porphyrobacter (3%), unassigned Sphingomonadaceae (3%), unassigned Xanthobacteraceae (2%), Brevundimonas (2%), Vibrio (1%), Methylobacterium (1%), unassigned Beijerinckiaceae (1%) and others (7%). With this in mind, the consortium was composed of a higher proportion of microorganisms able to metabolize PAHs, whereas on previous reports [22,76,77,78] it has been described that Chryseobacterium belonging to the family Weeksellaceae is able to increase its cell development on polluted soils with crude oil, with the presence of carbazole as a sole source of carbon and energy, and on natural asphalts [79]. Moreover, the Porphyrobacter genus related to the family Sphingomonadaceae has been reported [80,81,82] to have mineralization capacity with naphthalene, 1-methylnaphthalene, acenaphthene, dibenzothiophene, phenanthrene, anthracene, biphenyl, dibenzofuran and bisphenol A on wastewater. Xanthobacteraceae is scarcely described with the capacity to metabolize naphthalene [83]. Brevundimonas and Vibrio genera belonging to Caulobacteraceae and Vibrionaceae, respectively, [84] have been previously reported with a high biodegradation capacity for crude oil and PAHs, such as phenanthrene, fluorene, pyrene, acenaphthene, anthracene, and fluoranthene, among others.
Nonetheless, Methylobacterium and Beijerinckiaceae, members of the family Beijerinckiaceae, have been explored previously [85,86] and shown to be capable of excessive growth in presence of monocyclic aromatic hydrocarbons and PAHs, with optimal behavior in the presence of naphthalene.
To the best of our knowledge, Fischerella sp. PCC-9339 has no PAH biodegradation evidence and consequently, the results reported here are the first to describe its behavior under holoxenic conditions and with phenanthrene exposition as a model pollutant. Likewise, as we mentioned before in our study, Nostocaceae (55%) family was the most widely distributed. It is known that this cyanobacterium Nostocaceae with some genera, such as Anabaena sp. and Nostoc sp., can degrade aliphatic compounds contents of crude oil (decane, pentacosane, hexacosane, octacosane, and nonacosane) [87,88].
On the other hand, filamentous cyanobacteria, such as Microcoleus chthonoplastes and Lyngbya aestuarii, have been described in the literature [89] with the ability to develop biomass on crude oil contaminated sites; however, it is not yet defined whether cyanobacteria can play a direct role in the biodegradation of oil molecules. Our results suggest that the Nostocaceae family plays a main role during the process of biodegradation and bioavailability of phenanthrene.
4. Conclusions
This is the first study to report the phenanthrene biodegradation capacity of a heterotrophic aerobic bacterial consortium dominated by Fischerella sp. in order to determine the dynamics of the microbial process during PAH biodegradation. In light of the above discussion, phenanthrene mineralization behavior and biomass growth monitored during the five days of operation indicated phenanthrene consumption by microbial biodegradation. While the kinetic parameters were established as a function of biomass production and phenanthrene consumption as a substrate, these variables of the stoichiometric models described were based on the literature. Molecular characterization via Illumina sequencing of the 16S rRNA gene confirmed that the consortium includes several bacterial families reported in previous studies which are responsible for PAH biodegradation. However, as corroborated in this study and with other specific taxonomic studies, the Nostocaceae family has been shown to have a higher relative abundance with respect to the filamentous cyanobacterium Fischerella sp. and it offers clear indications as co-responsible for phenanthrene biodegradation. It is clear that the metabolic pathway of phenanthrene biodegradation described above could provide further insight into the biocatalytic nature of the cyanobacterium Fischerella sp. and of the bacteria present in the consortium. Therefore, a further quantification of the metabolic products would be key for a deeper understanding of this technology since it is virtually possible to produce, purify and immobilize its biocatalysts as a study target to deepen the oxidation of phenanthrene and other polycyclic aromatic hydrocarbons through an enzymatic format. In addition, it is important to isolate and characterize bacterial consortia from regions with a clear history of oil or hydrocarbon contamination to discover new microorganisms with more robust enzymatic mechanisms adapted to transform contaminants into simple molecules that are not harmful to natural matrices. Hence, this study provides results which provide a basis for future bioremediation studies with phenanthrene as a three-ring PAH model.
Author Contributions
J.M.M.-V., N.A.C., J.C.R.-S. and E.H.-N. contributed with several objectives of equal contributions, with regard to conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration and funding acquisition. E.H.-N. and N.A.C. were responsible for the project administration, J.Q.G.-M., N.N.C.-C. and S.C. were responsible for bioinformatic data curation, formal analysis, methodology, investigation, and visualization. Authorship is limited to those who have contributed substantially to the work reported. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by CONACyT (CB-2015-01) through the project 254321: “Diseño, síntesis y evaluación biológica de entidades químicas basadas en fármacos para diabetes, hipertensión y antiparasitarios”.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chandra, S.; Sharma, R.; Singh, K.; Sharma, A. Application of bioremediation technology in the environment contaminated with petroleum hydrocarbon. Ann. Microbiol. 2013, 63, 417–431. [Google Scholar] [CrossRef]
- Salleh, A.B.; Ghazali, F.M.; Noor, R.; Abd, Z.; Basri, M. Bioremediation of petroleum hydrocarbon pollution. Indian J. Biotechnol. 2003, 2, 411–425. [Google Scholar]
- Taylor, P.; Journal, B.; Strong, P.J.; Burgess, J.E.; Strong, P.J. Treatment methods for wine-related and distillery wastewaters: A review. Bioremediat. J. 2008, 12, 37–41. [Google Scholar] [CrossRef]
- Lowe, J.P.; Silverman, B.D. Predicting Carcinogenicity of Polycyclic Aromatic Hydrocarbons. Acc. Chem. Res. 1984, 17, 332–338. [Google Scholar] [CrossRef]
- Abdel-shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Habe, H.; Omori, T. Genetics of polycyclic aromatic hydrocarbon metabolism in diverse aerobic bacteria. Biosci. Biotechnol. Biochem. 2003, 67, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Ichor, T.; Gberikon, G.M.; Nevkaa, D. Biodegradation of phenanthrene by a consortium of aerobic heterotrophic bacteria and cyanobacteria in petroleum hydrocarbon polluted brackish water of Bodo Creek. Microbiol. J. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Cerniglia, C.E. Biodegradation of polycyclic aromatic hydrocarbons. Curr. Opin. Biotechnol. 1993, 4, 331–338. [Google Scholar] [CrossRef]
- Keith, L.H.; Telliard, W.A. Priority pollutants. Environ. Sci. Technol. 1979, 13, 416–423. [Google Scholar] [CrossRef]
- Maigari, A.U.; Maigari, M.U. Microbial metabolism of polycyclic aromatic hydrocarbons (PAHs): A review. Int. J. Sci. Eng. Res. 2015, 6, 1449–1459. [Google Scholar]
- Harmsen, J. Landfarming of Polycyclic Aromatic Hydrocarbons and Mineral Oil Contaminated Sediments; Wageningen University and Research: Wageningen, The Netherlands, 2004. [Google Scholar]
- Bamforth, S.M.; Singleton, I. Bioremediation of polycyclic aromatic hydrocarbons: Current knowledge and future directions. J. Chem. Technol. Biotechnol. 2005, 80, 723–736. [Google Scholar] [CrossRef]
- Hatzinger, P.B.; Alexander, M. Effect of aging of chemicals in soil on their biodegradability and extractability. Environ. Sci. Technol. 1995, 29, 537–545. [Google Scholar] [CrossRef]
- Kanaly, R.A.; Harayama, S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J. Bacteriol. 2000, 182, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Jerina, D.M.; Sayer, J.M.; Agarwal, S.K.; Yagi, H.; Levin, W.; Wood, A.W.; Conney, A.H.; Pruess-Schwartz, D.; Baird, W.M.; Pigott, M.A.; et al. Reactivity and Tumorigenicity of Bay-Region Diol Epoxides Derived from Polycyclic Aromatic Hydrocarbons. In Biological Reactive Intermediates III. Advances in Experimental Medicine and Biology; Kocsis, J.J., Jollow, D.J., Witmer, C.M., Nelson, J.O., Snyder, R., Eds.; Springer: Boston, MA, USA, 1986; pp. 11–30. [Google Scholar]
- Tam, N.F.Y.; Guo, C.L.; Yau, W.Y.; Wong, Y.S. Preliminary study on biodegradation of phenanthrene by bacteria isolated from mangrove sediments in Hong Kong. Mar. Pollut. Bull. 2002, 45, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Weis, L.M.; Rummel, A.M.; Masten, S.J.; Trosko, J.E.; Upham, B.L. Bay or baylike regions of polycyclic aromatic hydrocarbons were potent inhibitors of Gap junctional intercellular communication. Environ. Health Perspect. 1998, 106, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.J.B.; Oliveira, E.; Teixeira, A.S.; Gravato, C.S.; Loureiro, S.; Guilhermino, L.C.; Van Gestel, C.A.M.; Soares, A.M.V.M. Toxicity and bioaccumulation of phenanthrene in Enchytraeus albidus (Oligochaeta: Enchytraeidae). Environ. Toxicol. Chem. 2011, 30, 967–972. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Heitkamp, M.A. Microbial degradation of polycyclic aromatic hydrocarbons (PAH) in the aquatic environment. In Metabolism of Polycyclic Aromatic Hydrocarbons in the Aquatic Environment; Varanasi, U., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 41–68. [Google Scholar]
- Moody, J.D.; Freeman, J.P.; Doerge, D.R.; Cerniglia, C.E. Degradation of Phenanthrene and Anthracene by Cell Suspensions of Mycobacterium sp. Strain PYR-1. Appl. Environ. Microbiol. 2001, 67, 1476–1483. [Google Scholar] [CrossRef]
- Pashin, Y.V.; Bakhitova, L.M. Mutagenic and carcinogenic actions of some polycyclic aromatic hydrocarbons. Environ. Health Perspect. 1979, 30, 185–189. [Google Scholar] [CrossRef]
- Seo, J.S.; Keum, Y.S.; Li, Q.X. Bacterial degradation of aromatic compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef]
- Heitkamp, M.A.; Franklin, W.; Cerniglia, C.E. Microbial metabolism of polycyclic aromatic hydrocarbons: Isolation and characterization of a pyrene-degrading bacterium. Appl. Environ. Microbiol. 1988, 54, 2549–2555. [Google Scholar] [CrossRef]
- Catallo, W.J.; Portier, R.J. Use of Indigenous and Adapted Microbial Assemblages in the Removal of Organic Chemicals from Soils and Sediments. Water Sci. Technol. 1992, 25, 229–237. [Google Scholar] [CrossRef]
- Wilson, S.C.; Jones, K.C. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): A review. Environ. Pollut. 1993, 81, 229–249. [Google Scholar] [CrossRef]
- Borde, X.; Guieysse, B.; Delgado, O.; Muñoz, R.; Hatti-Kaul, R.; Nugier-Chauvin, C.; Patin, H.; Mattiasson, B. Synergistic relationships in algal-bacterial microcosms for the treatment of aromatic pollutants. Bioresour. Technol. 2003, 86, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.K. The interaction of algae and bacteria. In Microbial Interactions and Communitties; Bull, A.T., Slater, J.H., Eds.; Academic Press: New York, NY, USA, 1982; pp. 189–247. [Google Scholar]
- Colyer, C.L.; Klinkade, C.S.; Viskari, P.J.; Landers, J.P. Analysis of cyanobacterial pigments and proteins by electrophoretic and chromatographic methods. Anal. Bioanal. Chem. 2005, 382, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, E.I.; Galun, M. Desert algae, lichens, and fungi. In Desert Biology: Special Topics on the Physical and Biological Aspects of Arid Regions; Brown, G.W., Ed.; Academic Press: London, UK, 1974; pp. 165–204. Volume 2, ISBN 0121359026. [Google Scholar]
- Madigan, M.T.; Martinko, J.M.; Stahl, D.A.; David, P. Clark Brock Biology of Microorganisms, 13th ed.; Pearson: San Francisco, CA, USA, 2012. [Google Scholar]
- Tiwari, O.N.; Singh, B.V.; Mishra, U.; Singh, A.K.; Dhar, D.W.; Singh, P.K. Distribution and physiological characterization of cyanobacteria isolated from arid zones of Rajasthan. Trop. Ecol. 2005, 46, 165–171. [Google Scholar]
- Uyeda, J.C.; Harmon, L.J.; Blank, C.E. A comprehensive study of cyanobacterial morphological and ecological evolutionary dynamics through deep geologic time. PLoS ONE 2016, 11, e0162539. [Google Scholar] [CrossRef]
- Mouget, J.L.; Dakhama, A.; Lavoie, M.C.; de la Noüe, J. Algal growth enhancement by bacteria: Is consumption of photosynthetic oxygen involved? FEMS Microbiol. Ecol. 1995, 18, 35–43. [Google Scholar] [CrossRef]
- Radhakrishnan, L. Removal of Priority Pollutant Nitrobenzene by Algal Bacterial System in Rotating Biological Reactor. Ph.D. Dissertation, IIT Bombay, Mumbai, India, 1997. [Google Scholar]
- Ahmed, B.E.; Badawi, M.H.; Mostafa, S.S.; Higazy, A.M. Human anticancers and antidiabetic activities of the cyanobacterium Fischerella sp. BS1-EG isolated from river Nile, Egypt. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3473–3485. [Google Scholar] [CrossRef]
- Kreitlow, S.; Mundt, S.; Lindequist, U. Cyanobacteria--a potential source of new biologically active substances. J. Biotechnol. 1999, 70, 61–63. [Google Scholar] [CrossRef]
- Partners, T.I. Microalgae-Based Products Market ($2.81Bn by 2028) Growth Forecast at 7.9% CAGR During 2021 to 2028 COVID Impact and Global Analysis. Available online: https://www.globenewswire.com/en/news-release/2021/09/01/2290179/0/en/Microalgae-Based-Products-Market-2-81Bn-by-2028-Growth-Forecast-at-7-9-CAGR-During-2021-to-2028-COVID-Impact-and-Global-Analysis-by-TheInsightPartners-com.html (accessed on 8 February 2023).
- Pervez, M.T.; Hasnain, M.J.; Abbas, S.H.; Moustafa, M.F.; Aslam, N.; Shah, S.S.M. A Comprehensive Review of Performance of Next-Generation Sequencing Platforms. Biomed. Res. Int. 2022, 2022, 3457806. [Google Scholar] [CrossRef]
- Salipante, S.J.; Kawashima, T.; Rosenthal, C.; Hoogestraat, D.R.; Cummings, L.A.; Sengupta, D.J.; Harkins, T.T.; Cookson, B.T.; Hoffman, N.G. Performance comparison of Illumina and Ion Torrent next-generation sequencing platforms for 16S rRNA-based bacterial community profiling. Appl. Environ. Microbiol. 2014, 80, 7583–7591. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.B.; Chee, M.S.; Gunderson, K.L. Highly parallel genomic assays. Nat. Rev. Genet. 2006, 7, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Metzker, M.L. Sequencing technologies: The next generation. Nat. Rev. Genet. 2010, 11, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Shendure, J.; Porreca, G.J.; Reppas, N.B.; Lin, X.; McCutcheon, J.P.; Rosenbaum, A.M.; Wang, M.D.; Zhang, K.; Mitra, R.D.; Church, G.M. Accurate multiplex polony sequencing of an evolved bacterial genome. Science 2005, 309, 1728–1732. [Google Scholar] [CrossRef]
- Allen, M.M.; Stanier, R.Y. Growth and division of some unicellular blue-green algae. J. Gen. Microbiol 1968, 51, 199–202. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.M.M.; Oishi, B.O.; Pandey, A.; Soccol, C.R. Kinetics of Gibberella fujikuroi Growth and Gibberellic Acid Production by Solid-State Fermentation in a Packed-Bed Column Bioreactor. Biotechnol. Prog. 2004, 20, 1449–1453. [Google Scholar] [CrossRef]
- Muloiwa, M.; Nyende-Byakika, S.; Dinka, M. Comparison of unstructured kinetic bacterial growth models. S. Afr. J. Chem. Eng. 2020, 33, 141–150. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high- throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Mcmurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Wickham, H. ggplo2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 9780387981406. [Google Scholar]
- Oksanen, J.; Blanchet, F.; Kindt, K.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, H.; Szoecs, E.; et al. R Package, version 2.2-1 ed; Vegan: Community Ecology Package; R Core Team: Vienna, Austria, 2015; pp. 1–296. [Google Scholar]
- Al-Yousef, H.M.; Amina, M. Phytoconstituents and pharmacological activities of cyanobacterium Fischerella ambigua. Arab. J. Chem. 2021, 14, 103153. [Google Scholar] [CrossRef]
- Brunberg, A.K. Contribution of bacteria in the mucilage of Microcystis spp. (Cyanobacteria) to benthic and pelagic bacterial production in a hypereutrophic lake. FEMS Microbiol. Ecol. 1999, 29, 13–22. [Google Scholar] [CrossRef]
- Ashby, M.K. Survey of the number of two-component response regulator genes in the complete and annotated genome sequences of prokaryotes. FEMS Microbiol. Lett. 2004, 231, 277–281. [Google Scholar] [CrossRef]
- Okerentugba, P.O.; Ezeronye, O.U. Petroleum degrading potentials of single and mixed microbial cultures isolated from rivers and refinery effluent in Nigeria. Afr. J. Biotechnol. 2003, 2, 288–292. [Google Scholar] [CrossRef]
- Rahman, K.S.M.; Rahman, T.; Lakshmanaperumalsamy, P.; Banat, I.M. Occurrence of crude oil degrading bacteria in gasoline and diesel station soils. J. Basic Microbiol. 2002, 42, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Bateson, M.M.; Ward, D.M. Photoexcretion and fate of glycolate in a hot spring cyanobacterial mat. Appl. Environ. Microbiol. 1988, 54, 1738–1743. [Google Scholar] [CrossRef]
- Gamazo, C.; Sánchez, S.; Camacho, A.I. Microbiología Basada en la Experimentación, 1st ed.; Elsevier: Barcelona, Spain, 2013; ISBN 9788490220856. [Google Scholar]
- Xu, X.; Liu, W.; Wang, W.; Tian, S.; Jiang, P.; Qi, Q.; Li, F.; Li, H.; Wang, Q.; Li, H.; et al. Potential biodegradation of phenanthrene by isolated halotolerant bacterial strains from petroleum oil polluted soil in Yellow River Delta. Sci. Total Environ. 2019, 664, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.; Bracho, M.; Díaz-Borrego, L.; Araujo, I. Productos metabólicos de la degradación de fenantreno por Bacillus sp. Boletín Cent. Investig. Biológicas 2008, 42, 291–298. [Google Scholar]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef]
- Kumar, V.; Shahi, S.K.; Singh, S. Bioremediation: An Eco-sustainable Approach for Restoration of Contaminated Sites. In Microbial Bioprospecting for Sustainable Development; Singh, J., Sharma, D., Kumar, G., Sharma, N., Eds.; Springer: Singapore, 2018; pp. 115–136. ISBN 9789811300530. [Google Scholar]
- Markande, A.R.; Patel, D.; Varjani, S. A review on biosurfactants: Properties, applications and current developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef]
- Márquez-Villa, J.M.; Mateos-Díaz, J.C.; Rodríguez-González, J.A.; Camacho-Ruíz, R.M. Optimization of Lipopeptide Biosurfactant Production by Salibacterium sp. 4CTb in Batch Stirred-Tank Bioreactors. Microorganisms 2022, 10, 983. [Google Scholar] [CrossRef]
- Kumari, S.; Regar, R.K.; Manickam, N. Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour. Technol. 2018, 254, 174–179. [Google Scholar] [CrossRef]
- Brusseau, M.L. The impact of physical, chemical and biological factor on biodegradation: Implication for in situ bioremediation. In Proceedings of the International Conference on Biotechnology for Soil Remediation: Scientific Bases and Practical Applications; CIPASRL: Milan, Italy, 1998; pp. 81–98. [Google Scholar]
- Elnaker, N.A.; Elektorowicz, M.; Naddeo, V.; Hasan, S.W.; Yousef, A.F. Assessment of microbial community structure and function in serially passaged wastewater electro-bioreactor sludge: An approach to enhance sludge settleability. Sci. Rep. 2018, 8, 7013. [Google Scholar] [CrossRef]
- Izquierdo-Romero, A.R. Biodegradación de HAPs Durante la Biorremediación Aeróbica de Suelos Contaminados con Hidrocarburos del Petróleo. Análisis de Poblaciones Bacterianas y Genes Funcionales; Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Porwal, H.; Mane, A.V.; Velhal, S.G. Biodegradation of diary effluent by using microbial isolates obtained from activated sludge. Water Resour. Ind. 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Martirani-Von Abercron, S.M.; Marín, P.; Solsona-Ferraz, M.; Castañeda-Cataña, M.A.; Marqués, S. Naphthalene biodegradation under oxygen-limiting conditions: Community dynamics and the relevance of biofilm-forming capacity. Microb. Biotechnol. 2017, 10, 1781–1796. [Google Scholar] [CrossRef]
- Guo, W.; Li, D.; Tao, Y.; Gao, P.; Hu, J. Isolation and description of a stable carbazole-degrading microbial consortium consisting of Chryseobacterium sp. NCY and Achromobacter sp. NCW. Curr. Microbiol. 2008, 57, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Owsianiak, M.; Szulc, A.; Chrzanowski; Cyplik, P.; Bogacki, M.; Olejnik-Schmidt, A.K.; Heipieper, H.J. Biodegradation and surfactant-mediated biodegradation of diesel fuel by 218 microbial consortia are not correlated to cell surface hydrophobicity. Appl. Microbiol. Biotechnol. 2009, 84, 545–553. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Philip, L.; Bhallamudi, S.M. Biodegradation of various aromatic compounds by enriched bacterial cultures: Part a–monocyclic and polycyclic aromatic hydrocarbons. Appl. Biochem. Biotechnol. 2015, 176, 1870–1888. [Google Scholar] [CrossRef]
- Chirwa, E.; Bezza, F. Petroleum hydrocarbon spills in the environment and abundance of microbial community capable of biosurfactant production. J. Pet. Environ. Biotechnol. 2015, 6, 1–5. [Google Scholar] [CrossRef]
- Ike, M.; Jin, C.-S.; Fujita, M. Isolation and characterization of a novel bisphenol A-degrading bacterium Pseudomonas paucimobilis strain FJ-4. Jpn. J. Water Treat. Biol. 1995, 31, 203–212. [Google Scholar] [CrossRef]
- Muangchinda, C.; Pansri, R.; Wongwongsee, W.; Pinyakong, O. Assessment of polycyclic aromatic hydrocarbon biodegradation potential in mangrove sediment from Don Hoi Lot, Samut Songkram Province, Thailand. J. Appl. Microbiol. 2013, 114, 1311–1324. [Google Scholar] [CrossRef]
- Wang, S.; Cui, Z.; Zheng, L.; Gao, W.; Li, Q. Characterization of PAHs-degrading Bacterium Porphyrobacter sp. D22F and its ecological niche in oil-degrading consortium D22-1. Chin. J. Appl. Environ. Biol. 2012, 18, 122–127. [Google Scholar] [CrossRef]
- Jamieson, W.D.; Pehl, M.J.; Gregory, G.A.; Orwin, P.M. Coordinated surface activities in Variovorax paradoxus EPS. BMC Microbiol. 2009, 9, 124. [Google Scholar] [CrossRef]
- Alkanany, F.N.A.; Gmais, S.A.; Maki, A.A.; Altaee, A.M.R. Estimation of bacterial biodegradability of PAH in Khor Al-Zubair channel, Southern Iraq. Micro 2017, 7, 399–410. [Google Scholar] [CrossRef]
- Nzila, A.; Thukair, A.; Sankara, S.; Chanbasha, B.; Musa, M.M. Isolation and characterization of naphthalene biodegrading Methylobacterium radiotolerans bacterium from the eastern coastline of the Kingdom of Saudi Arabia. Arch. Environ. Prot. 2016, 42, 25–32. [Google Scholar] [CrossRef]
- Wong, M.L.; An, D.; Caffrey, S.M.; Soh, J.; Dong, X.; Sensen, C.W.; Thomas, T.B.; Larter, S.R.; Voordouw, G. Roles of thermophiles and fungi in bitumen degradation in mostly cold oil sands outcrops. Appl. Environ. Microbiol. 2015, 81, 6825–6838. [Google Scholar] [CrossRef] [PubMed]
- Ichor, T.; Okerentugba, P.O.; Okpokwasil, G.C. Biodegradation of total petroleum hydrocarbon by a consortium of cyanobacteria isolated from crude oil polluted brackish waters of Bodo Creeks in Ogoniland, Rivers State. Res. J. Environ. Toxicol. 2016, 10, 16–27. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Gibson, D.T.; Baalen, C. Van Oxidation of naphthalene by cyanobacteria and microalgae. J. Gen. Microbiol. 1980, 116, 495–500. [Google Scholar]
- Barth, H.J. The influence of cyanobacteria on oil polluted intertidal soils at the Saudi Arabian Gulf shores. Mar. Pollut. Bull. 2003, 46, 1245–1252. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).