Influence of Three Laser Wavelengths with Different Power Densities on the Mitochondrial Activity of Human Gingival Fibroblasts in Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture—Gingival Fibroblasts Isolation and Cultivation
2.2. Laser Irradiation Procedures
2.3. Experimental Groups
- (1)
- 3 J/cm2, 50 mW, time 18 s;
- (2)
- 25 J/cm2, 400 mW, time 18 s;
- (3)
- 64 J/cm2, 1000 mW, time 18 s;
- (4)
- non-irradiated control group.
- (1)
- 3 J/cm2 50 mW, time 18 s;
- (2)
- 25 J/cm2 400 mW, time 18 s;
- (3)
- 64 J/cm2 i 1000 mW, time 18 s;
- (4)
- non-irradiated control group.
- (1)
- 3 J/cm2 50 mW, time 18 s;
- (2)
- 25 J/cm2 400 mW, time 18 s;
- (3)
- non-irradiated control group.
2.4. Cell Viability Assay (CCK-8 Assay)
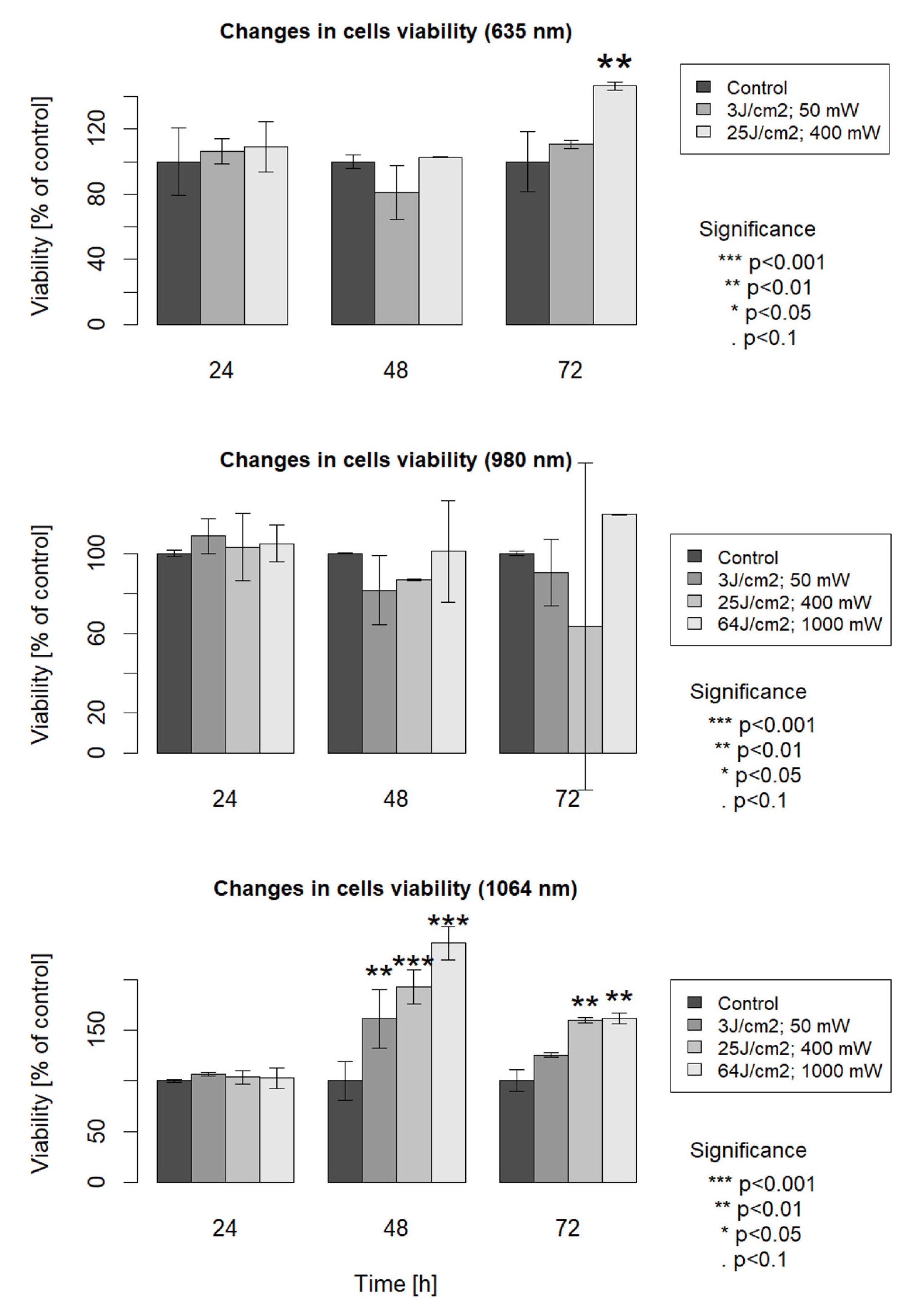
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, M.; Zhang, B.; Shen, N.; Wu, N.; Sun, J. A systematic review and meta-analysis of the effect of low-level laser therapy (LLLT) on chemotherapy-induced oral mucositis in pediatric and young patients. Eur. J. Pediatr. 2018, 177, 7–17. [Google Scholar] [CrossRef]
- Clijsen, R.; Brunner, A.; Barbero, M.; Clarys, P.; Taeymans, J. Effects of low-level laser therapy on pain in patients with musculoskeletal disorders: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2017, 53, 603–610. [Google Scholar] [CrossRef]
- Smoot, B.; Chiavola-Larson, L.; Lee, J.; Manibusan, H.; Allen, D. Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymphedema: A systematic review and meta-analysis. J. Cancer Surviv. 2015, 9, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, S.; Zamperlini-Netto, G.; Beyene, J.; Treister, N.S.; Sung, L. Effect of prophylactic low level laser therapy on oral mucositis: A systematic review and meta-analysis. PLoS ONE 2014, 9, e107418. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Aoki, A.; Iwasaki, K.; Mizutani, K.; Katagiri, S.; Suda, T.; Ichinose, S.; Ogita, M.; Pavlic, V.; Izumi, Y. Biological effects of Er:YAG laser irradiation on the proliferation of primary human gingival fibroblasts. J. Biophotonics 2018, 11, e201700157. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Włodarczak SLesiak, M.; Doroszko, A.; Włodarczak, A. Changes in cell biology under the influence of low-level laser therapy. Photonics 2022, 9, 502. [Google Scholar] [CrossRef]
- AlGhamdi, K.M.; Kumar, A.; Moussa, N.A. Low-level laser therapy: A useful technique for enhancing the proliferation of various cultured cells. Lasers Med. Sci. 2012, 27, 237–249. [Google Scholar] [CrossRef]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.; Takasaki, A.; Romanos, G.; Taniguchi, Y.; Sasaki, K.; Zeredo, J.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000 2015, 68, 217–269. [Google Scholar] [CrossRef]
- Crisan, B.; Soritau, O.; Baciut, M.; Campian, R.; Crisan, L.; Baciut, G. Influence of three laser wavelengths on human fibroblasts cell culture. Lasers Med. Sci. 2013, 28, 457–463. [Google Scholar] [CrossRef]
- Azevedo, L.H.; de Paula Eduardo, F.; Moreira, M.S.; de Paula Eduardo, C.; Marques, M.M. Influence of different power densities of LILT on cultured human fibroblast growth: A pilot study. Lasers Med. Sci. 2006, 21, 86–89. [Google Scholar] [CrossRef]
- Besser, M.; Schaeler, L.; Plattfaut, I.; Brill, F.; Kampe, A.; Geffken, M.; Smeets, R.; Debus, S.; Stuermer, E. Pulsed low-intensity laser treatment stimulates wound healing without enhancing biofilm development in vitro. J. Photochem. Photobiol. B 2021, 221, 112240. [Google Scholar] [CrossRef] [PubMed]
- Damante, C.A.; De Micheli, G.; Miyagi, S.P.; Feist, I.S.; Marques, M.M. Effect of laser phototherapy on the release of fibroblast growth factors by human gingival fibroblasts. Laser Med. Sci. 2009, 24, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Gasparyan, L. Laser irradiation of the blood. Laser Partn. Clin. 2003, 1, 1–4. [Google Scholar]
- Pyszora, A.; Adamczyk, A. Application of Low Level Laser Therapy (LLLT) for pain relief. Adv. Palliat. Med. 2005, 4, 127–132. [Google Scholar]
- van Coevorden, R.S. Zastosowanie niskoenergetycznego promieniowania laserowego w opiece paliatywnej. Adv. Pall. Med. 2009, 3, 83–89. [Google Scholar]
- Vijay Bakshi, P.; Badarinarayan Setty, S.; Raghavendra Kulkarni, M. Photobiomodulation of human gingival fibroblasts with diode laser—A systemic review. J. Indian Soc. Periodontol. 2022, 26, 5–12. [Google Scholar] [CrossRef]
- Da Ré Guerra, F.; Vieira, C.P.; Marques, P.P.; Oliveira, L.P.; Pimentel, E.R. Low level laser therapy accelerates the extracellular matrix reorganization of inflamed tendon. Tissue Cell 2017, 49, 483–488. [Google Scholar] [CrossRef]
- Yin, K.; Zhu, R.; Wang, S.; Zhao, R.C. Low-level laser effect on proliferation, migration and antiapoptosis of mesenchymal stem cells. Stem. Cells Dev. 2017, 26, 762–775. [Google Scholar] [CrossRef]
- de Oliveira, F.A.; Matos, A.A.; Matsuda, S.S.; Buzalaf, M.A.R.; Bagnato, V.S.; de Andrade Moreira Machado, M.A.; Damante, C.A.; de Oliveira, R.C.; Peres-Buzalaf, C. Low-level laser therapy modulates viability, alkaline phosphatase, and matrix metalloproteinase-2 activities of osteoblasts. J. Photochem. Photobiol. B 2017, 169, 35–40. [Google Scholar] [CrossRef]
- de Medeiros, M.L.; Araújo-Filho, I.; da Silva, E.M.N.; de Sousa Queiroz, W.S.; Soares, C.D.; de Carvalho, M.G.F.; Maciel, M.A.M. Effect of low-level laser therapy on angiogenesis and matrix metalloproteinase-2 immunoexpression in wound repair. Lasers Med. Sci. 2017, 32, 35–43. [Google Scholar] [CrossRef]
- Ren, C.; McGrath, C.; Jin, L.; Zhang, C.; Yang, Y. The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: A meta-analysis. J. Periodontal. Res. 2017, 52, 8–20. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, J.; Zhao, L. The effect of low-level laser therapy as an adjunct to periodontal surgery in the management of postoperative pain and wound healing: A systematic review and meta-analysis. Lasers Med. Sci. 2020, 36, 175–187. [Google Scholar] [CrossRef]
- Amid, R.; Kadkhodazadeh, M.; Ahsaie, M.G.; Hakakzadeh, A. Effect of low level laser therapy on proliferation and differentiation of the cells contributing in bone regeneration. J. Lasers Med. Sci. 2014, 5, 163–170. [Google Scholar]
- Huertas, R.M.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Leyva, F.M.; Ruiz, C.; García-Martínez, O. Effect and clinical implications of the low-energy diode laser on bone cell proliferation. Biol. Res. Nurs. 2013, 16, 191–196. [Google Scholar] [CrossRef]
- Safaa, T.; Wafaa, R.M.; Mai, A.; El-Gendy, A.; Mohamed, T. Tunable femtosecond laser suppresses the proliferation of breast cancer in vitro. J. Photochem. Photobiol. B 2023, 240, 112665. [Google Scholar]
- Yildiz, M.S.; Gunpinar, S. Free gingival graft adjunct with low-level laser therapy: A randomized placebo-controlled parallel group study. Clin. Oral Investig. 2019, 23, 1845–1854. [Google Scholar] [CrossRef]
- Alondra, L.; Cord, B. Wound photobiomodulation treatment outcomes in animal models. J. Vet. Intern. Med. 2019, 2019, 6320515. [Google Scholar]
- Gupta, A.; Dai, T.; Hamblin, M.R. Effect of red and near-infrared wavelengths on low-level laser (light) therapy-induced healing of partial-thickness dermal abrasion in mice. Lasers Med. Sci. 2014, 29, 257–265. [Google Scholar] [CrossRef]
- Musstaf, R.A.; Jenkins, D.F.L.; Jha, A.N. Assessing the impact of low-level laser therapy (LLLT) on biological systems: A review. Int. J. Radiat. Biol. 2019, 95, 120–143. [Google Scholar] [CrossRef]
- Martignago, C.C.S.; Tim, C.R.; Assis, L.; Da Silva, V.R.; Santos, E.C.B.D.; Vieira, F.N.; Parizotto, N.A.; Liebano, R.E. Effects of red and near-infrared LED light therapy on full-thickness skin graft in rats. Lasers Med. Sci. 2020, 35, 157–164. [Google Scholar] [CrossRef]
- Ohsugi, Y.; Niimi, H.; Shimohira, T.; Hatasa, M.; Katagiri, S.; Aoki, A.; Iwata, T. In vitro cytological responses against laser photobiomodulation for periodontal pegeneration. Int. J. Mol. Sci. 2020, 21, 9002. [Google Scholar] [CrossRef]
- Dehdashtizadeh, A.; Esnaashari, N.; Farhad, S.Z.; Ejeian, F.; Amini, S. The effect of laser irradiation and doxycycline application on the production of matrix metalloproteinase-8 and collagen I from cultured human periodontal ligament cells. Dent. Res. J. 2020, 23, 213–218. [Google Scholar]
- Lim, W.; Choi, H.; Kim, J.; Kim, S.; Jeon, S.; Zheng, H.; Kim, D.; Ko, Y.; Kim, D.; Sohn, H.; et al. Antiinflammatory effect of 635 nm irradiations on in vitro direct/indirect irradiation model. J. Oral. Pathol. Med. 2015, 44, 94–102. [Google Scholar] [CrossRef]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Photobiomodulation dose parameters in dentistry: A systematic review and meta-analysis. Dent. J. 2020, 8, 114. [Google Scholar] [CrossRef]
- Neves, L.; Gonçalves, E.; Cavalli, J.; Vieira, G.; Laurindo, L.; Simões, R.; Coelho, I.; Santos, A.; Marcolino, A.; Cola, M.; et al. Photobiomodulation therapy improves acute inflammatory response in mice: The role of cannabinoid receptors/ATP-Sensitive K+ channel/p38-MAPK signalling pathway. Mol. Neurobiol. 2018, 55, 5580–5593. [Google Scholar] [CrossRef]
- Śledziński, P.; Nowak-Terpiłowska, A.; Zeyland, J. Cannabinoids in Medicine: Cancer, Immunity, and Microbial Diseases. Int. J. Mol. Sci. 2021, 22, 263. [Google Scholar] [CrossRef]
- Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 nm), near-infrared (808 nm) and violet-blue (405 nm) photobiomodulation potentiality on human osteoblasts and mesenchymal stromal cells: A morphological and molecular in vitro study. Int. J. Mol. Sci. 2018, 19, 1946. [Google Scholar] [CrossRef]
- Dilsiz, A.; Aydin, T.; Canakci, V.; Cicek, Y. Root surface biomodification with Nd: YAG laser for the treatment of gingival recession with subepithelial connective tissue grafts. Photomed. Laser Surg. 2010, 28, 337–343. [Google Scholar] [CrossRef]
- Sun, G.; Tunér, J. Low-level laser therapy in dentistry. Dent. Clin. N. Am. 2004, 48, 1061–1076. [Google Scholar] [CrossRef]
| Laser Type | Diode 1064 nm Laser | Diode 980 nm Laser | Diode 635 nm Laser | |
|---|---|---|---|---|
| Power Density | ||||
| 1 | 3 J/cm2, 50 mW | 3 J/cm2, 50 mW | 3 J/cm2, 50 mW | |
| 2 | 25 J/cm2, 400 mW | 25 J/cm2, 400 mW | 25 J/cm2, 400 mW | |
| 3 | 64 J/cm2, 1000 mW | 64 J/cm2, 1000 mW | - | |
| 4 | non-irradiated control | non-irradiated control | non-irradiated control | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak-Terpiłowska, A.; Zeyland, J.; Hryhorowicz, M.; Śledziński, P.; Wyganowska, M. Influence of Three Laser Wavelengths with Different Power Densities on the Mitochondrial Activity of Human Gingival Fibroblasts in Cell Culture. Life 2023, 13, 1136. https://doi.org/10.3390/life13051136
Nowak-Terpiłowska A, Zeyland J, Hryhorowicz M, Śledziński P, Wyganowska M. Influence of Three Laser Wavelengths with Different Power Densities on the Mitochondrial Activity of Human Gingival Fibroblasts in Cell Culture. Life. 2023; 13(5):1136. https://doi.org/10.3390/life13051136
Chicago/Turabian StyleNowak-Terpiłowska, Agnieszka, Joanna Zeyland, Magdalena Hryhorowicz, Paweł Śledziński, and Marzena Wyganowska. 2023. "Influence of Three Laser Wavelengths with Different Power Densities on the Mitochondrial Activity of Human Gingival Fibroblasts in Cell Culture" Life 13, no. 5: 1136. https://doi.org/10.3390/life13051136





