Temporomandibular Joint Involvement in Juvenile Idiopathic Arthritis: The Results from a Retrospective Cohort Tertial Center Study
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Characteristics of JIA Patients Depending on the Presence of TMJ Arthritis
3.2. Factors Associated with TMJ Arthritis
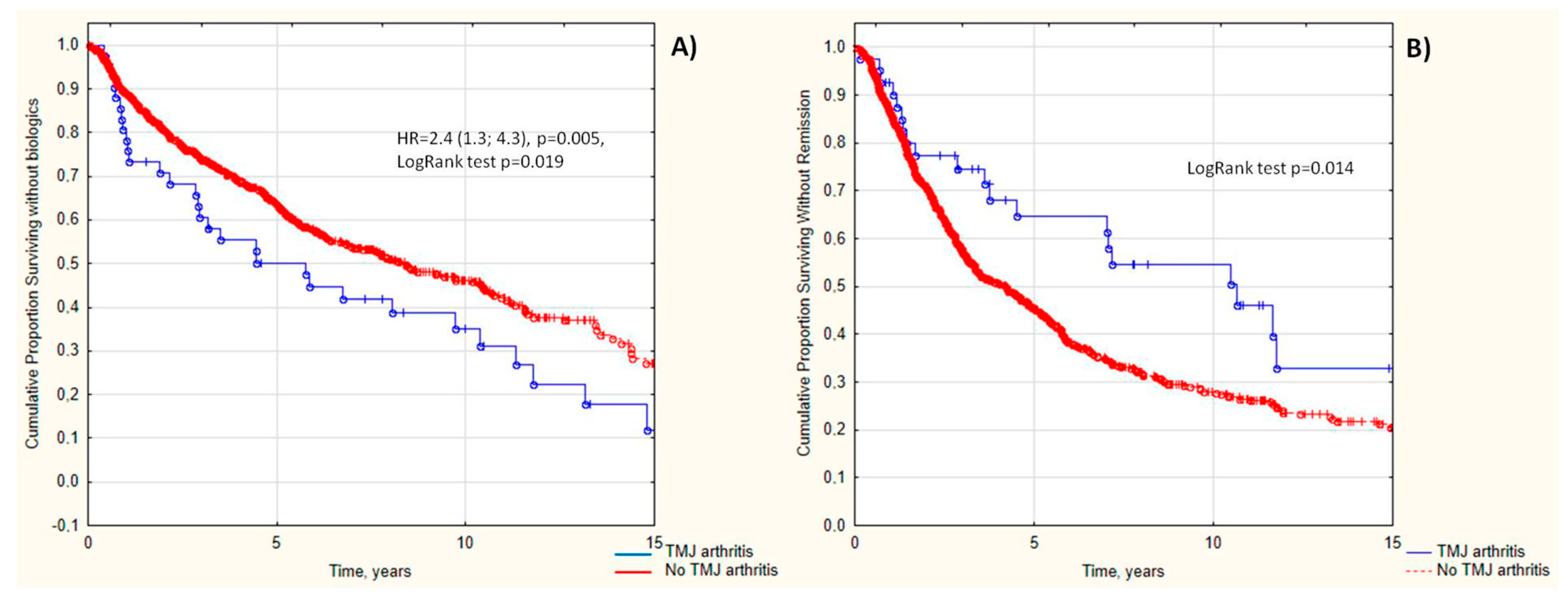
3.3. TMJ Arthritis as a Predictor of Poor JIA Outcomes
4. Discussion
4.1. The Prevalence of TMJ Arthritis in JIA Patients
4.2. TMJ Arthritis-Associated Outcomes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar] [PubMed]
- Ravelli, A.; Martini, A. Juvenile idiopathic arthritis. Lancet 2007, 369, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Ringold, S.; Nigrovic, P.A.; Feldman, B.M.; Tomlinson, G.A.; von Scheven, E.; Wallace, C.A.; Huber, A.M.; Schanberg, L.E.; Li, S.C.; Weiss, P.F.; et al. The Childhood Arthritis and Rheumatology Research Alliance Consensus Treatment Plans: Toward Comparative Effectiveness in the Pediatric Rheumatic Diseases. Arthritis Rheumatol. 2018, 70, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Stoustrup, P.; Glerup, M.; Bilgrau, A.E.; Küseler, A.; Verna, C.; Christensen, A.E.; Kristensen, K.D.; Nørholt, S.E.; Twilt, M.; Herlin, T.; et al. Cumulative Incidence of Orofacial Manifestations in Early Juvenile Idiopathic Arthritis: A Regional, Three-Year Cohort Study. Arthritis Care Res. 2020, 72, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Frid, P.; Nordal, E.; Bovis, F.; Giancane, G.; Larheim, T.A.; Rygg, M.; Pires Marafon, D.; De Angelis, D.; Palmisani, E.; Murray, K.J.; et al. Temporomandibular Joint Involvement in Association With Quality of Life, Disability, and High Disease Activity in Juvenile Idiopathic Arthritis. Arthritis Care Res. 2017, 69, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.; Twilt, M.; Herlin, T.; Spiegel, L.; Pedersen, T.K.; Küseler, A.; Stoustrup, P. Orofacial symptoms and oral health-related quality of life in juvenile idiopathic arthritis: A two-year prospective observational study. Pediatr. Rheumatol. Online J. 2018, 16, 47. [Google Scholar] [CrossRef]
- Valesan, L.F.; Da-Cas, C.D.; Réus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; de Souza, B.D.M. Prevalence of temporomandibular joint disorders: A systematic review and meta-analysis. Clin. Oral. Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef]
- Jin, L.J.; Lamster, I.B.; Greenspan, J.S.; Pitts, N.B.; Scully, C.; Warnakulasuriya, S. Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral. Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef]
- Weiss, P.F.; Arabshahi, B.; Johnson, A.; Bilaniuk, L.T.; Zarnow, D.; Cahill, A.M.; Feudtner, C.; Cron, R.Q. High prevalence of temporomandibular joint arthritis at disease onset in children with juvenile idiopathic arthritis, as detected by magnetic resonance imaging but not by ultrasound. Arthritis Rheum. 2008, 58, 1189–1196. [Google Scholar] [CrossRef]
- Glerup, M.; Stoustrup, P.; Matzen, L.H.; Rypdal, V.; Nordal, E.; Frid, P.; Arnstad, E.D.; Rygg, M.; Thorarensen, O.; Ekelund, M.; et al. Longterm Outcomes of Temporomandibular Joints in Juvenile Idiopathic Arthritis: 17 Years of Followup of a Nordic Juvenile Idiopathic Arthritis Cohort. J. Rheumatol. 2020, 47, 730–738. [Google Scholar] [CrossRef]
- Inarejos Clemente, E.J.; Tolend, M.; Navallas, M.; Doria, A.S.; Meyers, A.B. MRI of the temporomandibular joint in children with juvenile idiopathic arthritis: Protocol and findings. Pediatr. Radiol. 2023. Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Stoll, M.L.; Kau, C.H.; Waite, P.D.; Cron, R.Q. Temporomandibular joint arthritis in juvenile idiopathic arthritis, now what? Pediatr. Rheumatol. Online J. 2018, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Onel, K.B.; Horton, D.B.; Lovell, D.J.; Shenoi, S.; Cuello, C.A.; Angeles-Han, S.T.; Becker, M.L.; Cron, R.Q.; Feldman, B.M.; Ferguson, P.J.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Oligoarthritis, Temporomandibular Joint Arthritis, and Systemic Juvenile Idiopathic Arthritis. Arthritis Care Res. 2022, 74, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Ferrillo, M.; Nucci, L.; Giudice, A.; Calafiore, D.; Marotta, N.; Minervini, G.; d’Apuzzo, F.; Ammendolia, A.; Perillo, L.; de Sire, A. Efficacy of conservative approaches on pain relief in patients with temporomandibular joint disorders: A systematic review with network meta-analysis. Cranio 2022. Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Del Mondo, D.; Russo, D.; Cervino, G.; D’Amico, C.; Fiorillo, L. Stem Cells in Temporomandibular Joint Engineering: State of Art and Future Persectives. J. Craniofac Surg. 2022, 33, 2181–2187. [Google Scholar] [CrossRef]
- Stoll, M.L.; Sharpe, T.; Beukelman, T.; Good, J.; Young, D.; Cron, R.Q. Risk factors for temporomandibular joint arthritis in children with juvenile idiopathic arthritis. J. Rheumatol. 2012, 39, 1880–1887. [Google Scholar] [CrossRef]
- Bahabri, S.; Al-Sewairi, W.; Al-Mazyad, A.; Karrar, A.; Al-Ballaa, S.; El-Ramahai, K.; Al-Dalaan, A. Juvenile rheumatoid arthritis: The Saudi Experience. Ann. Saudi Med. 1997, 17, 413–418. [Google Scholar] [CrossRef]
- Al-Hemairi, M.H.; Albokhari, S.M.; Muzaffer, M.A. The Pattern of Juvenile Idiopathic Arthritis in a Single Tertiary Center in Saudi Arabia. Int. J. Inflam. 2016, 2016, 7802957. [Google Scholar] [CrossRef]
- Abramowicz, S.; Levy, J.M.; Prahalad, S.; Travers, C.D.; Angeles-Han, S.T. Temporomandibular joint involvement in children with juvenile idiopathic arthritis: A preliminary report. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2019, 127, 19–23. [Google Scholar] [CrossRef]
- Alqanatish, J.T.; Alrewaithi, B.S.; Alsewairi, W.M.; Khan, A.H.; Alsalman, M.J.; Alrasheed, A.A. Temporomandibular joint involvement in children with juvenile idiopathic arthritis: A single tertiary-center experience. Saudi Med. J. 2021, 42, 399–404. [Google Scholar] [CrossRef]
- Cannizzaro, E.; Schroeder, S.; Müller, L.M.; Kellenberger, C.J.; Saurenmann, R.K. Temporomandibular joint involvement in children with juvenile idiopathic arthritis. J. Rheumatol. 2011, 38, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Glerup, M.; Tagkli, A.; Küseler, A.; Christensen, A.E.; Verna, C.; Bilgrau, A.E.; Nørholt, S.E.; Herlin, T.; Pedersen, T.K.; Stoustrup, P. Incidence of orofacial manifestations of juvenile idiopathic arthritis from diagnosis to adult care transition: A population-based cohort study. Arthritis Rheumatol. 2023. Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, L.S.; Avrusin, I.S.; Raupov, R.K.; Lubimova, N.A.; Khrypov, S.V.; Kostik, M.M. Hip Involvement in Juvenile Idiopathic Arthritis: A Roadmap From Arthritis to Total Hip Arthroplasty or How Can We Prevent Hip Damage? Front. Pediatr. 2021, 9, 747779. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.B.; Haynes, K.; Denburg, M.R.; Thacker, M.M.; Rose, C.D.; Putt, M.E.; Leonard, M.B.; Strom, B.L. Oral glucocorticoid use and osteonecrosis in children and adults with chronic inflammatory diseases: A population-based cohort study. BMJ Open. 2017, 7, e016788. [Google Scholar] [CrossRef] [PubMed]
- Stoustrup, P.; Resnick, C.M.; Abramowicz, S.; Pedersen, T.K.; Michelotti, A.; Küseler, A.; Koos, B.; Verna, C.; Nordal, E.B.; Granquist, E.J.; et al. Management of Orofacial Manifestations of Juvenile Idiopathic Arthritis: Interdisciplinary Consensus-Based Recommendations. Arthritis Rheumatol. 2023, 75, 4–14. [Google Scholar] [CrossRef]
| Parameters | TMJ Arthritis | p-Value | |
|---|---|---|---|
| Yes, (n = 48) | No (n = 710) | ||
| Demography | |||
| JIA onset age, years, Me (25%; 75%) | 6.1 (2.8; 11.0) | 6.0 (3.0; 10.4) | 0.775 |
| Sex, females, n (%) | 30 (69.8) | 427 (60.1) | 0.209 |
| JIA duration, years, Me (25%; 75%) | 5.5 (2.7; 11.7) | 4.2 (1.9; 7.4) | 0.058 |
| Uveitis, n (%) | 1/32 (3.1) | 115/488 (23.6) | 0.007 |
| JIA categories, n (%) | 0.0006 | ||
| Oligoarthritis | 1 (2.3) | 203 (28.6) | |
| Polyarthritis | 25 (58.1) | 240 (33.8) | |
| Psoriatic arthritis | 2 (4.7) | 38 (5.4) | |
| Enthesitis-related arthritis | 9 (20.9) | 177 (24.9) | |
| Systemic arthritis | 6 (14.0) | 52 (7.3) | |
| Joint involvement | |||
| Active joints, Me (25%; 75%) | 17 (10; 42) | 6.0 (3.0; 11.0) | 0.0001 |
| Cervical spine, n (%) | 24 (50) | 77 (10.9) | 0.000001 |
| Shoulder, n (%) | 12 (25.0) | 44 (6.2) | 0.0000001 |
| Sterno-clavicular, n (%) | 4 (8.3) | 8 (1.1) | 0.00003 |
| Elbow, n (%) | 17 (35.4) | 103/707 (14.6%) | 0.00002 |
| Wrist, n (%) | 23 (47.9) | 181/710 (25.5) | 0.00006 |
| Metacarpophalangeal, n (%) | 22 (45.8) | 142/710 (20.0) | 0.000002 |
| Proximal interphalangeal, n (%) | 24/43 (50.0) | 168/710 (23.7) | 0.000003 |
| Distal interphalangeal, n (%) | 11 (22.9) | 59 (8.3) | 0.0002 |
| Hip, n (%) | 13 (27.1) | 140 (19.7) | 0.096 |
| Hip osteoarthritis, n (%) | 8/13 (61.5) | 40/140 (28.6) | 0.014 |
| Delay hip involvement, n (%) | 8/10 (80.0) | 58/125 (46.4) | 0.041 |
| Sacroiliac, n (%) | 7 (14.6) | 64 (9.0) | 0.114 |
| Knee, n (%) | 36 (75.0) | 499 (70.4) | 0.061 |
| Ankle, n (%) | 28 (58.3) | 295 (41.6) | 0.002 |
| Subtalar, n (%) | 6 (12.5) | 56 (7.9) | 0.160 |
| Tarsus, n (%) | 5 (10.4) | 38 (5.4) | 0.085 |
| Metatarsophalangeal, n (%) | 15 (31.3) | 83 (11.7) | 0.00001 |
| Foot interphalangeal, n (%) | 15 (31.3) | 79 (11.1) | 0.000005 |
| Laboratory data | |||
| Hemoglobin, g/L, Me (25%; 75%) | 120 (111; 128) | 125 (116; 133) | 0.020 |
| White blood cells, 109/L, Me (25%; 75%) | 7.4 (5.9; 11.4) | 7.1 (5.8; 9.2) | 0.164 |
| Platelets, 109/L, Me (25%; 75%) | 332 (285; 428) | 311 (255;386) | 0.161 |
| Erythrocyte sedimentation rate, mm/h, Me (25%; 75%) | 12 (3; 25) | 7 (3; 20) | 0.077 |
| C-reactive protein, mg/L, Me (25%; 75%) | 4.1 (0.0; 12.7) | 1.3 (0.2; 7.4) | 0.187 |
| Rheumatoid factor, n (%) | 1/25 (4.0) | 21/381 (5.5) | 0.808 |
| ANA-positivity, n (%) | 8/28 (28.6) | 204/432 (47.2) | 0.136 |
| HLA B27, n (%) | 4/17 (23.5) | 96/291 (33.0) | 0.694 |
| Treatment | |||
| NSAIDs, n (%) | 42 (87.5) | 613 (86.3) | 0.705 |
| Corticosteroids, n (%) | 19 (39.6) | 135 (19.0) | 0.0007 |
| Methylprednisolone pulse therapy, n (%) | 15 (31.2) | 122 (17.3) | 0.032 |
| Intra-articular corticosteroids, n (%) | 15 (31.2) | 301 (42.4) | 0.116 |
| Any corticosteroids, n (%) | 32 (66.7) | 416 (58.6) | 0.252 |
| Corticosteroid cumulative dose, mg | 2050 (1000; 5000) | 2850 (1000; 5000) | 0.802 |
| Non-biologic DMARD, n (%) | 42 (87.5) | 585 (82.4) | 0.314 |
| Biologic DMARD, n (%) | 34 (70.8) | 320 (45.1) | 0.0006 |
| Time before biologic, years | 3.9 (1.0; 9.7) | 4.2 (1.9; 7.6) | 0.912 |
| Outcomes | |||
| Remission, n (%) | 22 (45.8) | 155 (22.0) | 0.0004 |
| Time before remission, years, Me (25%; 75%) | 4.2 (1.7; 10.8) | 3.1 (1.5; 6.3) | 0.042 |
| JIA flares, n (%) | 1 (2.1) | 137 (19.3) | 0.005 |
| Predictors | Se | Sp | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Active joints >8 | 88.4 | 66.2 | 14.9 (5.8; 38.3) | 0.0000001 |
| Time before remission >7 years | 46.3 | 78.0 | 3.1 (1.6; 5.8) | 0.0004 |
| JIA duration >8 years | 42.9 | 78.6 | 2.8 (1.5; 5.2) | 0.001 |
| No uveitis | 96.9 | 23.6 | 9.6 (1.3; 70.8) | 0.007 |
| Joint involvement | ||||
| Cervical spine arthritis | 55.8 | 89.2 | 10.3 (5.4; 19.8) | 0.000001 |
| Sterno-clavicular arthritis | 9.3 | 98.9 | 9.0 (2.6; 31.2) | 0.00003 |
| Hip arthritis | 30.2 | 80.3 | 1.8 (0.9; 3.5) | 0.096 |
| Delayed hip involvement | 53.6 | 80.0 | 4.6 (0.9; 22.6) | 0.041 |
| Hip osteoarthritis | 61.5 | 71.4 | 4.0 (1.2; 13.0) | 0.014 |
| Shoulder arthritis | 27.9 | 93.8 | 5.9 (2.8; 12.2) | 0.0000001 |
| Elbow arthritis | 39.5 | 85.4 | 3.8 (2.1; 7,3) | 0.00002 |
| Wrist arthritis | 53.5 | 74.5 | 3.4 (1.8; 6.3) | 0.00006 |
| Metacarpophalangeal joints arthritis | 51.2 | 80.0 | 4.2 (2.2; 7.8) | 0.000002 |
| Proximal interphalangeal joints arthritis | 55.8 | 76.3 | 4.1 (2.2; 7.6) | 0.000003 |
| Distal interphalangeal joints arthritis | 25.6 | 91.7 | 3.8 (1.8; 7.9) | 0.0002 |
| Ankle arthritis | 65.1 | 58.5 | 2.6 (1.4; 5.0) | 0.002 |
| Metatarsophalangeal joints arthritis | 34.9 | 88.3 | 4.1 (2.1; 7.9) | 0.00001 |
| Interphalangeal foot joints arthritis | 34.9 | 88.9 | 4.1 (2.1; 7.9) | 0.000005 |
| Oral corticosteroids | 40.5 | 81.0 | 2.3 (1.2; 4.4) | 0.0007 |
| Corticosteroids pulse therapy | 30.2 | 82.7 | 2.2 (1.1; 4.1) | 0.032 |
| Cyclosporine | 30.8 | 89.0 | 3.6 (1.5; 8.8) | 0.003 |
| Biologic therapy | 72.1 | 54.9 | 3.2 (1.6; 6.2) | 0.0006 |
| Non-achievement of remission | 65.4 | 51.2 | 2.0 (1.1; 3.7) | 0.028 |
| No following JIA flares | 19.3 | 97.7 | 10.1 (1.4; 73.7) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artamonov, A.K.; Kaneva, M.A.; Gordeeva, N.A.; Sorokina, L.S.; Kostik, M.M. Temporomandibular Joint Involvement in Juvenile Idiopathic Arthritis: The Results from a Retrospective Cohort Tertial Center Study. Life 2023, 13, 1164. https://doi.org/10.3390/life13051164
Artamonov AK, Kaneva MA, Gordeeva NA, Sorokina LS, Kostik MM. Temporomandibular Joint Involvement in Juvenile Idiopathic Arthritis: The Results from a Retrospective Cohort Tertial Center Study. Life. 2023; 13(5):1164. https://doi.org/10.3390/life13051164
Chicago/Turabian StyleArtamonov, Artem K., Maria A. Kaneva, Natalia A. Gordeeva, Lubov S. Sorokina, and Mikhail M. Kostik. 2023. "Temporomandibular Joint Involvement in Juvenile Idiopathic Arthritis: The Results from a Retrospective Cohort Tertial Center Study" Life 13, no. 5: 1164. https://doi.org/10.3390/life13051164
APA StyleArtamonov, A. K., Kaneva, M. A., Gordeeva, N. A., Sorokina, L. S., & Kostik, M. M. (2023). Temporomandibular Joint Involvement in Juvenile Idiopathic Arthritis: The Results from a Retrospective Cohort Tertial Center Study. Life, 13(5), 1164. https://doi.org/10.3390/life13051164







