Effects of High-Intensity Functional Training (HIFT) on the Functional Capacity, Frailty, and Physical Condition of Older Adults with Mild Cognitive Impairment: A Blind Randomized Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
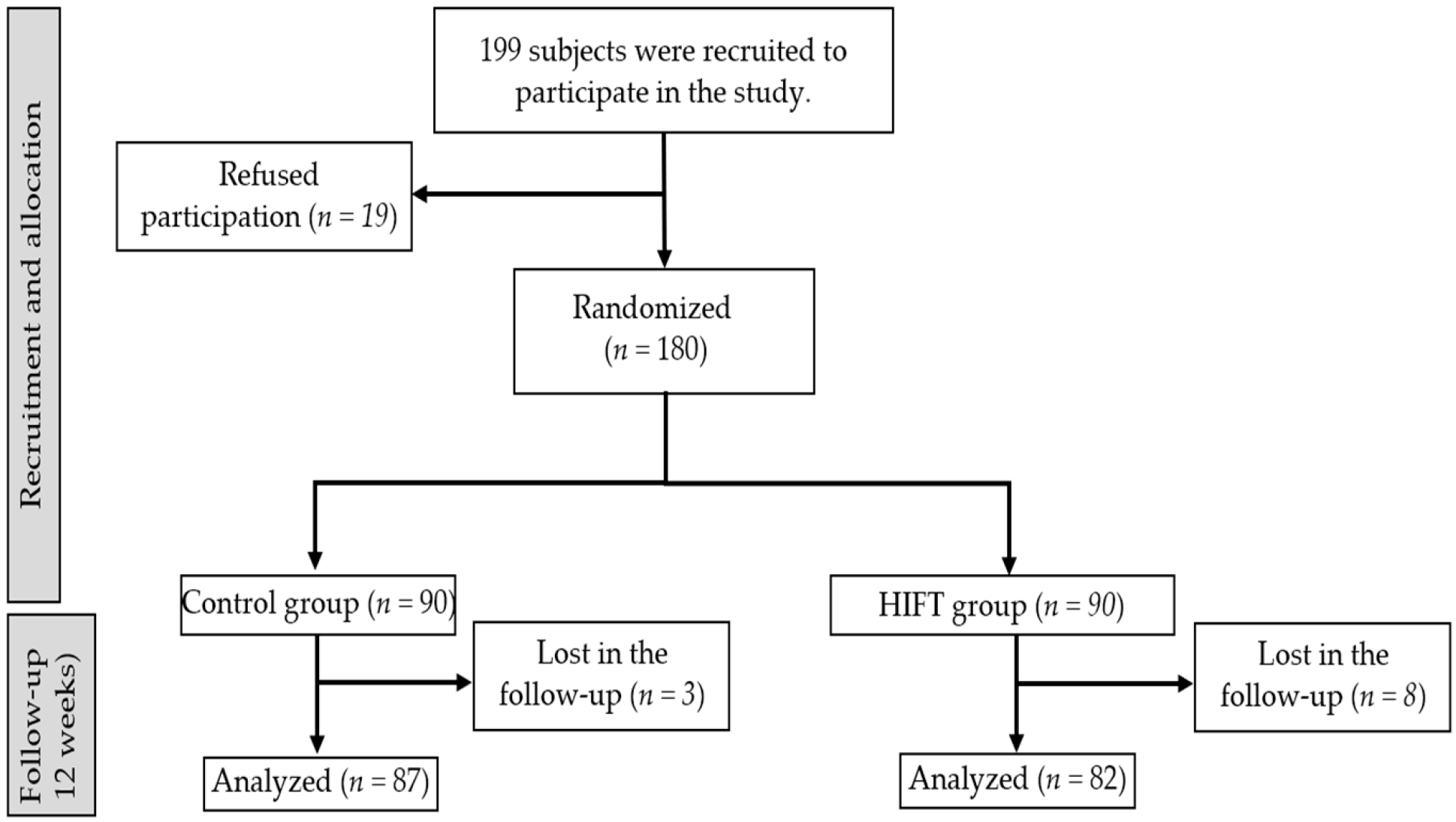
2.2. Participants
2.3. Intervention
2.3.1. Control Group (CG)
2.3.2. Intervention Group (IG)
2.4. Outcome Measurements
2.4.1. Physical Condition and Functional Capacity
2.4.2. Gait and Balance
2.4.3. Frailty
2.4.4. Functional Capacity and Independence
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
3.1. Functional Capacity
3.2. Gait and Balance
3.3. Fall Risk
3.4. Frailty
3.5. Independence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, W.; Goodkind, D.; Kowal, P.R. An Aging World: 2015; United States Census Bureau: Suitland-Silver Hill, MD, USA, 2016.
- World Health Organization. Ageing and Health Unit; WHO: Geneva, Switzerland, 2022; Volume 8.
- Fernández-Ortiz, Y.N.; Mora-Villalobos, C.A. Población Adulta Mayor en Colombia, 2020: Índice de envejecimiento poblacional, relación de dependencia demográfica y afiliación en Salud. (Elder Population in Colombia, 2020: Population Aging Index, Relationship of Demographic Dependence and Membership in Health). SSRN Electron. J. 2020, 1–38. [Google Scholar]
- GBD Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Bossers, W.J.; van der Woude, L.H.; Boersma, F.; Hortobágyi, T.; Scherder, E.J.; van Heuvelen, M.J. Comparison of Effect of Two Exercise Programs on Activities of Daily Living in Individuals with Dementia: A 9-Week Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2016, 64, 1258–1266. [Google Scholar] [CrossRef]
- Atherton, N.; Bridle, C.; Brown, D.; Collins, H.; Dosanjh, S.; Griffiths, F.; Hennings, S.; Khan, K.; Lall, R.; Lyle, S.; et al. Dementia and Physical Activity (DAPA)—An exercise intervention to improve cognition in people with mild to moderate dementia: Study protocol for a randomized controlled trial. Trials 2016, 17, 165. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Friedenreich, C.; Shiroma, E.J.; Lee, I.M. Physical inactivity and non-communicable disease burden in low-income, middle-income and high-income countries. Br. J. Sports Med. 2022, 56, 101–106. [Google Scholar] [CrossRef]
- O’Donovan, G.; Lee, I.M.; Hamer, M.; García-Garro, P.; Duran-Aniotz, C.; Ibáñez, A.; Sarmiento, O.L.; Hessel, P. The burden of mild cognitive impairment attributable to physical inactivity in Colombia. Eur. Rev. Aging Phys. Act. Off. J. Eur. Group Res. Elder. Phys. Act. 2022, 19, 28. [Google Scholar] [CrossRef]
- García-Garro, P.A.; Hita-Contreras, F.; Martínez-Amat, A.; Achalandabaso-Ochoa, A.; Jiménez-García, J.D.; Cruz-Díaz, D.; Aibar-Almazán, A. Effectiveness of A Pilates Training Program on Cognitive and Functional Abilities in Postmenopausal Women. Int. J. Environ. Res. Public Health 2020, 17, 3580. [Google Scholar] [CrossRef]
- Lam, F.M.; Huang, M.Z.; Liao, L.R.; Chung, R.C.; Kwok, T.C.; Pang, M.Y. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: A systematic review. J. Physiother. 2018, 64, 4–15. [Google Scholar] [CrossRef]
- Iijima, K.; Arai, H.; Akishita, M.; Endo, T.; Ogasawara, K.; Kashihara, N.; Hayashi, Y.K.; Yumura, W.; Yokode, M.; Ouchi, Y. Toward the development of a vibrant, super-aged society: The future of medicine and society in Japan. Geriatr. Gerontol. Int. 2021, 21, 601–613. [Google Scholar] [CrossRef]
- Maccarone, M.C.; Masiero, S.; Papathanasiou, J.; Panayotov, K.; Kashilskah, Y.; Prokopidis, K.; Papanastasiou, C.; Tyllianakis, M.; Dionyssiotis, Y. Frailty education: Promoting geriatric competencies among Physical Medicine and Rehabilitation residents. Am. J. Phys. Med. Rehabil. 2023. [Google Scholar] [CrossRef]
- Serafim, T.T.; Maffulli, N.; Migliorini, F.; Okubo, R. Epidemiology of High Intensity Functional Training (HIFT) injuries in Brazil. J. Orthop. Surg. Res. 2022, 17, 522. [Google Scholar] [CrossRef] [PubMed]
- Dominski, F.H.; Siqueira, T.C.; Serafim, T.T.; Andrade, A. Injury profile in CrossFit practitioners: Systematic review. Fisioter. Pesqui. 2018, 25, 229–239. [Google Scholar] [CrossRef]
- Heinrich, K.M.; Becker, C.; Carlisle, T.; Gilmore, K.; Hauser, J.; Frye, J.; Harms, C.A. High-intensity functional training improves functional movement and body composition among cancer survivors: A pilot study. Eur. J. Cancer Care 2015, 24, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-García, J.D.; Martínez-Amat, A.; De la Torre-Cruz, M.J.; Fábrega-Cuadros, R.; Cruz-Díaz, D.; Aibar-Almazán, A.; Achalandabaso-Ochoa, A.; Hita-Contreras, F. Suspension Training HIIT Improves Gait Speed, Strength and Quality of Life in Older Adults. Int. J. Sports Med. 2019, 40, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Feito, Y.; Heinrich, K.M.; Butcher, S.J.; Poston, W.S.C. High-Intensity Functional Training (HIFT): Definition and Research Implications for Improved Fitness. Sports 2018, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- GBD Dementia Forecasting Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Mejía, E.A.; Ochoa González, M.E.; Ruiz Castillo, L.Y.; Vargas Niño, D.M.; Sáenz Pacheco, A.M.; Sandoval-Cuellar, C. Confiabilidad del Senior Fitness Test versión en español, para población adulta mayor en Tunja-Colombia. Arch. Med. Deporte 2016, 33, 382–386. [Google Scholar]
- Rikli, R.E.; Jones, C.J. Senior Fitness Test Manual; Human Kinetics: Champaign, IL, USA, 2013. [Google Scholar]
- Rodríguez Guevara, C.; Lugo, L.H. Validez y confiabilidad de la Escala de Tinetti para población colombiana. Rev. Colomb. Reumatol. 2012, 19, 218–233. [Google Scholar]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Katz, S. The index of ADL: A standardized measure of biological and psychosocial function. J. Am. Med. Assoc. 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Selfmonitoring and instrumental activities of daily living. Gerontologist 1961, 9, 181. [Google Scholar]
- Vergara, I.; Bilbao, A.; Orive, M.; Garcia-Gutierrez, S.; Navarro, G.; Quintana, J.M. Validation of the Spanish version of the Lawton IADL Scale for its application in elderly people. Health Qual. Life Outcomes 2012, 10, 130. [Google Scholar] [CrossRef]
- Reuben, D.B.; Siu, A.L. An objective measure of physical function of elderly outpatients: The Physical Performance Test. J. Am. Geriatr. Soc. 1990, 38, 1105–1112. [Google Scholar] [CrossRef]
- Osuka, Y.; Matsubara, M.; Hamasaki, A.; Hiramatsu, Y.; Ohshima, H.; Tanaka, K. Development of low-volume, high-intensity, aerobic-type interval training for elderly Japanese men: A feasibility study. Eur. Rev. Aging Phys. Act. Off. J. Eur. Group Res. Elder. Phys. Act. 2017, 14, 14. [Google Scholar] [CrossRef]
- Marriott, C.F.S.; Petrella, A.F.M.; Marriott, E.C.S.; Boa Sorte Silva, N.C.; Petrella, R.J. High-Intensity Interval Training in Older Adults: A Scoping Review. Sports Med. Open 2021, 7, 49. [Google Scholar] [CrossRef]
- Coswig, V.S.; Barbalho, M.; Raiol, R.; Del Vecchio, F.B.; Ramirez-Campillo, R.; Gentil, P. Effects of high vs moderate-intensity intermittent training on functionality, resting heart rate and blood pressure of elderly women. J. Transl. Med. 2020, 18, 88. [Google Scholar] [CrossRef]
- Hwang, C.L.; Yoo, J.K.; Kim, H.K.; Hwang, M.H.; Handberg, E.M.; Petersen, J.W.; Christou, D.D. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp. Gerontol. 2016, 82, 112–119. [Google Scholar] [CrossRef]
- Atakan, M.M.; Li, Y.; Koşar, Ş.N.; Turnagöl, H.H.; Yan, X. Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective. Int. J. Environ. Res. Public Health 2021, 18, 7201. [Google Scholar] [CrossRef]
- Xie, B.; Yan, X.; Cai, X.; Li, J. Effects of High-Intensity Interval Training on Aerobic Capacity in Cardiac Patients: A Systematic Review with Meta-Analysis. Biomed. Res. Int. 2017, 2017, 5420840. [Google Scholar] [CrossRef]
- Granata, C.; Oliveira, R.S.; Little, J.P.; Renner, K.; Bishop, D.J. Training intensity modulates changes in PGC-1α and p53 protein content and mitochondrial respiration, but not markers of mitochondrial content in human skeletal muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 959–970. [Google Scholar] [CrossRef]
- Gbiri, C.A.O.; Amusa, B.F. Progressive task-oriented circuit training for cognition, physical functioning and societal participation in individuals with dementia. Physiother. Res. Int. J. Res. Clin. Phys. Ther. 2020, 25, e1866. [Google Scholar] [CrossRef]
- Tibana, R.A.; de Almeida, L.M.; Frade de Sousa, N.M.; Nascimento, D.d.C.; Neto, I.V.d.S.; de Almeida, J.A.; de Souza, V.C.; Lopes, M.d.F.T.P.L.; Nobrega, O.d.T.; Vieira, D.C.L.; et al. Corrigendum: Two Consecutive Days of Extreme Conditioning Program Training Affects Pro and Anti-inflammatory Cytokines and Osteoprotegerin without Impairments in Muscle Power. Front. Physiol. 2018, 9, 771. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Godfrey, R.J.; Whyte, G.P.; Buckley, J.; Quinlivan, R. The role of lactate in the exercise-induced human growth hormone response: Evidence from McArdle disease. Br. J. Sports Med. 2009, 43, 521–525. [Google Scholar] [CrossRef]
- Ohno, Y.; Ando, K.; Ito, T.; Suda, Y.; Matsui, Y.; Oyama, A.; Kaneko, H.; Yokoyama, S.; Egawa, T.; Goto, K. Lactate Stimulates a Potential for Hypertrophy and Regeneration of Mouse Skeletal Muscle. Nutrients 2019, 11, 869. [Google Scholar] [CrossRef]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an energy allocator in muscle tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef]
- de Resende-Neto, A.G.; Oliveira Andrade, B.C.; Cyrino, E.S.; Behm, D.G.; De-Santana, J.M.; Da Silva-Grigoletto, M.E. Effects of functional and traditional training in body composition and muscle strength components in older women: A randomized controlled trial. Arch. Gerontol. Geriatr. 2019, 84, 103902. [Google Scholar] [CrossRef]
- Mile, M.; Balogh, L.; Papp, G.; Pucsok, J.M.; Szabó, K.; Barna, L.; Csiki, Z.; Lekli, I. Effects of Functional Training on Sarcopenia in Elderly Women in the Presence or Absence of ACE Inhibitors. Int. J. Environ. Res. Public Health 2021, 18, 6594. [Google Scholar] [CrossRef]
- Aboarrage Junior, A.M.; Teixeira, C.V.S.; Dos Santos, R.N.; Machado, A.F.; Evangelista, A.L.; Rica, R.L.; Alonso, A.C.; Barroso, J.A.; Serra, A.J.; Baker, J.S.; et al. A High-Intensity Jump-Based Aquatic Exercise Program Improves Bone Mineral Density and Functional Fitness in Postmenopausal Women. Rejuvenation Res. 2018, 21, 535–540. [Google Scholar] [CrossRef]
- Bruseghini, P.; Calabria, E.; Tam, E.; Milanese, C.; Oliboni, E.; Pezzato, A.; Pogliaghi, S.; Salvagno, G.L.; Schena, F.; Mucelli, R.P.; et al. Effects of eight weeks of aerobic interval training and of isoinertial resistance training on risk factors of cardiometabolic diseases and exercise capacity in healthy elderly subjects. Oncotarget 2015, 6, 16998–17015. [Google Scholar] [CrossRef]
- Wilke, J.; Mohr, L. Chronic effects of high-intensity functional training on motor function: A systematic review with multilevel meta-analysis. Sci. Rep. 2020, 10, 21680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chung, P.-K. Differences in Functional Fitness Among Older Adults With and Without Risk of Falling. Asian Nurs. Res. 2016, 10, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Li, F. The effects of Tai Ji Quan training on limits of stability in older adults. Clin. Interv. Aging 2014, 9, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Zouita, S.; Zouhal, H.; Ferchichi, H.; Paillard, T.; Dziri, C.; Hackney, A.C.; Laher, I.; Granacher, U.; Ben Moussa Zouita, A. Effects of Combined Balance and Strength Training on Measures of Balance and Muscle Strength in Older Women with a History of Falls. Front. Physiol. 2020, 11, 619016. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Park, S.Y. Balance improvement by strength training for the elderly. J. Phys. Ther. Sci. 2013, 25, 1591–1593. [Google Scholar] [CrossRef]
- Buchner, D.M.; Cress, M.E.; de Lateur, B.J.; Esselman, P.C.; Margherita, A.J.; Price, R.; Wagner, E.H. The effect of strength and endurance training on gait, balance, fall risk, and health services use in community-living older adults. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, M218–M224. [Google Scholar] [CrossRef]
- Jiménez-García, J.D.; Hita-Contreras, F.; de la Torre-Cruz, M.; Fábrega-Cuadros, R.; Aibar-Almazán, A.; Cruz-Díaz, D.; Martínez-Amat, A. Risk of Falls in Healthy Older Adults: Benefits of High-Intensity Interval Training Using Lower Body Suspension Exercises. J. Aging Phys. Act. 2019, 27, 325–333. [Google Scholar] [CrossRef]
- Telenius, E.W.; Engedal, K.; Bergland, A. Effect of a high-intensity exercise program on physical function and mental health in nursing home residents with dementia: An assessor blinded randomized controlled trial. PLoS ONE 2015, 10, e0126102. [Google Scholar] [CrossRef]
- Sobhani, A.; Sharifi, F.; Fadayevatan, R.; Kamrani, A.A.A.; Moodi, M.; Khorashadizadeh, M.; Kazemi, T.; Khodabakhshi, H.; Fakhrzadeh, H.; Arzaghi, M.; et al. Low physical activity is the strongest factor associated with frailty phenotype and frailty index: Data from baseline phase of Birjand Longitudinal Aging Study (BLAS). BMC Geriatr. 2022, 22, 498. [Google Scholar] [CrossRef]
- Suikkanen, S.; Soukkio, P.; Kautiainen, H.; Kääriä, S.; Hupli, M.T.; Sipilä, S.; Pitkälä, K.; Aartolahti, E.; Kukkonen-Harjula, K. Changes in the Severity of Frailty Among Older Adults After 12 Months of Supervised Home-Based Physical Exercise: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2022, 23, 1717.e9–1717.e15. [Google Scholar] [CrossRef]
- Buto, M.S.S.; Fiogbé, E.; Vassimon-Barroso, V.; Rossi, P.G.; Farche, A.C.; Carnavale, B.F.; Takahashi, A.C. Pre-Frail Multicomponent Training Intervention project for complexity of biological signals, functional capacity and cognition improvement in pre-frail older adults: A blinded randomized controlled study protocol. Geriatr. Gerontol. Int. 2019, 19, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Witham, M.D.; Chawner, M.; Biase, S.; Offord, N.; Todd, O.; Clegg, A.; Sayer, A.A. Content of exercise programmes targeting older people with sarcopenia or frailty—Findings from a UK survey. J. Frailty Sarcopenia Falls 2020, 5, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Jadczak, A.D.; Makwana, N.; Luscombe-Marsh, N.; Visvanathan, R.; Schultz, T.J. Effectiveness of exercise interventions on physical function in community-dwelling frail older people: An umbrella review of systematic reviews. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 752–775. [Google Scholar] [CrossRef]
- Marshall-McKenna, R.; Campbell, E.; Ho, F.; Banger, M.; Ireland, J.; Rowe, P.; McAlpine, C.; McArthur, K.; Quinn, T.J.; Gray, S.R. Resistance exercise training at different loads in frail and healthy older adults: A randomised feasibility trial. Exp. Gerontol. 2021, 153, 111496. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Pinto, R.S.; Radaelli, R.; Rech, A.; Grazioli, R.; Izquierdo, M.; Cadore, E.L. Benefits of resistance training in physically frail elderly: A systematic review. Aging Clin. Exp. Res. 2018, 30, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Tina, L.; Miroljub, J. Performance-Based Screening Tools for Physical Frailty in Community Settings. In Frailty in the Elderly; Sara, P., Ed.; IntechOpen: Rijeka, Croatia, 2020; p. 4. [Google Scholar]
- Fitten, L.J. Psychological Frailty in the Aging Patient; Nestle Nutrition Institute Workshop Series; Karger: Basel, Switzerland, 2015; Volume 83, pp. 45–53. [Google Scholar] [CrossRef]
- Bunt, S.; Steverink, N.; Olthof, J.; van der Schans, C.P.; Hobbelen, J.S.M. Social frailty in older adults: A scoping review. Eur. J. Ageing 2017, 14, 323–334. [Google Scholar] [CrossRef]
- Silva, R.B.; Aldoradin-Cabeza, H.; Eslick, G.D.; Phu, S.; Duque, G. The Effect of Physical Exercise on Frail Older Persons: A Systematic Review. J. Frailty Aging 2017, 6, 91–96. [Google Scholar] [CrossRef]
- Sebastiano, M.D.K. Functional Capacity, Disability, and Status. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 903–905. [Google Scholar]
- Palma, R.; de Conti, M.H.; Quintino, N.M.; Gatti, M.A.; Simeão, S.F.; de Vitta, A. Functional capacity and its associated factors in the elderly with low back pain. Acta Ortop. Bras. 2014, 22, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Takase, M.; Takahashi, K.; Ogino, R.; Nitanai, R.; Tanaka, T.; Saisho, S.; Goto, J.; Iijima, K. Functional capacity in community-dwelling older adults maintained by a higher friend network than family network: Implications from a two-year longitudinal study. BMC Res. Notes 2022, 15, 319. [Google Scholar] [CrossRef]
- Matteo, C.; Yuka, S.; Zee, A.H.; Monica, P.; Hyobum, J.; Andrew, B.; Jotheeswaran Amuthavalli, T.; Ritu, S.; Anshu, B. Implementing care for healthy ageing. BMJ Glob. Health 2022, 7, e007778. [Google Scholar] [CrossRef]
- Parra-Rizo, M.A.; Sanchís-Soler, G. Physical Activity and the Improvement of Autonomy, Functional Ability, Subjective Health, and Social Relationships in Women over the Age of 60. Int. J. Environ. Res. Public Health 2021, 18, 6926. [Google Scholar] [CrossRef]
- Ramos, A.M.; Marcos-Pardo, P.J.; Vale, R.G.S.; Vieira-Souza, L.M.; Camilo, B.F.; Martin-Dantas, E.H. Resistance Circuit Training or Walking Training: Which Program Improves Muscle Strength and Functional Autonomy More in Older Women? Int. J. Environ. Res. Public Health 2022, 19, 8828. [Google Scholar] [CrossRef]
- Sugano, K.; Yokogawa, M.; Yuki, S.; Dohmoto, C.; Yoshita, M.; Hamaguchi, T.; Yanase, D.; Iwasa, K.; Komai, K.; Yamada, M. Effect of cognitive and aerobic training intervention on older adults with mild or no cognitive impairment: A derivative study of the nakajima project. Dement. Geriatr. Cogn. Disord. Extra 2012, 2, 69–80. [Google Scholar] [CrossRef]
- Heinrich, K.M.; Crawford, D.A.; Langford, C.R.; Kehler, A.; Andrews, V. High-Intensity Functional Training Shows Promise for Improving Physical Functioning and Activity in Community-Dwelling Older Adults: A Pilot Study. J. Geriatr. Phys. Ther. 2021, 44, 9–17. [Google Scholar] [CrossRef]
| Total (n = 169) | CG (n = 87) | IG (n = 82) | p Value | ||
|---|---|---|---|---|---|
| Age. Mean (SD) | 77.1 (7.41) | 76.8 (7.4) | 77.4 (7.3) | 0.616 | |
| MMSE. Mean (SD) | 21.6 (1.4) | 21.1 (1.2) | 21.5 (1.5) | 0.689 | |
| BMI. Mean (SD) | 27.9 (4.7) | 27.8 (5) | 28.1 (4.2) | 0.657 | |
| Sex. n (%) | Female | 103 (60.9) | 53 (60.9) | 50 (61) | 0.990 |
| Male | 66 (39.1) | 34 (39.1) | 32 (39.0) | ||
| Socioeconomic strata. n (%) | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.884 |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 3 | 53 (31.4) | 29 (33.3) | 24 (29.3) | ||
| 4 | 71 (42.0) | 34 (39.1) | 37 (45.1) | ||
| 5 | 28 (16.6) | 15 (17.2) | 13 (15.8) | ||
| 6 | 17 (10) | 9 (10.3) | 8 (9.8) | ||
| Education level. n (%) | Elementary school | 25 (14.8) | 14 (16.1) | 11 (13.4) | 0.820 |
| High school | 95 (56.2) | 47 (54.0) | 48 (58.5) | ||
| College | 46 (27.2) | 25 (28.7) | 21 (25.6) | ||
| Postgraduate | 3 (1.8) | 1 (1.1) | 2 (2.4) | ||
| Marital status. n (%) | Single | 20 (11.8) | 10 (11.5) | 10 (12.2) | 0.411 |
| Married | 87 (51.5) | 48 (55.1) | 39 (47.5) | ||
| Divorced | 18 (10.7) | 6 (6.9) | 12 (14.6) | ||
| Widowed | 44 (26.0) | 23 (26.4) | 21 (25.6) | ||
| Laterality. n (%) | Right | 161 (95.3) | 83 (95.4) | 78 (95.1) | 0.562 |
| Left | 7 (4.1) | 4 (4.6) | 3 (3.6) | ||
| Both | 1 (0.6) | 0 (0.0) | 1 (1.2) | ||
| Health status. n (%) | High | 108 (63.9) | 55 (63.2) | 53 (64.6) | 0.848 |
| Low | 61 (36.1) | 32 (36.8) | 29 (35.4) |
| Quantitative Outcomes | Pre | Post | Group | Time | Group × Time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CG | IG | p-Value | CG | IG | p Value | F | P-Value | η2 | F | p-Value | η2 | F | p-Value | η2 | ||
| Chair Stand Test. Mean (SD) | 9.7 (2.0) | 9.4 (2.3) | 0.342 | 9.7 (7.7) | 11.5 (2.0) | 0.490 | 2.41 | 0.123 | 0.070 | 4.81 | 0.03 | 0.014 | 4.70 | 0.032 | 0.014 | |
| Arm Curl Test. Mean (SD) | 11.3 (2.6) | 11.1 (2.8) | 0.751 | 10.2 (2.1) | 10.8 (2.5) | 0.217 | 1.51 | 0.221 | 0.004 | 1.21 | 0.274 | 0.004 | 1.56 | 0.213 | 0.005 | |
| Six-Minute Walk Test. Mean (SD) | 387.4 (49.2) | 389.8 (53.0) | 0.759 | 386.0 (67.5) | 475.1 (69.0) | <0.001 | 43.42 | <0.001 | 1.040 | 46.50 | <0.001 | 0.087 | 49.60 | <0.001 | 0.093 | |
| Chair Sit-and-Reach Test. Mean (SD) | −5.1 (1.8) | −5.1 (1.6) | 0.947 | −5.9 (1.7) | −4.0 (1.7) | <0.001 | 17.81 | <0.001 | 0.067 | 1.21 | 0.272 | 0.002 | 41.00 | <0.001 | 0.064 | |
| Back Scratch Test. Mean (SD) | −4.8 (1.7) | −5.0 (1.6) | 0.445 | −5.1 (1.7) | −4.1 (1.6) | <0.001 | 2.84 | 0.094 | 0.010 | 7.79 | 0.006 | 0.010 | 22.95 | <0.001 | 0.029 | |
| Eight-Foot Up-and-Go Test. Mean (SD) | 9.6 (2.5) | 9.5 (2.1) | 0.845 | 11.4 (2.9) | 4.6 (1.9) | <0.001 | 89.72 | <0.001 | 0.240 | 5.16 | <0.001 | 0.050 | 24.22 | <0.001 | 0.230 | |
| Tinetti Gait. Mean (SD) | 9.5 (1.6) | 10.0 (1.6) | 0.850 | 9.3 (1.6) | 10.4 (1.4) | <0.001 | 10.80 | 0.001 | 0.057 | 3.52 | 0.062 | 0.001 | 36.01 | <0.001 | 0.011 | |
| Tinetti Balance. Mean (SD) | 11.0 (2.2) | 10.8 (2.3) | 0.663 | 10.5 (2.1) | 19.8 (3.3) | <0.001 | 5.73 | 0.018 | 0.029 | 29.8 | <0.001 | 0.013 | 97.00 | <0.001 | 0.042 | |
| Tinetti Total. Mean (SD) | 20.4 (3.4) | 20.7 (3.8) | 0.521 | 19.8 (3.3) | 22.6 (2.9) | <0.001 | 9.54 | 0.002 | 0.049 | 28.4 | <0.001 | 0.009 | 94.4 | <0.001 | 0.029 | |
| Categorical Outcomes | Pre | Post | Group | Time | Group × Time | |||||||||||
| CG | IG | p value | CG | IG | p value | Odds | IC 95% | R2 | Odds | IC 95% | R2 | Odds | IC 95% | R2 | ||
| Tinetti. n (%) | Without risk | 12 (13.8) | 17 (20.7) | 0.483 | 8 (9.2) | 20 (24.4) | 0.002 | Reference | 0.01 | Reference | 0.004 | Reference | 0.010 | |||
| Minimum risk | 46 (52.9) | 39 (47.6) | 49 (56.3) | 50 (61.0) | 0.68 | (0.60 to 0.76) | −0.188 | (−0.19 to 1.01) | −1.463 | (−1.49 to −1.40) | ||||||
| High risk | 29 (33.3) | 26 (31.7) | 30 (34.5) | 12 (14.6) | −0.37 | (−0.31 to 1.08) | −0.435 | (−0.45 to 1.09) | −0.269 | (−0.33 to 1.07) | ||||||
| Frailty. n (%) | Non-Frail | 14 (16.1) | 7 (8.5) | 0.320 | 14 (16.1) | 15 (18.3) | 0.170 | Reference | 0.009 | Reference | 0.006 | Reference | 0.002 | |||
| Moderate Frail | 25 (28.7) | 27 (32.9) | 26 (29.9) | 36 (43.9) | 5.92 | (0.99 to 6.2) | 0.907 | (0.86 to 1.75) | 0.68 | (0.67 to 1.06) | ||||||
| Frail | 48 (55.2) | 48 (58.5) | 47 (54.0) | 31 (37.8) | 1.22 | (1.18 to 1.25) | 2.190 | (0.89 to 2.66) | 1.42 | (1.39 to 1.45) | ||||||
| Katz Index. n (%) | Without dependence | 38 (43.8) | 43 (52.4) | 0.475 | 38 (43.7) | 57 (69.5) | 0.003 | Reference | 0.010 | Reference | 0.021 | Reference | 0.020 | |||
| Mild dependence | 36 (41.4) | 27 (32.9) | 30 (34.5) | 17 (20.7) | −0.68 | (−0.71 to −0.61) | −4.524 | (−4.90 to 1.36) | −0.855 | (−0.95 to −0.89) | ||||||
| Moderate dependence | 13 (14.9) | 12 (14.6) | 19 (21.8) | 8 (9.8) | −0.74 | (−0.81 to−0.67) | −0.825 | (−0.99 to 1.27)) | −0.8151 | (−0.86 to −0.80). | ||||||
| Lawton and Brody Index. n (%) | Mild dependence | 73 (83.9) | 70 (85.4) | 0.793 | 65 (74.7) | 82 (100.0) | <0.001 | Reference | 0.004 | Reference | 0.004 | Reference | 0.004 | |||
| Moderate dependence | 14 (16.1) | 12 (14.6) | 22 (25.3) | 0 (0.0) | 1.20 | (1.93 to 1.28) | 0.195 | (0.192 to 0.199) | 1.197 | (1.14 to 1.22) | ||||||
| Siu and Reubens Scale. n (%) | Autonomous | 19 (21.8) | 27 (32.9) | 0.106 | 21 (24.1) | 44 (53.7) | <0.001 | Reference | 0.030 | Reference | 0.010 | Reference | 0.040 | |||
| Not autonomous | 68 (78.2) | 55 (67.1) | 66 (75.9) | 38 (46.3) | 0.93 | (0.90 to 0.95) | 0.501 | (0.43 to 0.62) | 0.954 | (0.93 to 0.96) | ||||||
| Wilks’ Lambda Value | F | p-Value | |
|---|---|---|---|
| Group | 0.173 | 88.231 | <0.001 |
| Charlson | 0.965 | 0.661 | 0.725 |
| Sex | 0.956 | 0.845 | 0.564 |
| Group × Charlson | 0.973 | 0.513 | 0.845 |
| Group × Sex | 0.942 | 1.141 | 0.339 |
| Charlson × Sex | 0.957 | 0.841 | 0.568 |
| Group × Charlson × Sex | 0.960 | 0.770 | 0.630 |
| Age | 0.539 | 15.826 | <0.001 |
| BMI | 0.967 | 0.631 | 0.750 |
| MMSE | 0.971 | 0.556 | 0.812 |
| RISK OF FALLING * | Multinomial Model | p-Value | Odds Ratio | η2 |
|---|---|---|---|---|
| Minimum | Group: IG–CG | 0.015 | 2.60 | 0.082 |
| Without risk | Group: IG–CG | <0.001 | 6.95 | |
| FRAILTY * | Multinomial Model | p-value | Odds ratio | |
| Moderate Frail | Group: IG–CG | 0.130 | 2.53 | 0.252 |
| Non-Frail | Group: IG–CG | 0.760 | 2.68 | |
| INDEPENDENCE ON ADL * | Multinomial Model | p value | Odds ratio | |
| Without dependence | Group: IG–CG | <0.001 | 16.69 | 0.289 |
| Mild dependence | Group: IG–CG | 0.760 | 3.06 | |
| IADL ** | Binomial Model | p-value | Odds Ratio | |
| Age | <0.001 | 0.61 | ||
| MMSE | 0.381 | 0.81 | 0.319 | |
| BMI | 0.69 | 0.97 | ||
| Group: IG–CG | 0.054 | 1.51 | ||
| Health condition: low–high | 0.696 | 0.73 | ||
| AADL *** | Binomial Model | p-value | Odds Ratio | |
| Age | <0.001 | 0.91 | 0.211 | |
| MMSE | 0.258 | 0.86 | ||
| BMI | 0.331 | 1.04 | ||
| Group: IG–CG | 0.042 | 1.91 | ||
| Health condition: low–high | 0.140 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas-Campo, Y.; Aibar-Almazán, A.; Afanador-Restrepo, D.F.; García-Garro, P.A.; Vega-Ávila, G.C.; Rodríguez-López, C.; Castellote-Caballero, Y.; Carcelén-Fraile, M.d.C.; Lavilla-Lerma, M.L. Effects of High-Intensity Functional Training (HIFT) on the Functional Capacity, Frailty, and Physical Condition of Older Adults with Mild Cognitive Impairment: A Blind Randomized Controlled Clinical Trial. Life 2023, 13, 1224. https://doi.org/10.3390/life13051224
Rivas-Campo Y, Aibar-Almazán A, Afanador-Restrepo DF, García-Garro PA, Vega-Ávila GC, Rodríguez-López C, Castellote-Caballero Y, Carcelén-Fraile MdC, Lavilla-Lerma ML. Effects of High-Intensity Functional Training (HIFT) on the Functional Capacity, Frailty, and Physical Condition of Older Adults with Mild Cognitive Impairment: A Blind Randomized Controlled Clinical Trial. Life. 2023; 13(5):1224. https://doi.org/10.3390/life13051224
Chicago/Turabian StyleRivas-Campo, Yulieth, Agustín Aibar-Almazán, Diego Fernando Afanador-Restrepo, Patricia Alexandra García-Garro, Gloria Cecilia Vega-Ávila, Carlos Rodríguez-López, Yolanda Castellote-Caballero, María del Carmen Carcelén-Fraile, and María Leyre Lavilla-Lerma. 2023. "Effects of High-Intensity Functional Training (HIFT) on the Functional Capacity, Frailty, and Physical Condition of Older Adults with Mild Cognitive Impairment: A Blind Randomized Controlled Clinical Trial" Life 13, no. 5: 1224. https://doi.org/10.3390/life13051224
APA StyleRivas-Campo, Y., Aibar-Almazán, A., Afanador-Restrepo, D. F., García-Garro, P. A., Vega-Ávila, G. C., Rodríguez-López, C., Castellote-Caballero, Y., Carcelén-Fraile, M. d. C., & Lavilla-Lerma, M. L. (2023). Effects of High-Intensity Functional Training (HIFT) on the Functional Capacity, Frailty, and Physical Condition of Older Adults with Mild Cognitive Impairment: A Blind Randomized Controlled Clinical Trial. Life, 13(5), 1224. https://doi.org/10.3390/life13051224