Nasal Patency in Sitting, Supine, and Prone Positions in Individuals with and without Allergic Rhinitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants with and without Allergic Rhinitis
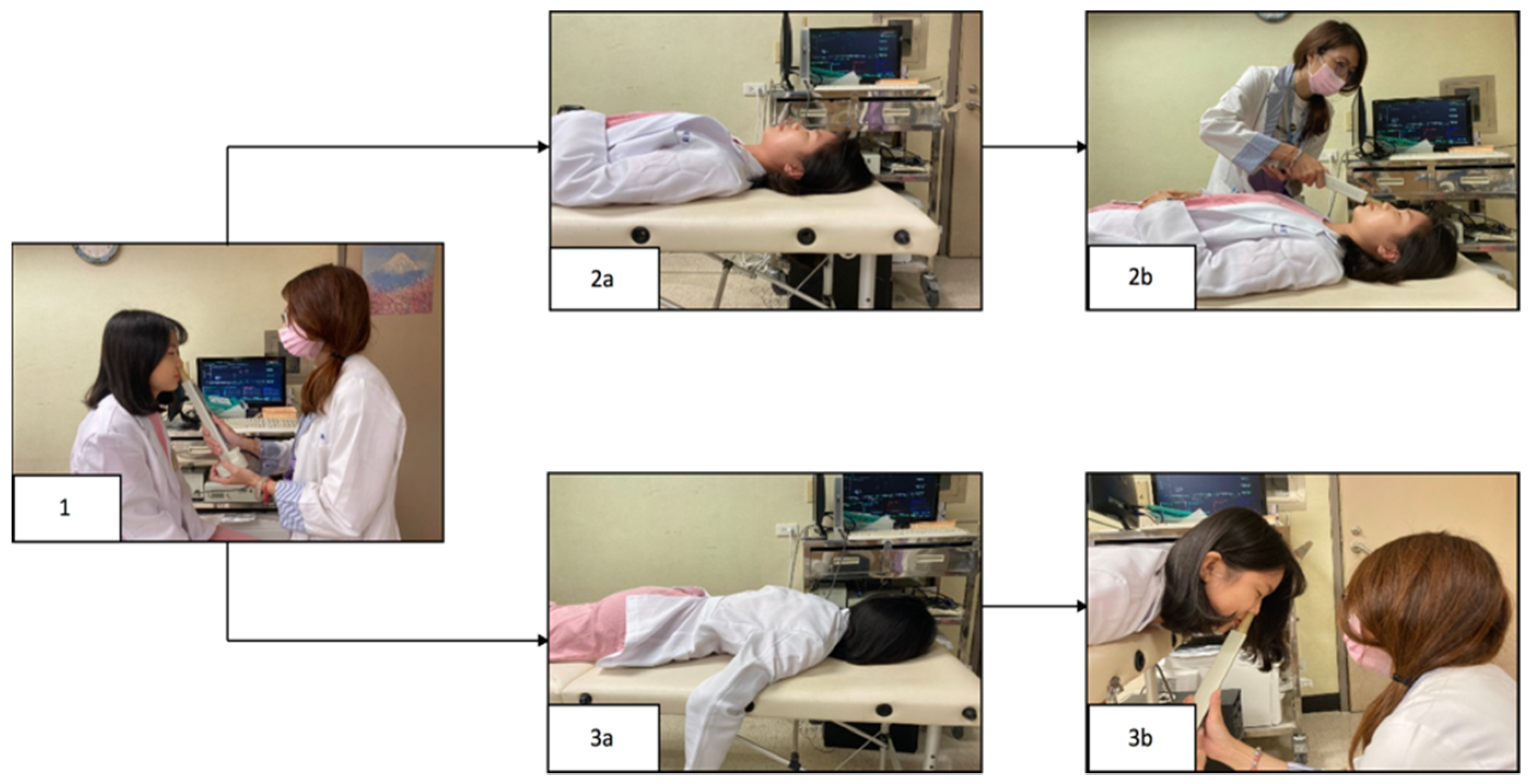
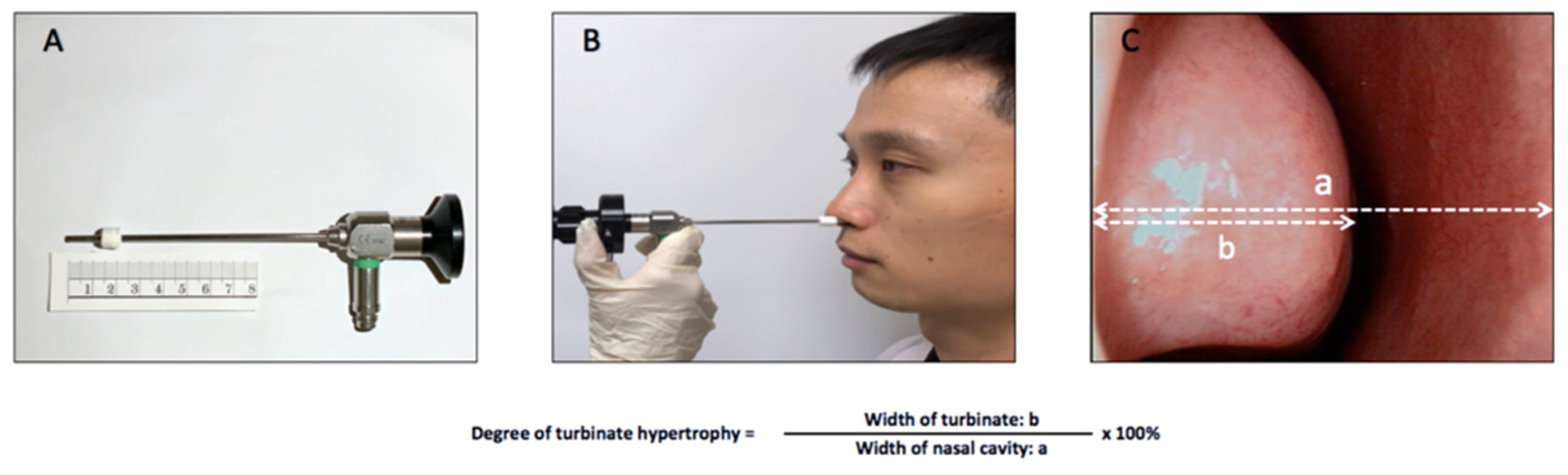
2.2. Subjective and Objective Assessments of Sleeping Positions
2.3. Statistical Analysis
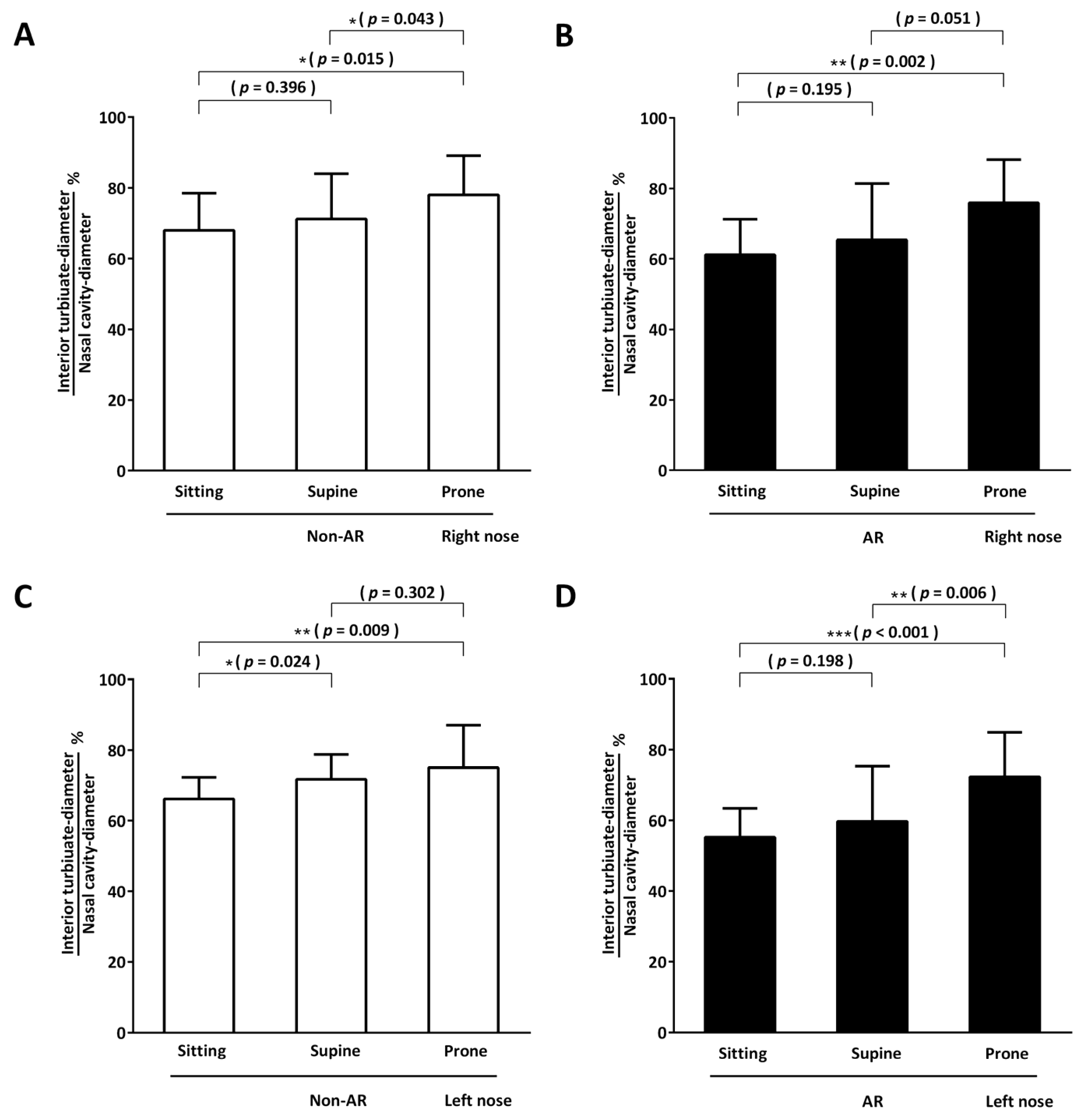
3. Results
3.1. Study Population
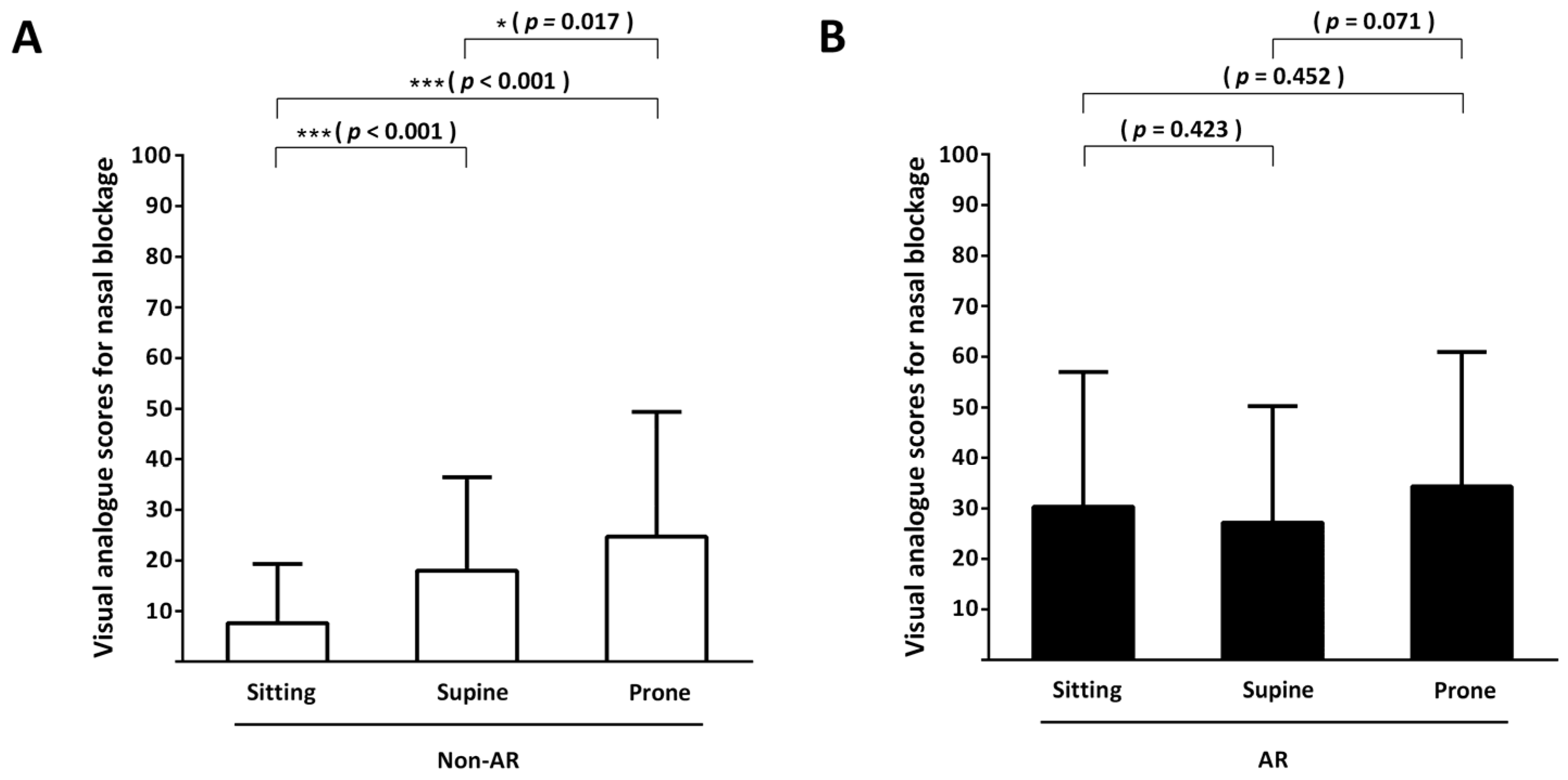
3.2. Subjective Assessment: Primary Outcome
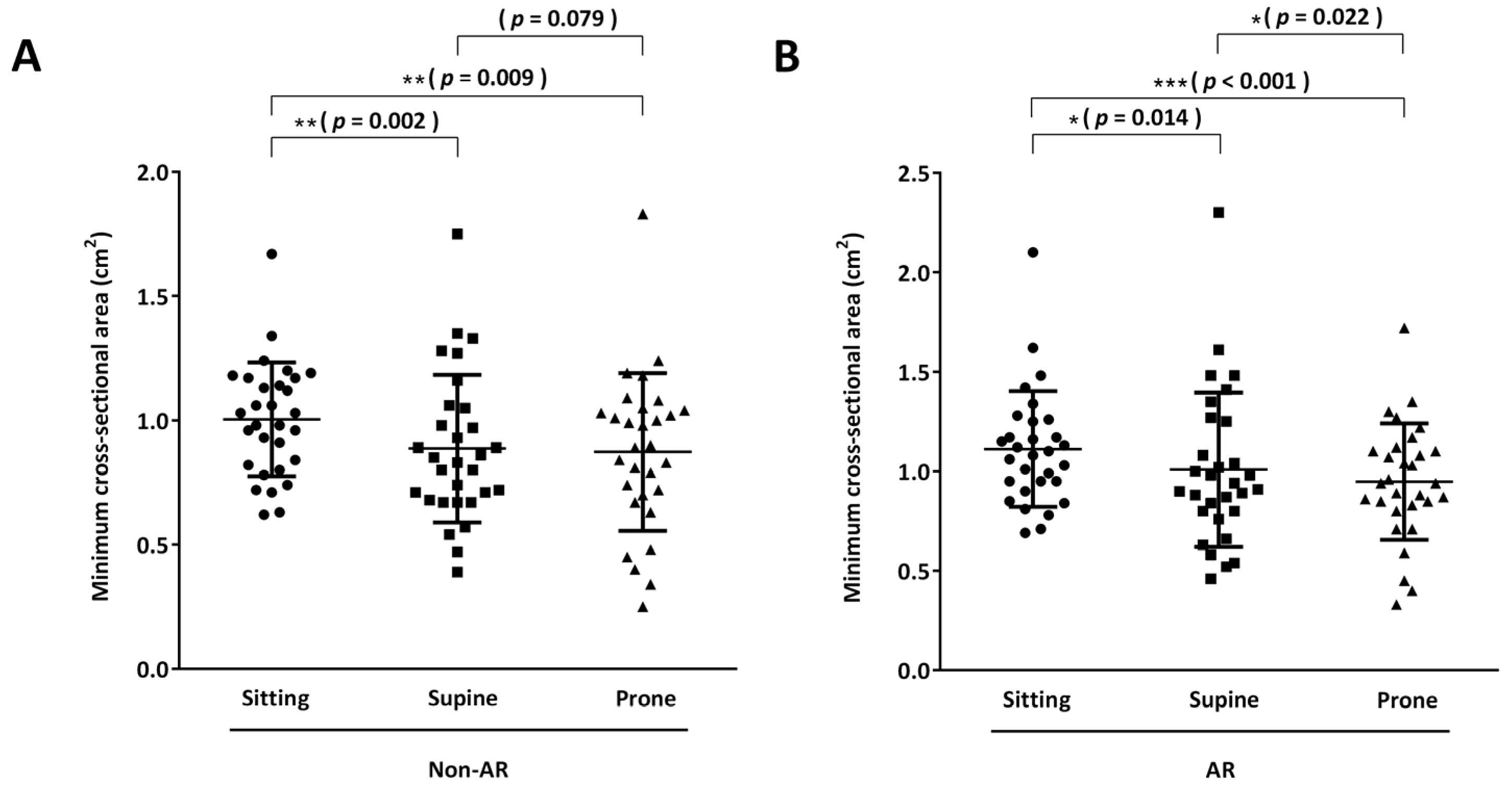
3.3. Objective Assessments: Secondary Outcomes
3.3.1. Acoustic Rhinometry
3.3.2. Endoscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hellgren, J.; Yee, B.J.; Dungan, G.; Grunstein, R.R. Altered positional regulation of nasal patency in patients with obstructive sleep apnoea syndrome. Eur. Arch. Otorhinolaryngol. 2009, 266, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Valero, A.; Navarro, A.M.; Del Cuvillo, A.; Alobid, I.; Benito, J.R.; Colás, C.; Santos, G.D.L.; Liesa, R.F.; García-Lliberós, A.; González-Pérez, R.; et al. Position paper on nasal obstruction: Evaluation and treatment. J. Investig. Allergol. Clin. Immunol. 2018, 28, 67–90. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, R.D. Effect of sleep position on sleep apnea severity. Sleep 1984, 7, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Joosten, S.A.; O’Driscoll, D.M.; Berger, P.J.; Hamilton, G.S. Supine position related obstructive sleep apnea in adults: Pathogenesis and treatment. Sleep Med. Rev. 2014, 18, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Roithmann, R.; Demeneghi, P.; Faggiano, R.; Cury, A. Effects of posture change on nasal patency. Braz. J. Otorhinolaryngol. 2005, 71, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Kuo, T.B.; Lee, G.S. Effect of postural change on nasal airway and autonomic nervous system established by rhinomanometry and heart rate variability analysis. Am. J. Rhinol. 2008, 22, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.-H.; Hsu, C.-M.; Huang, E.I.; Tsai, H.-Y.; Wang, Y.-T.; Tsai, M.-S.; Chang, P.-J.; Tsai, Y.-T. Effects of Supine and Prone Positions on Nasal Patency in Healthy Individuals. Ear. Nose Throat J. 2021, 01455613211015437. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.H.; Lin, Y.S.; Hsu, K.H.; Cheng, Y.C.; Yang, P.R.; Tsai, M.S.; Tsai, Y.T.; Hsu, C.M.; Chang, P.J.; Shi, C.S.; et al. Nasal irrigation with Glycyrrhiza glabra extract for treatment of allergic rhinitis—A study of in vitro, in vivo and clinical trial. J. Ethnopharmacol. 2021, 275, 114116. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; La Mantia, I. VAS for assessing the perception of antihistamines use in allergic rhinitis. Acta Biomed. 2019, 90, 41–44. [Google Scholar] [PubMed]
- Klimek, L.; Bergmann, K.C.; Biedermann, T.; Bousquet, J.; Hellings, P.; Jung, K.; Merk, H.; Olze, H.; Schlenter, W.; Stock, P.; et al. Visual analogue scales (VAS): Measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care. Allergo. J. Int. 2017, 26, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. S23), 1–250. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.W.; Schleimer, R.P.; Kern, R.C. Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2016, 4, 565–572. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, C.; Gozal, D.; Bruni, O.; Goudouris, E.; Meira, E.; Cruz, M. Allergic rhinitis and sleep disorders in children—Coexistence and reciprocal interactions. J. Pediatr. Rio. J. 2022, 98, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Goldwater, P.N. Sudden Infant Death Syndrome, Infection, Prone Sleep Position, and Vagal Neuroimmunology. Front. Pediatr. 2017, 5, 223. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Isono, S.; Aiba, J.; Tanaka, A.; Nishino, T. Prone position increases collapsibility of the passive pharynx in infants and small children. Am. J. Respir. Crit. Care Med. 2002, 166, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Galland, B.C.; Taylor, B.J.; Bolton, D.P. Prone versus supine sleep position: A review of the physiological studies in SIDS research. J. Paediatr. Child Health 2002, 38, 332–338. [Google Scholar] [CrossRef] [PubMed]
| Variable | Non-AR (n = 30) | AR (n = 30) | p-Value * |
|---|---|---|---|
| Sex | 0.201 | ||
| Women | 19 | 15 | |
| Men | 11 | 15 | |
| Age (years) | 31.1 ± 7.3 # | 37.7 ± 13.4 | 0.022 |
| IgE (kU/L) | 40.4 ± 24.9 | 247.6 ± 422.4 | 0.001 |
| VAS (0–100) | |||
| Nasal blockage | 7.7 ± 11.7 | 30.3 ± 26.6 | <0.001 |
| Rhinorrhea | 4.3 ± 10.1 | 14.3 ± 21.4 | 0.026 |
| Sneezing | 1.7 ± 4.6 | 6.0 ± 11.0 | 0.054 |
| Itchy nose | 7.0 ± 13.4 | 15.7 ± 23.0 | 0.080 |
| mCSA (cm2) | 1.0 ± 0.2 | 1.1 ± 0.3 | 0.269 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-T.; Tsai, Y.-T.; Hsu, C.-M.; Tsai, M.-S.; Tsai, H.-Y.; Chang, G.-H. Nasal Patency in Sitting, Supine, and Prone Positions in Individuals with and without Allergic Rhinitis. Life 2023, 13, 1226. https://doi.org/10.3390/life13051226
Wang Y-T, Tsai Y-T, Hsu C-M, Tsai M-S, Tsai H-Y, Chang G-H. Nasal Patency in Sitting, Supine, and Prone Positions in Individuals with and without Allergic Rhinitis. Life. 2023; 13(5):1226. https://doi.org/10.3390/life13051226
Chicago/Turabian StyleWang, Yun-Ting, Yao-Te Tsai, Cheng-Ming Hsu, Ming-Shao Tsai, Hsin-Yi Tsai, and Geng-He Chang. 2023. "Nasal Patency in Sitting, Supine, and Prone Positions in Individuals with and without Allergic Rhinitis" Life 13, no. 5: 1226. https://doi.org/10.3390/life13051226
APA StyleWang, Y. -T., Tsai, Y. -T., Hsu, C. -M., Tsai, M. -S., Tsai, H. -Y., & Chang, G. -H. (2023). Nasal Patency in Sitting, Supine, and Prone Positions in Individuals with and without Allergic Rhinitis. Life, 13(5), 1226. https://doi.org/10.3390/life13051226








