Early Detection of Secondary Bladder Urothelial Carcinoma and Disseminated Bone Metastases with Normal Prostate-Specific Antigen Level after Pelvic Salvage Radiotherapy in Prostate Cancer
Abstract
:1. Introduction
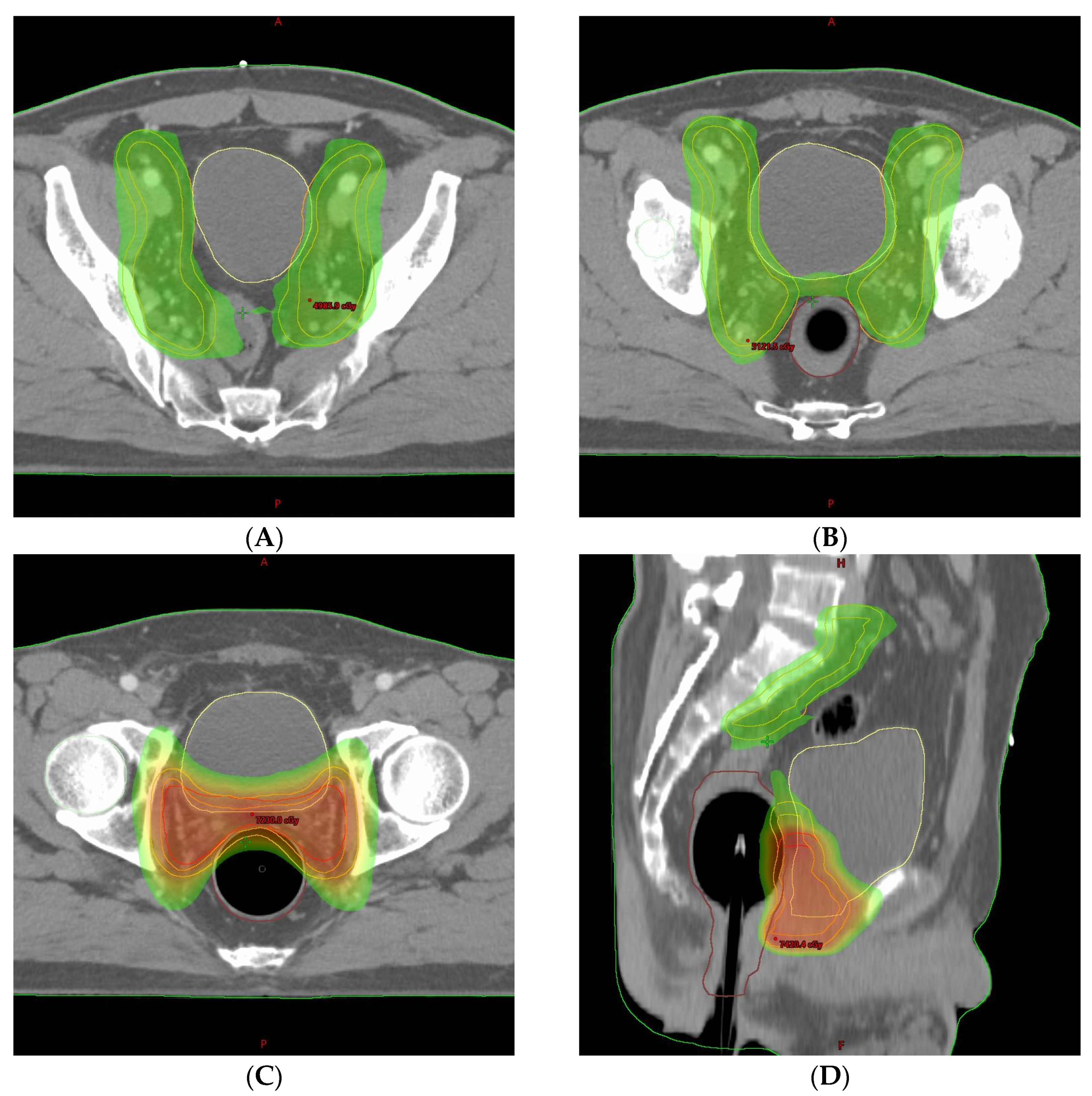
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef]
- Raicu, M.; Vral, A.; Thierens, H.; De Ridder, L. Radiation damage to endothelial cells in vitro, as judged by the micronucleus assay. Mutagenesis 1993, 8, 335–339. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Z.; Wang, J.; Hu, W. Dosimetric impact of different bladder and rectum filling during prostate cancer radiotherapy. Radiat. Oncol. 2016, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Miralbell, R.; Roberts, S.A.; Zubizarreta, E.; Hendry, J.H. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5969 patients in seven international institutional datasets: α/β = 1.4 (0.9–2.2) Gy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e17–e24. [Google Scholar] [CrossRef] [PubMed]
- Chorbińska, J.; Krajewski, W.; Zdrojowy, R. Urological complications after radiation therapy-nothing ventured, nothing gained: A Narrative Review. Transl. Cancer Res. 2021, 10, 1096–1118. [Google Scholar] [CrossRef] [PubMed]
- Leibovici, D.; Spiess, P.E.; Agarwal, P.K.; Tu, S.M.; Pettaway, C.A.; Hitzhusen, K.; Millikan, R.E.; Pisters, L.L. Prostate cancer progression in the presence of undetectable or low serum prostate-specific antigen level. Cancer 2007, 109, 198–204. [Google Scholar] [CrossRef]
- Birtle, A.J.; Freeman, A.; Masters, J.R.; Payne, H.A.; Harland, S.J. Clinical features of patients who present with metastatic prostate carcinoma and serum prostate-specific antigen (PSA) levels < 10 ng/mL: The “PSA negative” patients. Cancer 2003, 98, 2362–2367. [Google Scholar] [PubMed]
- Oefelein, M.G.; Smith, N.; Carter, M.; Dalton, D.; Schaeffer, A. The incidence of prostate cancer progression with undetectable serum prostate specific antigen in a series of 394 radical prostatectomies. J. Urol. 1995, 154, 2128–2131. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Ito, T.; Akiyama, A.; Aizawa, T.; Miki, M.; Tachibana, M. M1 prostate cancer with a serum level of prostate-specific antigen less than 10 ng/mL. Int. J. Urol. 2001, 8, 374–379. [Google Scholar] [CrossRef]
- Stephenson, A.J.; Kattan, M.W.; Eastham, J.A.; Dotan, Z.A.; Bianco, F.J., Jr.; Lilja, H.; Scardino, P.T. Defining biochemical recurrence of prostate cancer after radical prostatectomy: A proposal for a standardized definition. J. Clin. Oncol. 2006, 24, 3973–3978. [Google Scholar] [CrossRef]
- Small, W., Jr.; Bosch, W.R.; Harkenrider, M.M.; Strauss, J.B.; Abu-Rustum, N.; Albuquerque, K.V.; Beriwal, S.; Creutzberg, C.L.; Eifel, P.J.; Erickson, B.A.; et al. NRG Oncology/RTOG Consensus Guidelines for Delineation of Clinical Target Volume for Intensity Modulated Pelvic Radiation Therapy in Postoperative Treatment of Endometrial and Cervical Cancer: An Update. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 413–424. [Google Scholar] [CrossRef]
- Suriano, F.; Altobelli, E.; Sergi, F.; Buscarini, M. Bladder cancer after radiotherapy for prostate cancer. Rev. Urol. 2013, 15, 108–112. [Google Scholar] [PubMed]
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Smit, S.G.; Heyns, C.F. Management of radiation cystitis. Nat. Rev. Urol. 2010, 7, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Dautruche, A.; Delouya, G. A contemporary review about the management of radiation-induced hemorrhagic cystitis. Curr. Opin. Support Palliat. Care 2018, 12, 344–350. [Google Scholar] [CrossRef]
- Wallis, C.J.; Mahar, A.L.; Choo, R.; Herschorn, S.; Kodama, R.T.; Shah, P.S.; Danjoux, C.; Narod, S.A.; Nam, R.K. Second malignancies after radiotherapy for prostate cancer: Systematic review and meta-analysis. BMJ 2016, 352, i851. [Google Scholar] [CrossRef]
- Moon, K.; Stukenborg, G.J.; Keim, J.; Theodorescu, D. Cancer incidence after localized therapy for prostate cancer. Cancer 2006, 107, 991–998. [Google Scholar] [CrossRef]
- Boorjian, S.; Cowan, J.E.; Konety, B.R.; DuChane, J.; Tewari, A.; Carroll, P.R.; Kane, C.J. Cancer of the Prostate Strategic Urologic Research Endeavor Investigators. Bladder cancer incidence and risk factors in men with prostate cancer: Results from Cancer of the Prostate Strategic Urologic Research Endeavor. J. Urol. 2007, 177, 883–888. [Google Scholar] [CrossRef]
- Huang, J.; Kestin, L.L.; Ye, H.; Wallace, M.; Martinez, A.A.; Vicini, F.A. Analysis of second malignancies after modern radiotherapy versus prostatectomy for localized prostate cancer. Radiother. Oncol. 2011, 98, 81–86. [Google Scholar] [CrossRef]
- Chrouser, K.; Leibovich, B.; Bergstralh, E.; Zincke, H.; Blute, M. Bladder cancer risk following primary and adjuvant external beam radiation for prostate cancer. J. Urol. 2005, 174, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.; Henry, A.; Hoskin, P.; Siebert, F.A.; Venselaar, J. Second primary cancers after radiation for prostate cancer: A systematic review of the clinical data and impact of treatment technique. Radiother. Oncol. 2014, 110, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.S.; Shariat, S.F.; Lowrance, W.T.; Sterbis, J.R.; Vora, K.C.; Bochner, B.H.; Donat, S.M.; Herr, H.W.; Dalbagni, G.; Sandhu, J.S. Impact of previous radiotherapy for prostate cancer on clinical outcomes of patients with bladder cancer. J. Urol. 2010, 183, 1751–1756. [Google Scholar] [CrossRef]
- Jiang, X.; Castelao, J.E.; Yuan, J.M.; Stern, M.C.; Conti, D.V.; Cortessis, V.K.; Pike, M.C.; Gago-Dominguez, M. Cigarette smoking and subtypes of bladder cancer. Int. J. Cancer 2012, 130, 896–901. [Google Scholar] [CrossRef]
- Guan, X.; Wei, R.; Yang, R.; Lu, Z.; Liu, E.; Zhao, Z.; Chen, H.; Yang, M.; Liu, Z.; Jiang, Z.; et al. Risk and Prognosis of Secondary Bladder Cancer After Radiation Therapy for Rectal Cancer: A Large Population-Based Cohort Study. Front. Oncol. 2020, 10, 586401. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhong, G.; Ren, M. Increased risk of secondary bladder cancer after radiation therapy for endometrial cancer. Sci. Rep. 2022, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Schalken, J.A. Molecular and cellular prostate biology: Origin of prostate-specific antigen expression and implications for benign prostatic hyperplasia. BJU Int. 2004, 93 (Suppl. S1), 5–9. [Google Scholar] [CrossRef]
- Kageyama, Y.; Kihara, K.; Kamata, S.; Nagahama, K.; Yonese, J.; Fukuda, H.; Tosaka, A.; Nagamatsu, H.; Ishizaka, K.; Tsujii, T.; et al. Relationship between pretreatment serum levels of prostate specific antigen and bone metastasis in prostate cancer. Hinyokika Kiyo 1996, 42, 197–199. [Google Scholar] [PubMed]
- Combes, A.D.; Palma, C.A.; Calopedos, R.; Wen, L.; Woo, H.; Fulham, M.; Leslie, S. PSMA PET-CT in the Diagnosis and Staging of Prostate Cancer. Diagnostics 2022, 12, 2594. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, T.J.; Choe, M.; Kim, B.H.; Byun, S.J. Early Detection of Secondary Bladder Urothelial Carcinoma and Disseminated Bone Metastases with Normal Prostate-Specific Antigen Level after Pelvic Salvage Radiotherapy in Prostate Cancer. Life 2023, 13, 1249. https://doi.org/10.3390/life13061249
Shin TJ, Choe M, Kim BH, Byun SJ. Early Detection of Secondary Bladder Urothelial Carcinoma and Disseminated Bone Metastases with Normal Prostate-Specific Antigen Level after Pelvic Salvage Radiotherapy in Prostate Cancer. Life. 2023; 13(6):1249. https://doi.org/10.3390/life13061249
Chicago/Turabian StyleShin, Teak Jun, Misun Choe, Byung Hoon Kim, and Sang Jun Byun. 2023. "Early Detection of Secondary Bladder Urothelial Carcinoma and Disseminated Bone Metastases with Normal Prostate-Specific Antigen Level after Pelvic Salvage Radiotherapy in Prostate Cancer" Life 13, no. 6: 1249. https://doi.org/10.3390/life13061249




