Phytase Supplementation of Growing-Finishing Pig Diets with Extruded Soya Seeds and Rapeseed Meal Improves Bone Mineralization and Carcass and Meat Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Enzyme
2.3. Ethical Statement
2.4. Animals, Diets, and Experimental Design
2.5. Analytical Procedures
2.5.1. Feed Analyses
2.5.2. Carcass and Meat Analyses
2.5.3. Bone Analysis
2.5.4. Meat Fatty Acid Profile
2.5.5. Statistical Analysis
3. Results
3.1. Performance Parameters
3.2. Carcass and Meat Quality
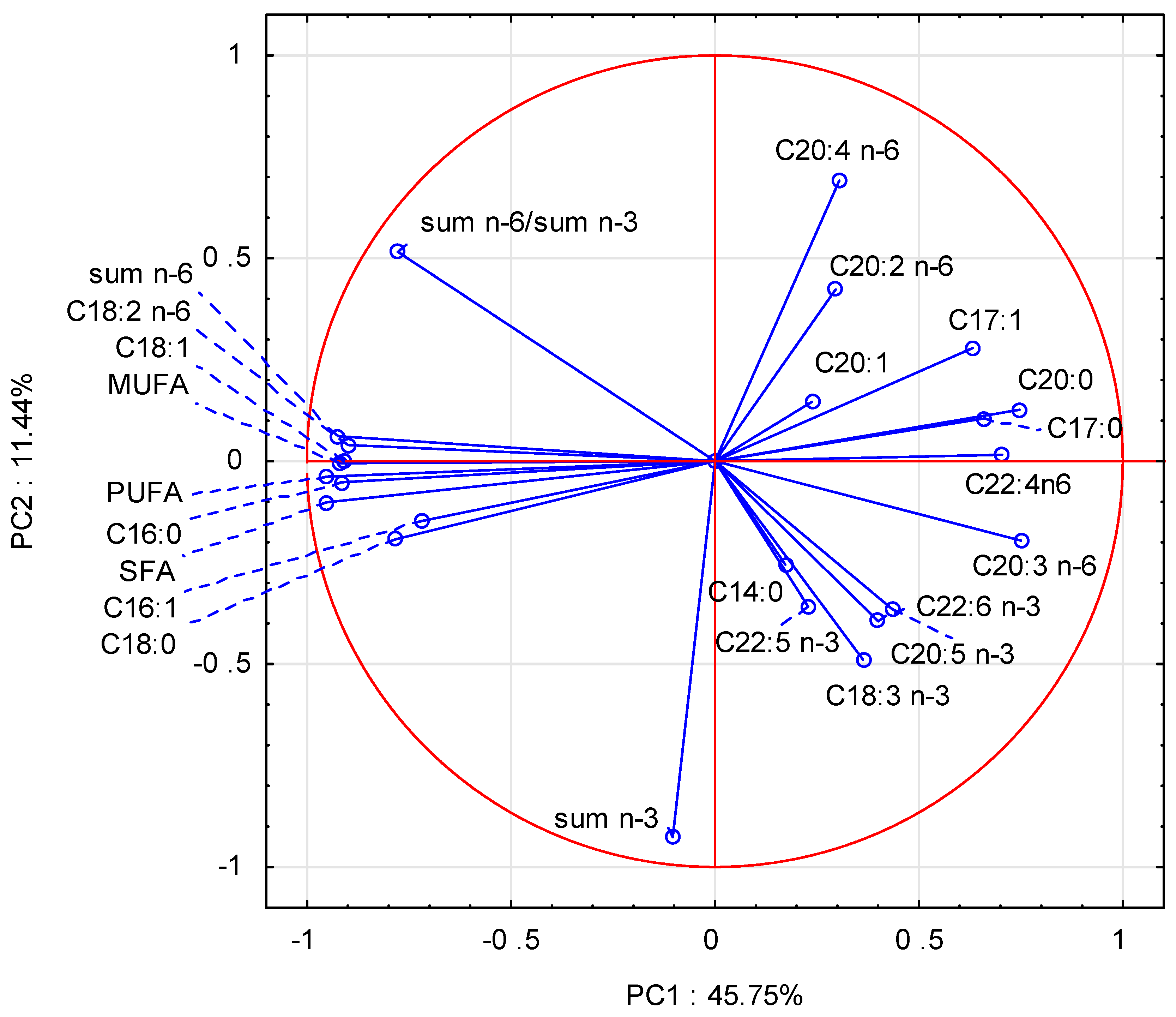
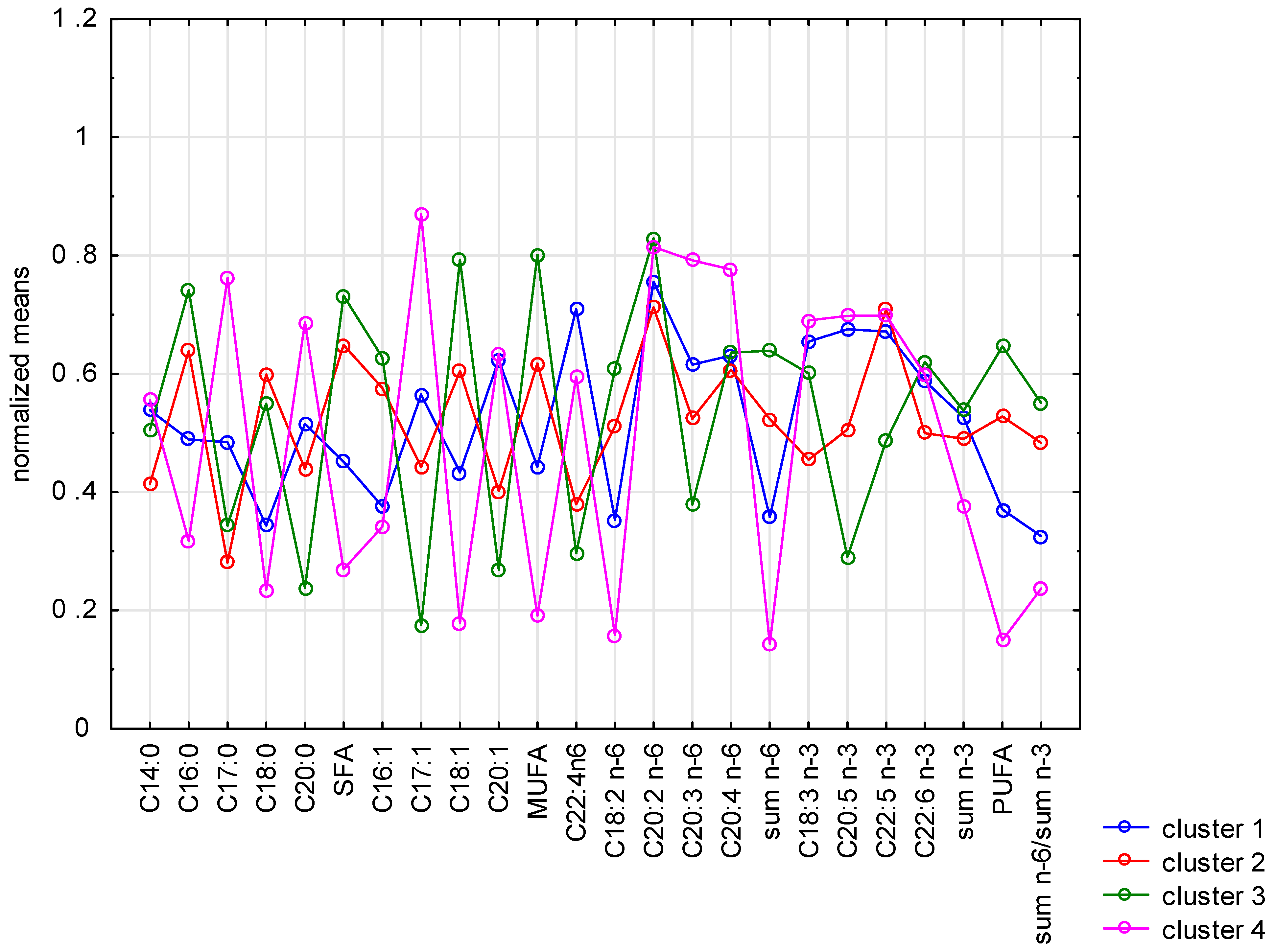
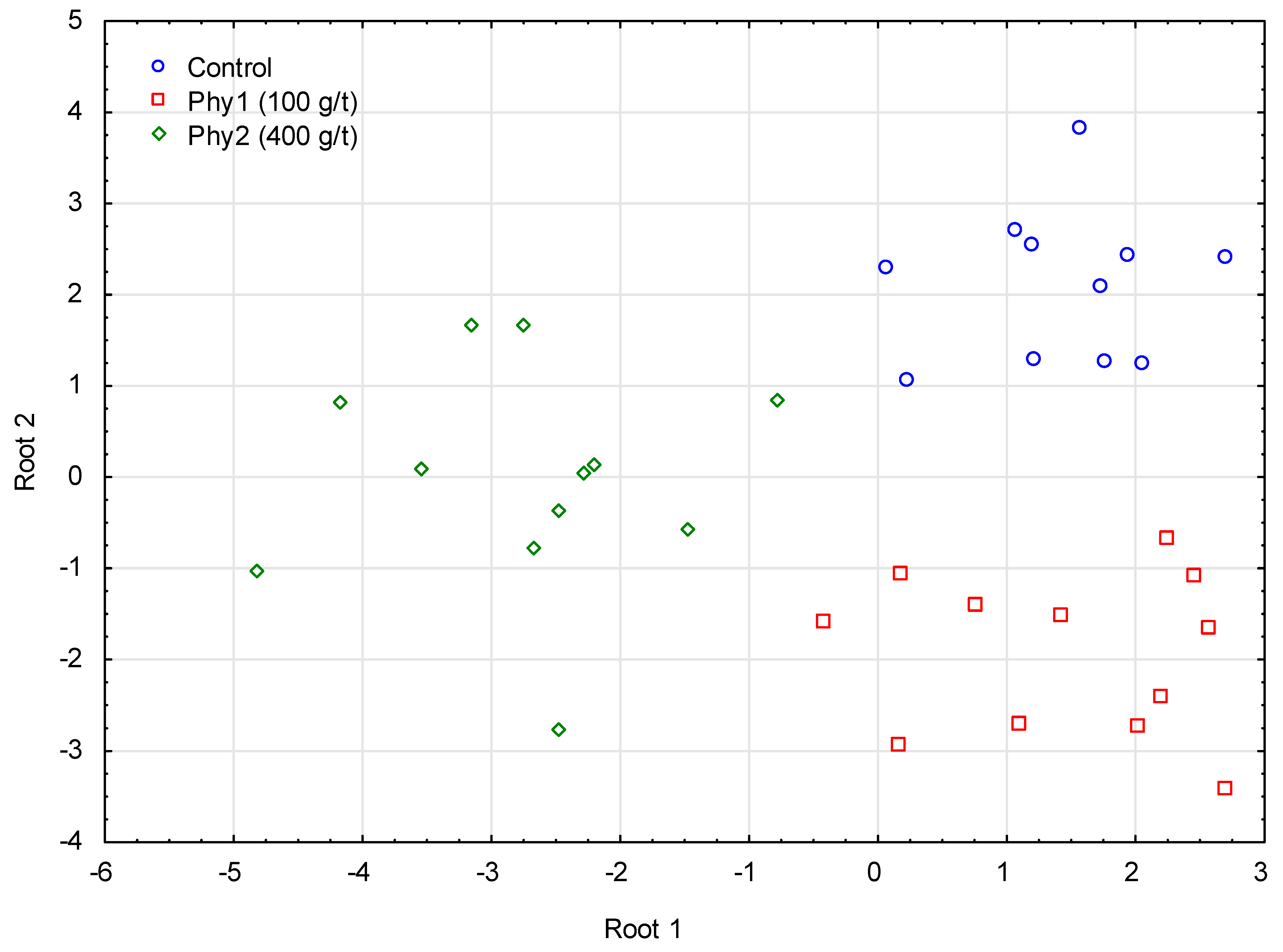
3.3. Fatty Acid Profile
3.4. Pig Metacarpal Bone Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raport InterPIG, AHDB—Cost Pig Production in 2021. 2022. Available online: https://ahdb.org.uk/cost-of-production-in-selected-countries (accessed on 24 April 2023).
- Sieradzki, Z.; Mazur, M.; Król, B.; Kwiatek, K. Prevalence of genetically modified soybean in animal feeding stuffs in Poland. Pol. J. Vet. Res. 2021, 65, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 276, 33.
- Choct, M.; Dersjant-Li, Y.; McLeish, J.; Peisker, M. Soy oligosaccharides and soluble non-starch polysaccharides: A review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian-Australas. J. Anim. Sci. 2010, 23, 1386–1398. [Google Scholar] [CrossRef]
- Radcliffe, J.S.; Brito, L.F.; Reddivari, L.; Schmidt, M.; Herman, E.M.; Schinckel, A.P. A swine model of soy protein-induced food allergenicity: Implications in human and swine nutrition. Anim. Front. 2019, 9, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Zaworska-Zakrzewska, A.; Kasprowicz-Potocka, M.; Twarużek, M.; Kosicki, R.; Grajewski, J.; Wiśniewska, Z.; Rutkowski, A. A comparison of the composition and contamination of soybean cultivated in Europe and limitation of raw soy seed content in weaned pigs’ diets. Animals 2020, 10, 1972. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz-Potocka, M.; Zaworska-Zakrzewska, A.; Rutkowski, A. Effect of phytase on digestibility and performance of growing and finishing pigs fed diets with lupins and rapeseed meal. J. Agric. Sci. Technol. A 2020, 10, 121–131. [Google Scholar] [CrossRef]
- Kasprowicz-Potocka, M.; Gulewicz, P.; Zaworska-Zakrzewska, A. The content of raffinose oligosaccharides in legumes and their importance for animals. J. Anim. Feed Sci. 2022, 31, 265–275. [Google Scholar] [CrossRef]
- Selle, P.H.; Ravindran, V.; Bryden, W.L.; Scott, T. Influence of dietary phytate and exogenous phytase on amino acid digestibility in poultry: A review. Poult. Sci. J. 2006, 43, 89–103. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Wealleans, A.L.; Barnard, L.P.; Lane, S. Effect of increasing Buttiauxella phytase dose on nutrient digestibility and performance in weaned piglets fed corn or wheat-based diets. Anim. Feed Sci. Technol. 2017, 234, 101–109. [Google Scholar] [CrossRef]
- Wiśniewska, Z.; Nollet, L.; Lanckriet, A.; Vanderbeke, E.; Petkov, S.; Outchkourov, N.; Kasprowicz-Potocka, M.; Zaworska-Zakrzewska, A.; Kaczmarek, S.A. Effect of phytase derived from the e. Coli appa gene on weaned piglet performance, apparent total tract digestibility and bone mineralization. Animals 2020, 10, 121. [Google Scholar] [CrossRef]
- Ravindran, V.; Son, J.H. Feed enzyme technology: Present status and future developments. Recent Pat. Food Nutr. Agric. 2011, 3, 102–109. [Google Scholar]
- Lalpanmawia, H.; Elangovan, A.V.; Sridhar, M.; Shet, D.; Ajith, S.; Pal, D.T. Efficacy of phytase on growth performance, nutrient utilization and bone mineralization in broiler chicken. Anim. Feed Sci. Technol. 2014, 19, 81–89. [Google Scholar] [CrossRef]
- Wilcock, P.; Bradley, L.; Chewning, J.J.; Walk, C.L. The effect of superdosing phytase on inositol and phytate concentration in the gastrointestinal tract and its effect on pig performance. J. Anim. Sci. 2014, 92, 383. [Google Scholar]
- Moran, K.; Boyd, R.D.; Zier-Rush, C.; Wilcock, P.; Bajjalieh, N.; Van Heugten, E. Effects of high inclusion of soybean meal and a phytase superdose on growth performance of weaned pigs housed under the rigors of commercial conditions. J. Anim. Sci. 2017, 95, 5455–5465. [Google Scholar] [CrossRef]
- Dang, D.X.; Kim, I.H. Effects of adding high-dosing Aspergillus oryzae phytase to corn–wheat–soybean meal-based basal diet on growth performance, nutrient digestibility, faecal gas emission, carcass traits and meat quality in growing-finishing pigs. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1056–1062. [Google Scholar] [CrossRef]
- Cauble, R.N.; Greene, E.S.; Orlowski, S.; Walk, C.; Bedford, M.; Apple, J.; Kidd, M.T.; Dridi, S. Research Note: Dietary phytase reduces broiler woody breast severity via potential modulation of breast muscle fatty acid profiles. Poult. Sci. 2020, 99, 4009–4015. [Google Scholar] [CrossRef]
- Gebert, S.; Bee, G.; Pfirter, H.P.; Wenk, C. Phytase and vitamin E in the feed of growing pigs: 2. Influence on carcass characteristics, meat and fat quality. J. Anim. Physiol. Anim. Nutr. 1998, 81, 20–30. [Google Scholar] [CrossRef]
- Liu, N.; Ru, Y.; Wang, J.; Xu, T. Effect of dietary sodium phytate and microbial phytase on the lipase activity and lipid metabolism of broiler chickens. Br. J. Nutr. 2010, 103, 862–868. [Google Scholar] [CrossRef]
- Biswas, P.; Pal, A.K.; Sahu, N.P.; Reddy, A.K.; Prusty, A.K.; Misra, S. Lysine and/or phytase supplementation in the diet of Penaeus monodon (Fabricius) juveniles: Effect on growth, body composition and lipid profile. Aquaculture 2007, 265, 253–260. [Google Scholar] [CrossRef]
- Gesellschaft für Ernährungsphysiologie; Society of Nutrition Physiology. Recommendations for the Supply of Energy and Nutrients to Pigs. Energy and Nutrients Requirements for Livestock; Committee for Requirement Standards of the Society of Nutrition Physiology, Ed.; DLG-Verlags GmbH: Frankfurt, Germany, 2006. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analysis Chemists International: Rockville, MD, USA, 2016; 3172. [Google Scholar]
- Grau, I.; Hamm, G. Die Bestimmung der Wasserbindung des Fleisches mittels der Preβmethode. Fleischwirtschaft 1956, 8, 733–734. [Google Scholar]
- Kaczmarek, S.A.; Cowieson, A.J.; Hejdysz, M.; Rutkowski, A. Microbial phytase improves performance and bone traits in broilers fed diets based on soybean meal and containing lupin meal. Anim. Prod. Sci. 2016, 56, 1669–1676. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Wondołowska-Grabowska, A. Quality Evaluation Indices for Soybean Oil in Relation to Cultivar, Application of N Fertiliser and Seed Inoculation with Bradyrhizobium japonicum. Foods 2022, 11, 762. [Google Scholar] [CrossRef] [PubMed]
- Mondal, N.; Bhat, K.V.; Srivastava, P.S. Variation in fatty acid composition in Indian germplasm of sesame. J. Am. Oil Chem. Soc. 2010, 87, 1263–1269. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Alonso-Salces, R.M.; Héberger, K. Supervised pattern recognition in food analysis. J. Chrom. A 2007, 1158, 196–214. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ruan, Z.; Zhang, Y.G.; Hou, Y.Q.; Yin, Y.L.; Li, T.J.; Huang, R.L.; Chu, W.Y.; Kong, X.F.; Gao, B.; et al. True digestibility of phosphorus in different resources of feed ingredients in growing pigs. Asian-Australas. J. Anim. Sci. 2020, 21, 107–119. [Google Scholar] [CrossRef]
- Cervantes, M.; Gómez, R.; Fierro, S.; Barrera, M.A.; Morales, A.; Araiza, B.A.; Zijlstra, R.T.; Sánchez, J.E.; Sauer, W.C. Ileal digestibility of amino acids, phosphorus, phytate and energy in pigs fed sorghum-based diets supplemented with phytase and Pancreatin®. J. Anim. Physiol. Anim. Nutr. 2011, 95, 179–186. [Google Scholar] [CrossRef]
- Reis de Souza, T.C.; Escobar, K.; Aguilera, G.A.; Ramírez, B.E.; Mariscal-Landín, R.G. Sesame meal as the first protein source in piglet starter diets and advantages of a phytase: A digestive study. S. Afr. J. Anim. Sci. 2017, 47, 606–615. [Google Scholar] [CrossRef]
- Grela, E.R.; Muszynski, S.; Czech, A.; Donaldson, J.; Stanislawski, P.; Kapica, M.; Brezvyn, O.; Musyka, V.; Kotsyumbas, I.; Tomaszewska, E. Influence of phytase supplementation at increasing grower—Finisher pigs fed phosphorus-deficient diets. Animals 2020, 10, 847. [Google Scholar] [CrossRef]
- Guggenbuhl, P.; Calvo, E.P.; Fru, F. Effect of high dietary doses of a bacterial 6-phytase in piglets fed a corn-soybean meal diet. J. Anim. Sci. 2016, 94, 307–309. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Q.; Tian, Q.; Zhao, P.; Xu, X.; Yu, S.; Piao, X. Super high dosing with a novel buttiauxella phytase continuously improves growth performance, nutrient digestibility, and mineral status of weaned pigs. Biol. Trace Elem. Res. 2015, 168, 103–109. [Google Scholar] [CrossRef]
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625. [Google Scholar] [CrossRef]
- Holloway, C.L.; Dean Boyd, R.; Koehler, D.; Gould, S.A.; Li, Q.; Patience, J.F. The impact of “super-dosing” phytase in pig diets on growth performance during the nursery and grow-out periods. Transl. Anim. Sci. 2019, 3, 419–428. [Google Scholar] [CrossRef]
- Szczuko, M.; Kaczkan, M.; Małgorzewicz, S.; Rutkowski, P.; Dębska-Ślizień, A.; Stachowska, E. The C18:3n6/C22:4n6 ratio is a good lipid marker of chronic kidney disease (CKD) progression. Lipids Health Dis. 2020, 19, 77. [Google Scholar] [CrossRef]
- Sońta, M.; Rekiel, A.; Więcek, J.; Batorska, M.; Puppel, K. Alternative Protein Sources vs. GM Soybean Meal as Feedstuff for Pigs-Meat Quality and Health-Promoting Indicators. Animals 2021, 11, 177. [Google Scholar] [CrossRef]
- Häuser, A.M. Einfluss von Fett und Tocopherol im Futter Sowie von Fleischtechnologischen Behandlungen auf die Oxidationsstabilität von Schweinefleisch-Patties. Ph.D. Dissertation, ETH Zurich, Zürich, Switzerland, 1991. [Google Scholar]
| Components (%)/Period | Starter | Grower | Finisher |
|---|---|---|---|
| Wheat | 25.00 | - | - |
| Maize | 20.27 | 44.65 | 40.57 |
| RSM | 11.50 | 15.00 | 13.00 |
| FFES | 10.00 | 7.50 | 5.00 |
| Rye | 10.00 | 20.00 | 25.00 |
| Barley | 10.00 | - | - |
| Wheat bran | 9.50 | 10.00 | 14.00 |
| Limestone | 1.45 | 1.40 | 1.30 |
| 1-Calcium phosphate | 0.30 | 0.25 | - |
| Sodium chloride | 0.40 | 0.40 | 0.40 |
| Premix grower 0.2% * | 0.20 | 0.20 | 0.15 |
| Magnesium oxide | 0.20 | 0.20 | 0.20 |
| HCL-lysine 78.5% | 0.51 | 0.36 | 0.36 |
| DL-methionine 99% | 0.22 | - | - |
| L-threonine 98.5% | 0.03 | 0.02 | - |
| L-tryptophan 98% | 0.03 | - | - |
| Liquid acidifier ** | 0.35 | - | - |
| Choline chloride | 0.04 | 0.02 | 0.02 |
| Calculated nutritional value (g/kg) | |||
| Crude protein | 170.32 | 171.60 | 153.20 |
| Crude fat | 41.70 | 41.20 | 34.20 |
| Crude fiber | 48.84 | 55.50 | 48.80 |
| Ca | 8.29 | 6.10 | 6.70 |
| P | 5.85 | 5.30 | 4.90 |
| Ca/P | 1.2–1.4: 1 | ||
| Indices | Formula | References |
|---|---|---|
| TI | Szpunar-Krok et al. [25] | |
| S/P | ||
| OL/(LA + ALA) | ||
| CI | ||
| ODR | Mondal et al. [26] | |
| LDR | ||
| COX | Szpunar-Krok et al., 2022 [25] | |
| DFA | ||
| OFA |
| Phase/Variable | Con | Phy1 | Phy2 | SEM | p |
|---|---|---|---|---|---|
| Starter | |||||
| IBW (kg) | 30.84 ± 1.04 | 31.33 ± 0.98 | 31.35 ± 1.02 | 0.392 | >0.05 |
| FBW (kg) | 51.37 ± 6.87 | 53.20 ± 8.07 | 53.80 ± 6.08 | 0.910 | >0.05 |
| DBWG (kg) | 0.821 ± 0.13 | 0.875 ± 0.16 | 0.898 ± 0.13 | 0.018 | >0.05 |
| FCR (kg/kg) | 2.38 ± 0.42 | 2.32 ± 0.47 | 2.25 ± 0.35 | 0.050 | >0.05 |
| Grower | |||||
| FBW (kg) | 90.58 ± 12.08 | 95.40 ± 12.37 | 97.28 ± 11.06 | 0.562 | >0.05 |
| DBWG (kg) | 1.09 ± 0.21 | 1.17 ± 0.18 | 1.21 ± 0.14 | 0.024 | >0.05 |
| FCR (kg/kg) | 2.60 ± 0.61 | 2.43 ± 0.40 | 2.38 ± 0.29 | 0.588 | >0.05 |
| Finisher | |||||
| FBW (kg) | 124.05 ± 15.31 | 131.35 ± 13.98 | 131.55 ± 12.86 | 1.860 | >0.05 |
| DBWG (kg) | 1.01 ± 1.20 | 1.09 ± 0.14 | 1.04 ± 0.16 | 0.022 | >0.05 |
| FCR (kg/kg) | 4.97 ± 0.61 | 4.04 ± 0.40 | 4.31 ± 0.29 | 0.200 | >0.05 |
| Total | |||||
| BWG (kg) | 93.21 ± 12.96 | 100.03 ± 11.17 | 100.20 ± 9.71 | 1.510 | >0.05 |
| DBWG (kg) | 1.00 ± 0.14 | 1.08 ± 0.12 | 1.08 ± 0.10 | 0.016 | >0.05 |
| FCR (kg/kg) | 3.25 a ± 0.51 | 2.96 b ± 0.46 | 2.98 b ± 0.31 | 0.054 | 0.049 |
| Phase/Variable | Con | Phy1 | Phy2 | SEM | p |
|---|---|---|---|---|---|
| Carcass weight (kg) | 97.91 ± 12.94 | 102.24 ± 11.71 | 102.83 ± 11.01 | 1.550 | >0.05 |
| Meatiness (%) | 57.30 ± 3.06 | 57.64 ± 2.55 | 56.35 ± 2.31 | 0.350 | >0.05 |
| Cold dressing yield (%) | 78.85 ± 1.35 | 77.80 ± 1.46 | 78.14 ± 1.13 | 0.240 | >0.05 |
| Mean thickness of pork loin (mm) | 66.90 ± 7.30 | 66.10 ± 6.30 | 66.30 ± 6.50 | 0.900 | >0.05 |
| Mean back-fat thickness (mm) | 17.32 ± 4.56 | 16.62 ± 3.61 | 18.72 ± 3.18 | 0.500 | >0.05 |
| pH 45 min | 6.473 ± 0.22 | 6.57 ± 0.23 | 6.45 ± 0.30 | 0.040 | >0.05 |
| Back-fat thickness K III (mm) | 20.50 ± 6.16 | 20.50 ± 6.46 | 22.84 ± 5.64 | 1.000 | >0.05 |
| Back-fat thickness K II (mm) | 17.75 ± 5.66 | 16.67 ± 5.76 | 18.42 ± 4.17 | 0.858 | >0.05 |
| Back-fat thickness K I (mm) | 24.67 ± 5.55 | 23.00 ± 6.72 | 25.25 ± 3.33 | 0.886 | >0.05 |
| GMT (mm) | 72.67 ± 7.11 | 71.42 ± 7.14 | 74.17 ± 7.16 | 1.170 | >0.05 |
| Back (cm) | 21.00 ± 5.17 | 20.00 ± 3.46 | 22.17 ± 3.88 | 0.700 | >0.05 |
| Shoulder (cm) | 43.08 ± 7.82 | 43.67 ± 6.54 | 47.00 ± 6.19 | 1.150 | >0.05 |
| Carcass length (cm) | 89.67 ± 3.28 | 89.08 ± 3.03 | 88.92 ± 3.26 | 0.520 | >0.05 |
| Carcass width (cm) | 39.34 ± 1.15 | 38.08 ± 2.35 | 38.67 ± 1.15 | 0.280 | >0.05 |
| EC (mS/cm) | 4.54 ± 0.76 | 4.79 ± 1.20 | 4.32 ± 1.13 | 0.170 | >0.05 |
| Variable | Con | Phy1 | Phy2 | SEM | p |
|---|---|---|---|---|---|
| Fat (%) | 1.81 ± 0.57 | 2.31 ± 0.59 | 2.19 ± 0.57 | 0.101 | >0.05 |
| Protein (%) | 24.21 ± 0.84 | 23.54 ± 1.30 | 24.21 ± 0.66 | 0.166 | >0.05 |
| Water (%) | 72.76 ± 0.77 | 72.82 ± 0.80 | 72.42 ± 0.84 | 0.134 | >0.05 |
| Dry matter (%) | 27.24 ± 0.77 | 27.18 ± 0.80 | 27.59 ± 0.84 | 0.134 | >0.05 |
| P (%) | 1.05 b ± 0.04 | 1.19 a ± 0.04 | 1.19 a ± 0.03 | 0.01 | <0.001 |
| pH 24 h | 5.53 ± 0.08 | 5.53 ± 0.12 | 5.48 ± 0.10 | 0.017 | >0.05 |
| L*—lightness | 46.83 ± 1.81 | 46.42 ± 2.83 | 47.86 ± 3.37 | 0.457 | >0.05 |
| a*—redness | 5.12 ± 0.76 | 5.48 ± 0.96 | 5.57 ± 1.14 | 0.160 | >0.05 |
| b*—yellowness | 1.96 ± 1.13 | 1.79 ± 0.90 | 2.84 ± 1.15 | 0.191 | >0.05 |
| Cooking loss (%) | 26.38 ± 5.19 | 26.12 ± 4.22 | 28.16 ± 4.34 | 0.760 | >0.05 |
| Tenderness (%) | 27.09 ± 5.07 | 26.04 ± 8.40 | 26.31 ± 4.26 | 1.000 | >0.05 |
| Drip loss (%) | 5.21 ± 1.74 | 5.10 ± 1.39 | 6.15 ± 1.21 | 0.250 | >0.05 |
| Water-holding capacity (%) | 28.07 ± 4.16 | 28.26 ± 1.81 | 28.36 ± 2.93 | 0.510 | >0.05 |
| Variable | Con | Phy1 | Phy2 | SEM | p |
|---|---|---|---|---|---|
| C14:0 | 1.32 ± 0.14 | 1.32 ± 0.14 | 1.26 ± 0.09 | 0.021 | >0.05 |
| C16:0 | 23.78 ± 0.96 | 23.68 ± 1.04 | 23.67 ± 0.84 | 0.154 | >0.05 |
| C17:0 | 0.22 ± 0.02 | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.003 | >0.05 |
| C18:0 | 13.36 ± 1.02 | 13.27 ± 1.04 | 13.72 ± 0.78 | 0.157 | >0.05 |
| C20:0 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.22 ± 0.01 | 0.002 | >0.05 |
| C16:1 | 2.32 ± 0.10 | 2.36 ± 0.09 | 2.37 ± 0.09 | 0.016 | >0.05 |
| C17:1 | 0.12 ± 0.02 | 0.12 ± 0.02 | 0.12 ± 0.02 | 0.003 | >0.05 |
| C18:1 | 43.59 ± 1.72 | 43.77 ± 1.70 | 43.501 ± 1.22 | 0.254 | >0.05 |
| C20:1 | 1.12 ± 0.04 | 1.09 ± 0.05 | 1.08 ± 0.06 | 0.008 | >0.05 |
| C22:4 n-6 | 0.12 b ± 0.01 | 0.11 a ± 0.01 | 0.11 a ± 0.01 | 0.001 | 0.008 |
| C18:2 n-6 | 10.89 ± 0.66 | 10.90 ± 0.63 | 10.86 ± 0.51 | 0.098 | >0.05 |
| C20:2 n-6 | 0.33 ± 0.06 | 0.34 ± 0.07 | 0.33 ± 0.03 | 0.009 | >0.05 |
| C20:3 n-6 | 0.21 ± 0.08 | 0.21 ± 0.06 | 0.20 ± 0.06 | 0.011 | >0.05 |
| C20:4 n-6 | 0.14 ± 0.04 | 0.15 ± 0.04 | 0.13 ± 0.02 | 0.005 | >0.05 |
| C18:3 n-3 | 0.92 ± 0.05 | 0.92 ± 0.06 | 0.89 ± 0.05 | 0.009 | >0.05 |
| C20:5 n-3 | 0.42 ± 0.06 | 0.38 ± 0.07 | 0.39 ± 0.07 | 0.011 | >0.05 |
| C22:5 n-3 | 0.44 ± 0.06 | 0.42 ± 0.05 | 0.45 ± 0.04 | 0.008 | >0.05 |
| C22:6 n-3 | 0.49 ± 0.05 | 0.51 ± 0.06 | 0.49 ± 0.06 | 0.009 | >0.05 |
| SFA | 38.91 ± 1.69 | 38.72 ± 1.41 | 39.08 ± 1.31 | 0.240 | >0.05 |
| MUFA | 47.14 ± 1.70 | 47.35 ± 1.62 | 47.06 ± 1.25 | 0.249 | >0.05 |
| PUFA | 13.95 ± 0.66 | 13.94 ± 0.65 | 13.85 ± 0.46 | 0.097 | >0.05 |
| Σ n-3 | 2.28 ± 0.10 | 2.24 ± 0.12 | 2.22 ± 0.12 | 0.019 | >0.05 |
| Σ n-6 | 11.77 ± 0.67 | 11.70 ± 0.58 | 11.64 ± 0.51 | 0.096 | >0.05 |
| Σ n-6/Σ n-3 | 5.14 ± 0.42 | 5.20 ± 0.29 | 5.26 ± 0.44 | 0.060 | >0.05 |
| Indices | Con | Phy1 | Phy2 | SEM | p |
|---|---|---|---|---|---|
| TI | 1.06 ± 0.07 | 1.05 ± 0.06 | 1.07 ± 0.06 | 0.061 | >0.05 |
| S/P | 0.63 ± 0.05 | 0.63 ± 0.04 | 0.64 ± 0.04 | 0.039 | >0.05 |
| OL/(LA + ALA) | 3.70 ± 0.27 | 3.72 ± 0.32 | 3.71 ± 0.19 | 0.260 | >0.05 |
| CI | 1.83 ± 0.10 | 1.81 ± 0.10 | 1.77 ± 0.13 | 0.110 | >0.05 |
| ODR | 21.32 ± 1.26 | 21.28 ± 1.44 | 21.27 ± 0.87 | 1.177 | >0.05 |
| LDR | 7.78 ± 0.65 | 7.82 ± 0.39 | 7.56 ± 0.58 | 0.549 | >0.05 |
| COX | 1.84 ± 0.06 | 1.84 ± 0.07 | 1.82 ± 0.06 | 0.061 | >0.05 |
| DFA | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.23 ± 0.02 | 0.020 | >0.05 |
| OFA | 25.11 ± 0.97 | 25.00 ± 1.00 | 24.93 ± 0.88 | 0.926 | >0.05 |
| Variable | Con | Phy1 | Phy2 | SEM | p |
|---|---|---|---|---|---|
| Ash (%) | 52.47 ± 3.07 | 53.90 ± 2.60 | 52.60 ± 2.31 | 0.447 | >0.05 |
| Ca (%) | 33.89 ± 1.38 | 33.27 ± 1.19 | 33.25 ± 1.46 | 0.050 | >0.05 |
| P (%) | 16.71 a ± 0.24 | 17.28 b ± 0.13 | 17.28 b ± 0.12 | 0.224 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzek, A.; Zaworska-Zakrzewska, A.; Muzolf-Panek, M.; Łodyga, D.; Lisiak, D.; Kasprowicz-Potocka, M. Phytase Supplementation of Growing-Finishing Pig Diets with Extruded Soya Seeds and Rapeseed Meal Improves Bone Mineralization and Carcass and Meat Quality. Life 2023, 13, 1275. https://doi.org/10.3390/life13061275
Buzek A, Zaworska-Zakrzewska A, Muzolf-Panek M, Łodyga D, Lisiak D, Kasprowicz-Potocka M. Phytase Supplementation of Growing-Finishing Pig Diets with Extruded Soya Seeds and Rapeseed Meal Improves Bone Mineralization and Carcass and Meat Quality. Life. 2023; 13(6):1275. https://doi.org/10.3390/life13061275
Chicago/Turabian StyleBuzek, Anna, Anita Zaworska-Zakrzewska, Małgorzata Muzolf-Panek, Dagmara Łodyga, Dariusz Lisiak, and Małgorzata Kasprowicz-Potocka. 2023. "Phytase Supplementation of Growing-Finishing Pig Diets with Extruded Soya Seeds and Rapeseed Meal Improves Bone Mineralization and Carcass and Meat Quality" Life 13, no. 6: 1275. https://doi.org/10.3390/life13061275









