The Development of Nonalcoholic Fatty Liver Disease and Metabolic Syndromes in Diet-Induced Rodent Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diet
2.3. Assessment of Body Weight (BW) and Food Consumption
2.4. Rat Euthanization and Sample Collection
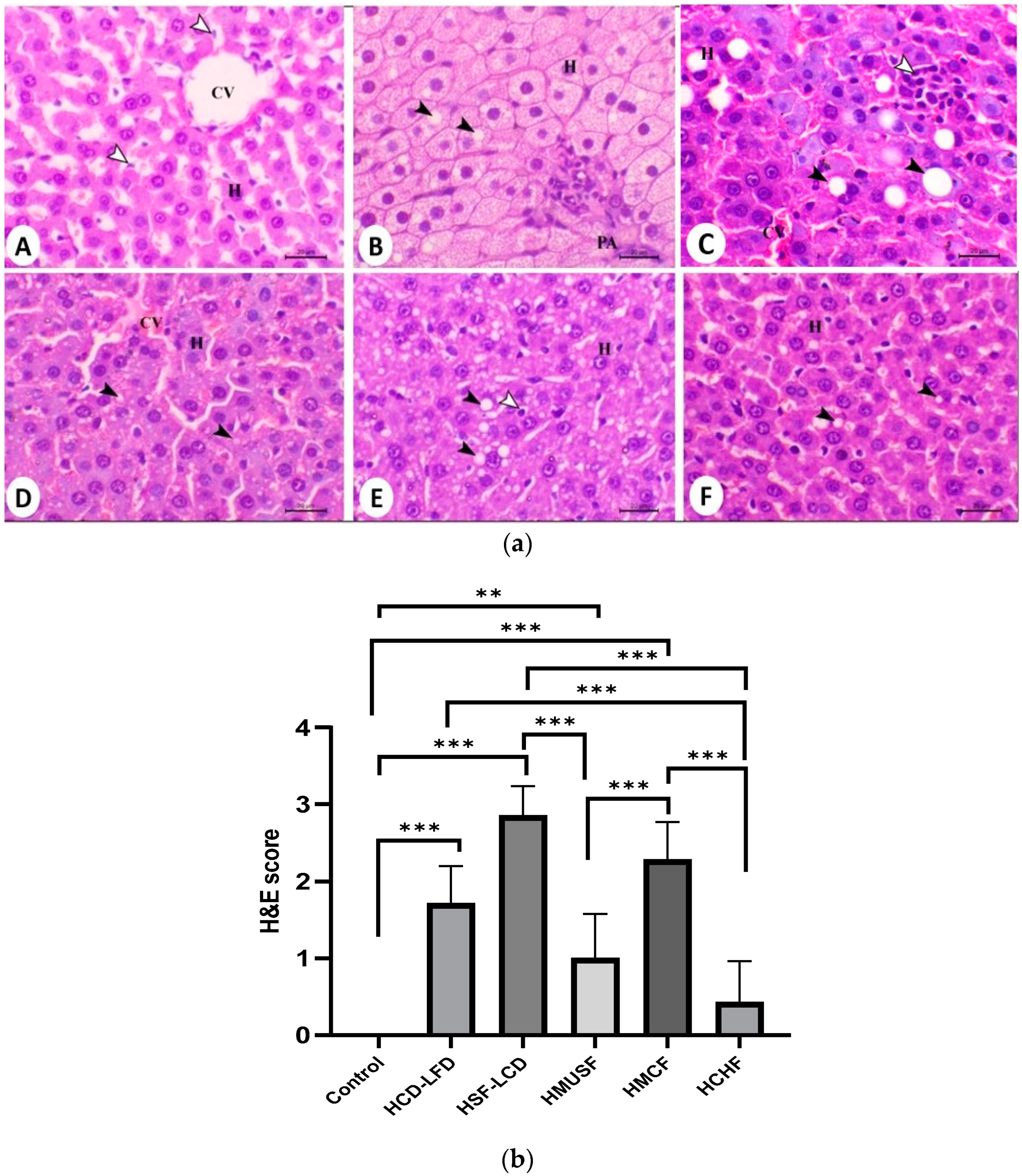
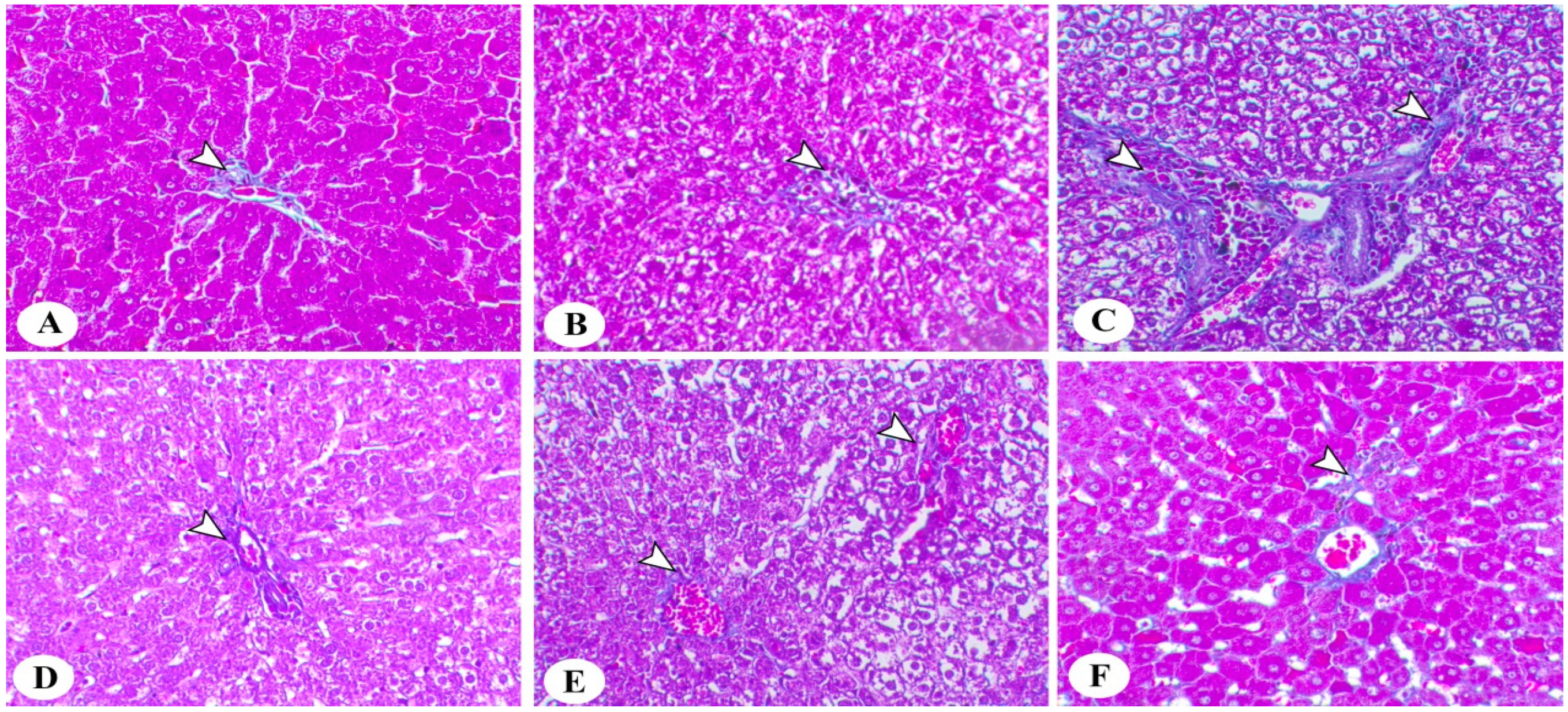
2.5. Liver Histology
2.6. Biochemical Analysis
2.7. Statistical Analysis
3. Results
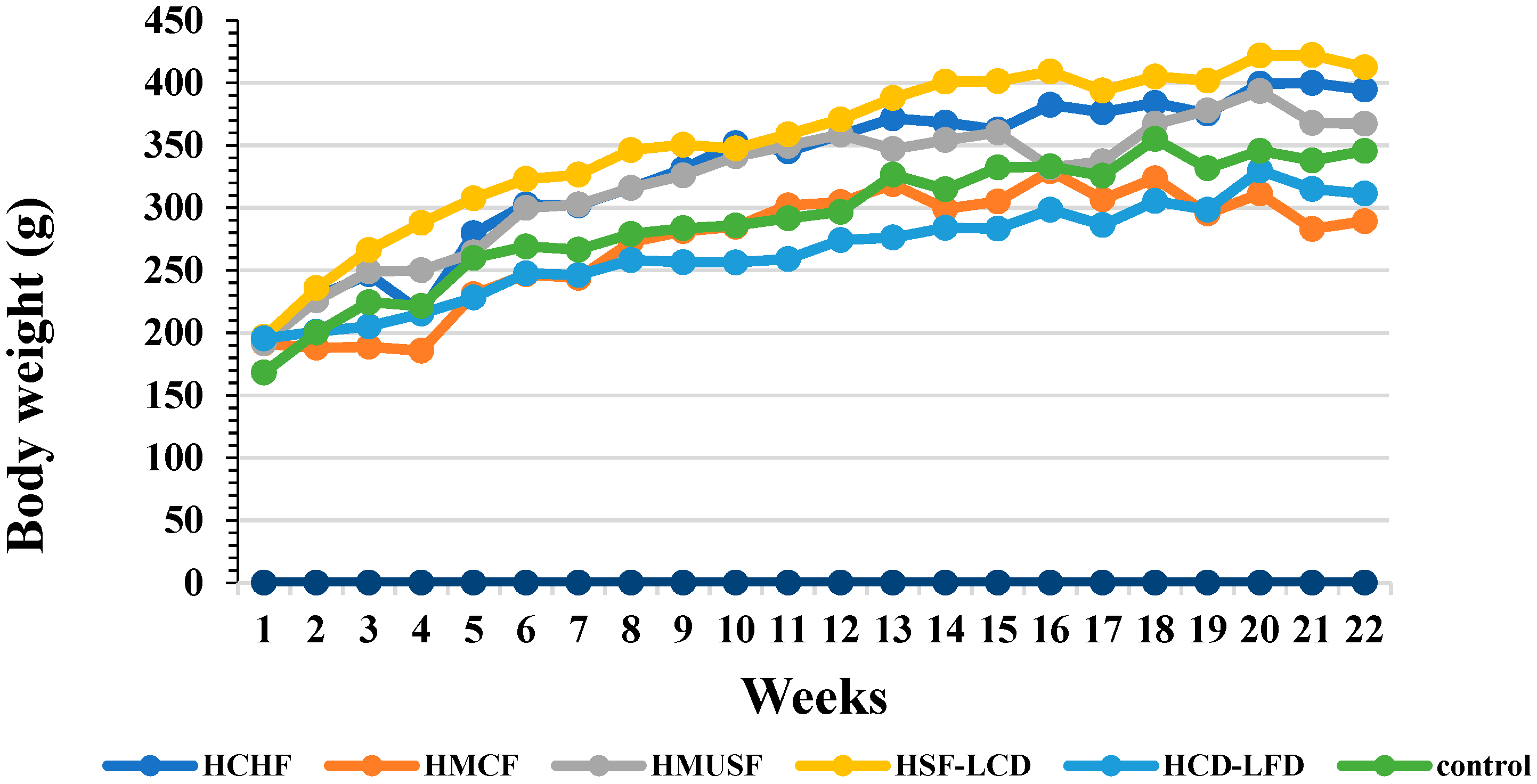
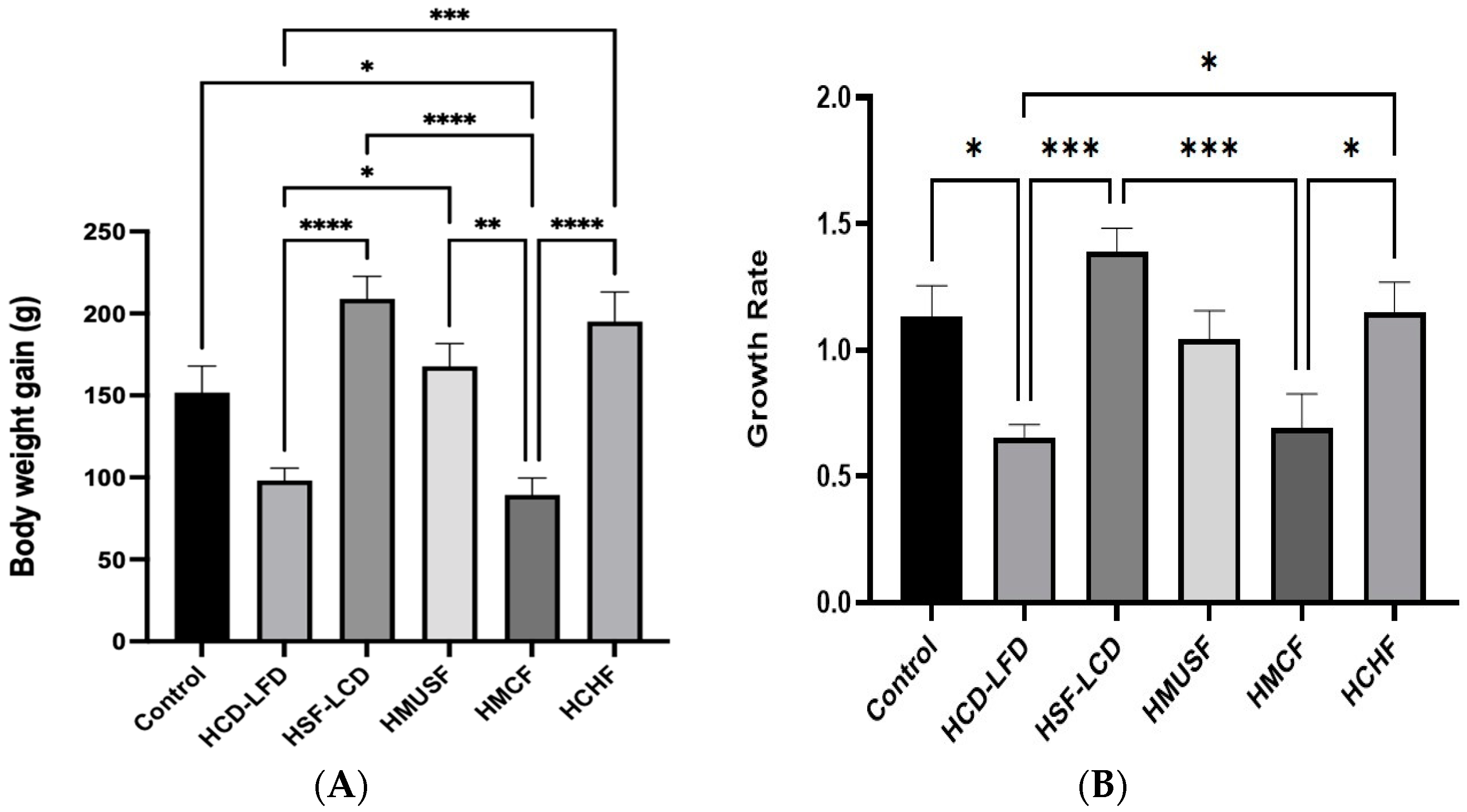
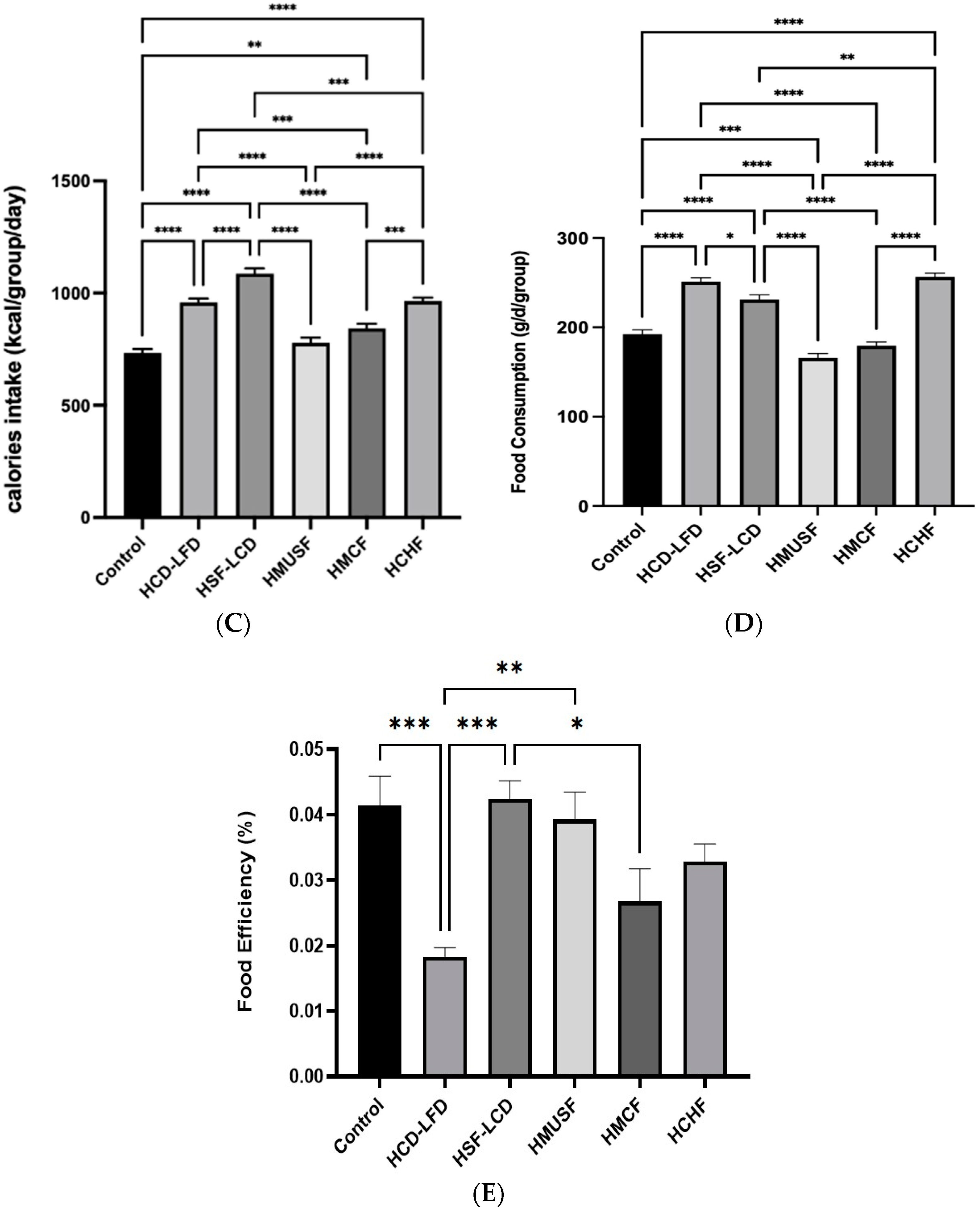
3.1. Effect of Diets on BW and Food Consumption
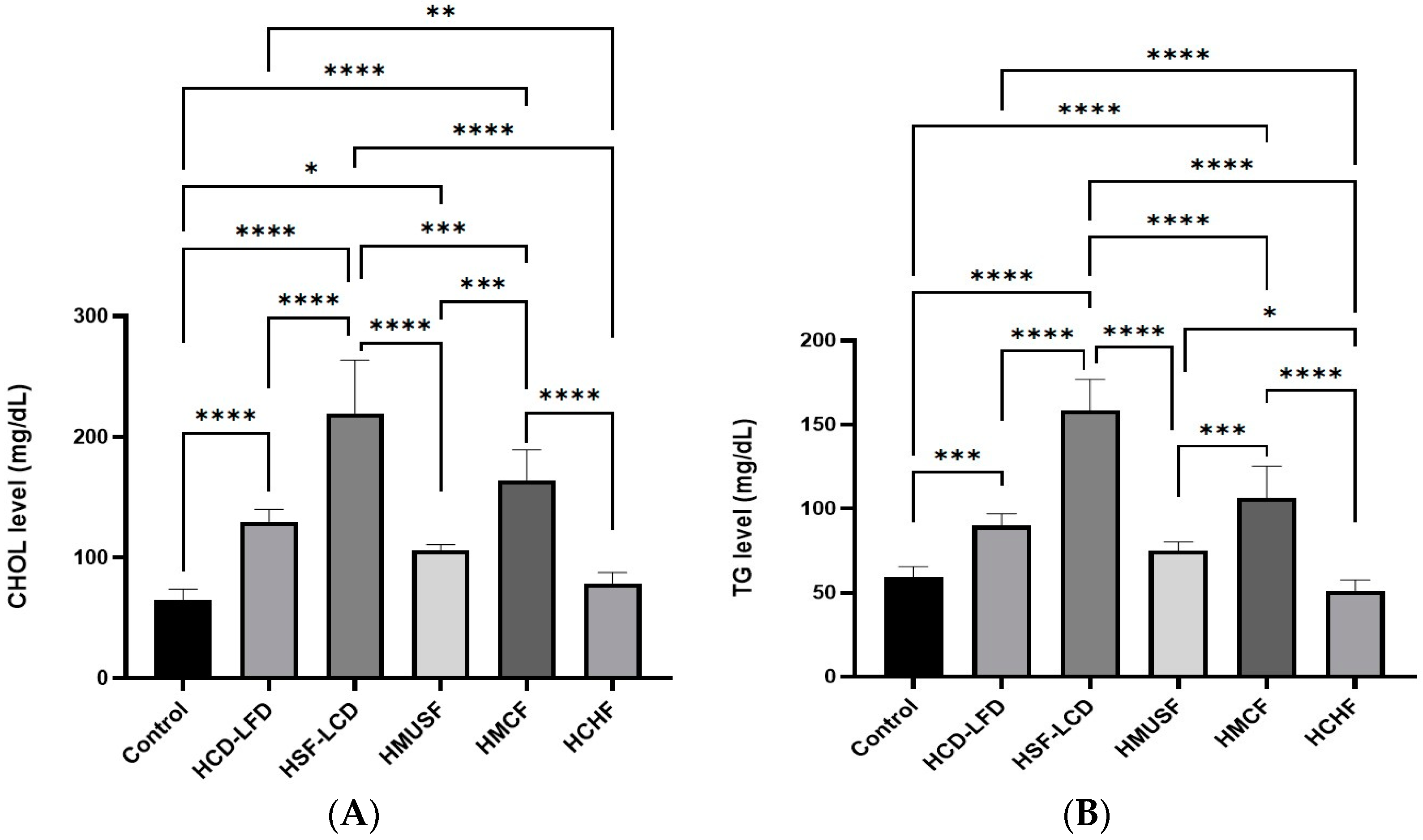
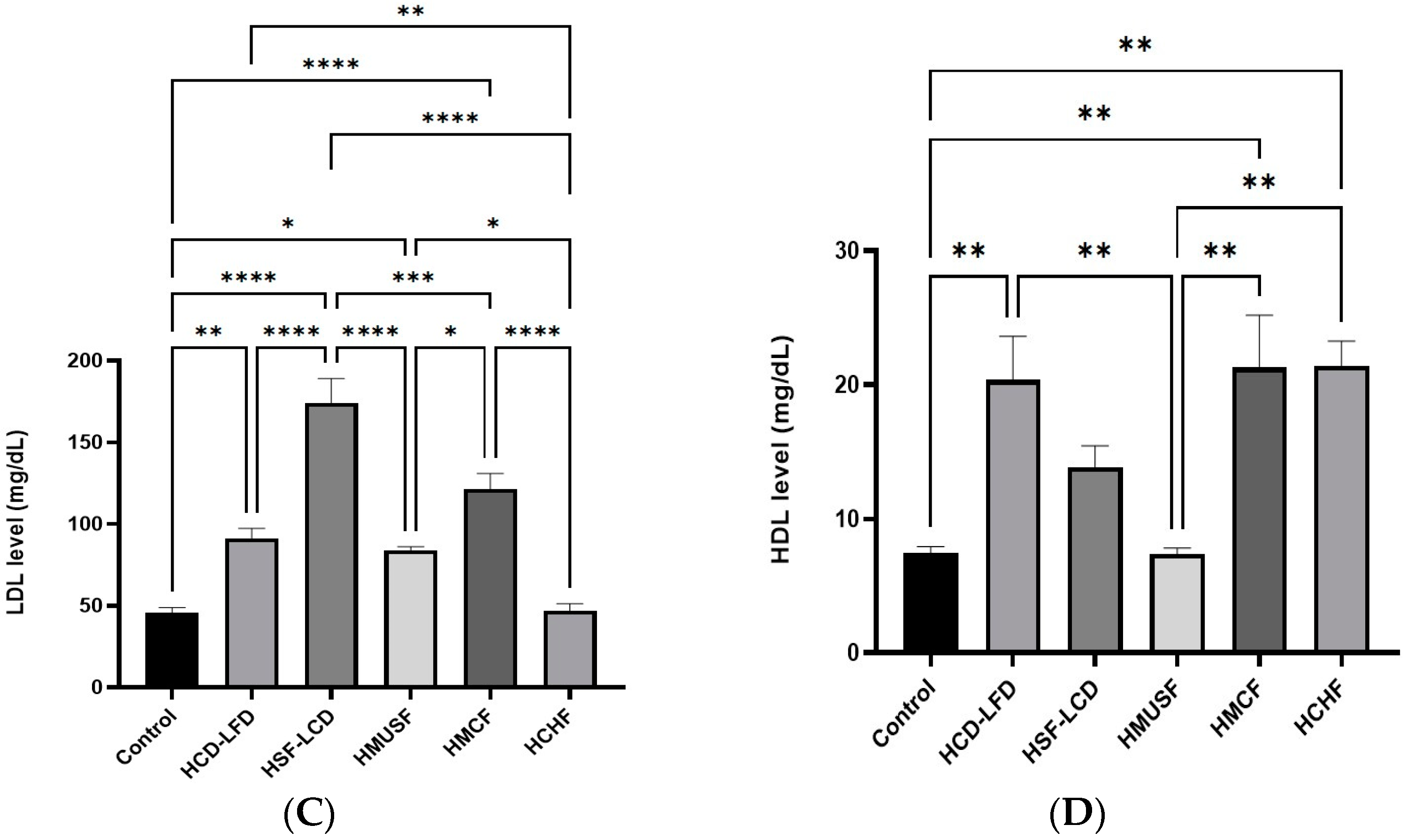
3.2. Effect of Diets on Lipid Profile
3.3. Effect of Diets on Glycemic Control
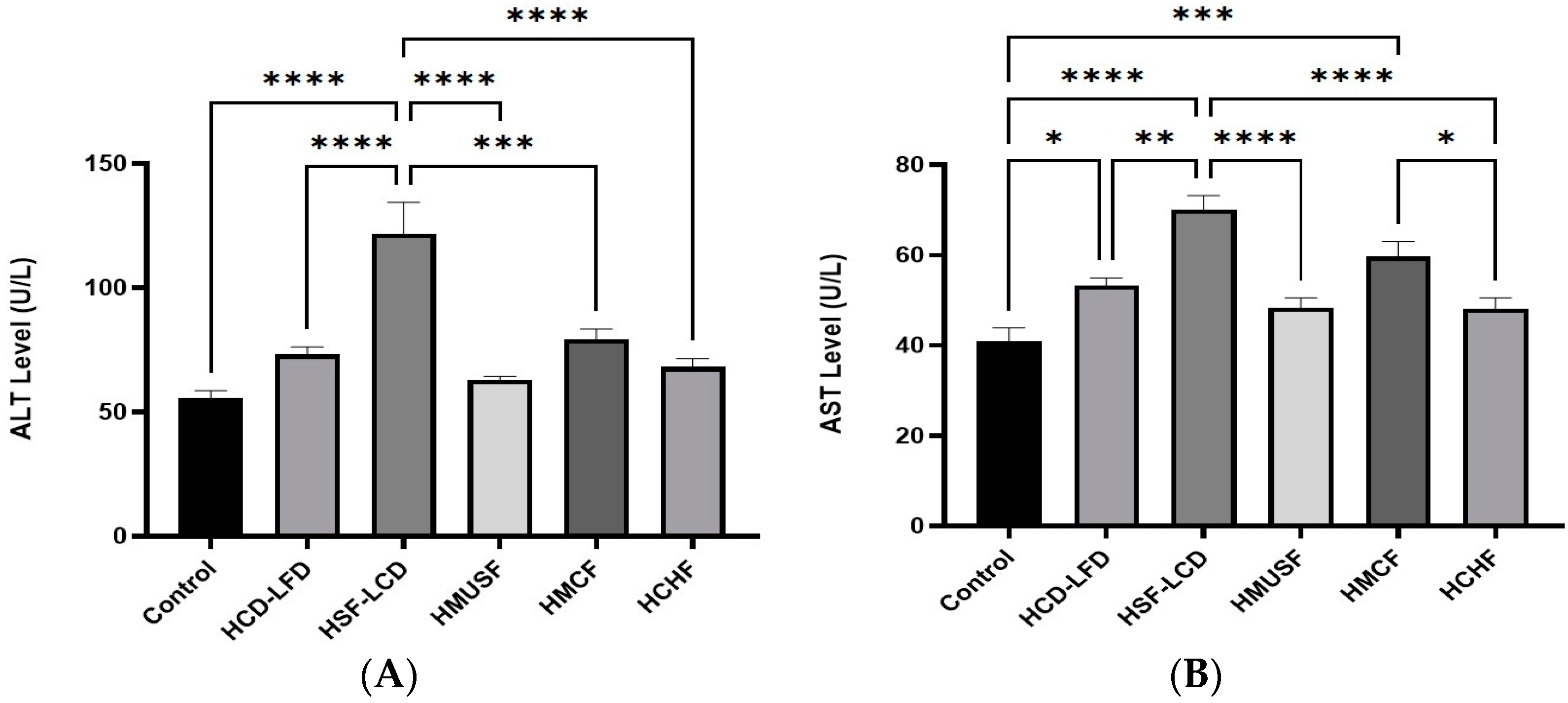
3.4. Effect of Diets on Liver Enzymes
3.5. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- Katsiki, N.; Mikhailidis, D.P.; Mantzoros, C.S. Non-Alcoholic Fatty Liver Disease and Dyslipidemia: An Update. Metabolism 2016, 65, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Scaglioni, F.; Marino, M.; Bedogni, G. Epidemiology of Non-Alcoholic Fatty Liver Disease. Dig. Dis. 2010, 28, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of Non-Alcoholic and Alcoholic Fatty Liver Diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a Continuum: From Obesity to Metabolic Syndrome and Diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Alswat, K.; Aljumah, A.A.; Sanai, F.M.; Abaalkhail, F.; Alghamdi, M.; Al Hamoudi, W.K.; Al Khathlan, A.; Al Quraishi, H.; Al Rifai, A.; Al Zaabi, M.; et al. Nonalcoholic Fatty Liver Disease Burden—Saudi Arabia and United Arab Emirates, 2017–2030. Saudi J. Gastroenterol. 2018, 24, 211–219. [Google Scholar] [CrossRef]
- Kucera, O.; Cervinkova, Z. Experimental Models of Non-Alcoholic Fatty Liver Disease in Rats. World J. Gastroenterol. 2014, 20, 8364–8376. [Google Scholar] [CrossRef]
- Hadi, H.E.; Vettor, R.; Rossato, M. Vitamin E as a Treatment for Nonalcoholic Fatty Liver Disease: Reality or Myth? Antioxidants 2018, 7, 12. [Google Scholar] [CrossRef]
- Huby, T.; Gautier, E.L. Immune Cell-Mediated Features of Non-Alcoholic Steatohepatitis. Nat. Rev. Immunol. 2022, 22, 429–443. [Google Scholar] [CrossRef]
- Pasarín, M.; Abraldes, J.G.; Liguori, E.; Kok, B.; La Mura, V. Intrahepatic Vascular Changes in Non-Alcoholic Fatty Liver Disease: Potential Role of Insulin-Resistance and Endothelial Dysfunction. World J. Gastroenterol. 2017, 23, 6777–6787. [Google Scholar] [CrossRef]
- Ziolkowska, S.; Binienda, A.; Jabłkowski, M.; Szemraj, J.; Czarny, P. The Interplay between Insulin Resistance, Inflammation, Oxidative Stress, Base Excision Repair and Metabolic Syndrome in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2021, 22, 11128. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A Tale of Two “Hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Carreres, L.; Jílková, Z.M.; Vial, G.; Marche, P.N.; Decaens, T.; Lerat, H. Modeling Diet-Induced NAFLD and NASH in Rats: A Comprehensive Review. Biomedicines 2021, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Evolution of Inflammation in Nonalcoholic Fatty Liver Disease: The Multiple Parallel Hits Hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Mells, J.E.; Fu, P.P.; Kumar, P.; Smith, T.; Karpen, S.J.; Anania, F.A. Saturated Fat and Cholesterol Are Critical to Inducing Murine Metabolic Syndrome with Robust Nonalcoholic Steatohepatitis. J. Nutr. Biochem. 2015, 26, 285–292. [Google Scholar] [CrossRef]
- Flessa, C.-M.; Nasiri-Ansari, N.; Kyrou, I.; Leca, B.M.; Lianou, M.; Chatzigeorgiou, A.; Kaltsas, G.; Kassi, E.; Randeva, H.S. Genetic and Diet-Induced Animal Models for Non-Alcoholic Fatty Liver Disease (NAFLD) Research. Int. J. Mol. Sci. 2022, 23, 15791. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Goldin, R.D. Mouse Models in Non-Alcoholic Fatty Liver Disease and Steatohepatitis Research. Int. J. Exp. Pathol. 2006, 87, 1–16. [Google Scholar] [CrossRef]
- Lau, J.K.C.; Zhang, X.; Yu, J. Animal Models of Non-alcoholic Fatty Liver Disease: Current Perspectives and Recent Advances. J. Pathol. 2017, 241, 36–44. [Google Scholar] [CrossRef]
- Eng, J.M.; Estall, J.L. Diet-Induced Models of Non-Alcoholic Fatty Liver Disease: Food for Thought on Sugar, Fat, and Cholesterol. Cells 2021, 10, 1805. [Google Scholar] [CrossRef]
- Jensen, V.S.; Hvid, H.; Damgaard, J.; Nygaard, H.; Ingvorsen, C.; Wulff, E.M.; Lykkesfeldt, J.; Fledelius, C. Dietary Fat Stimulates Development of NAFLD More Potently than Dietary Fructose in Sprague–Dawley Rats. Diabetol. Metab. Syndr. 2018, 10, 4. [Google Scholar] [CrossRef]
- Vancells Lujan, P.; Viñas Esmel, E.; Sacanella Meseguer, E. Overview of Non-Alcoholic Fatty Liver Disease (NAFLD) and the Role of Sugary Food Consumption and Other Dietary Components in Its Development. Nutrients 2021, 13, 1442. [Google Scholar] [CrossRef]
- Nseir, W.; Hellou, E.; Assy, N. Role of Diet and Lifestyle Changes in Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 9338–9344. [Google Scholar] [CrossRef]
- Kosmalski, M.; Frankowski, R.; Ziółkowska, S.; Różycka-Kosmalska, M.; Pietras, T. What’s New in the Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD). J. Clin. Med. 2023, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Casini, A. Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: New Therapeutic Option around the Corner? World J. Gastroenterol. 2014, 20, 7339–7346. [Google Scholar] [CrossRef] [PubMed]
- Berná, G.; Romero-Gomez, M. The Role of Nutrition in Non-Alcoholic Fatty Liver Disease: Pathophysiology and Management. Liver Int. 2020, 40, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ble-Castillo, J.L.; Aparicio-Trapala, M.A.; Juárez-Rojop, I.E.; Torres-Lopez, J.E.; Mendez, J.D.; Aguilar-Mariscal, H.; Olvera-Hernández, V.; Palma-Cordova, L.C.; Diaz-Zagoya, J.C. Differential Effects of High-Carbohydrate and High-Fat Diet Composition on Metabolic Control and Insulin Resistance in Normal Rats. Int. J. Environ. Res. Public Health 2012, 9, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Bancroft’s Theory and Practice of Histological T-9780702068645. Available online: https://www.us.elsevierhealth.com/bancrofts-theory-and-practice-of-histological-techniques-9780702068645.html (accessed on 26 April 2023).
- Serdjebi, C.; Bertotti, K.; Huang, P.; Wei, G.; Skelton-Badlani, D.; Leclercq, I.A.; Barbes, D.; Lepoivre, B.; Popov, Y.V.; Julé, Y. Automated Whole Slide Image Analysis for a Translational Quantification of Liver Fibrosis. Sci. Rep. 2022, 12, 17935. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Tetri, L.H.; Basaranoglu, M.; Brunt, E.M.; Yerian, L.M.; Neuschwander-Tetri, B.A. Severe NAFLD with Hepatic Necroinflammatory Changes in Mice Fed Trans Fats and a High-Fructose Corn Syrup Equivalent. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G987–G995. [Google Scholar] [CrossRef]
- Milić, S.; Lulić, D.; Štimac, D. Non-Alcoholic Fatty Liver Disease and Obesity: Biochemical, Metabolic and Clinical Presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar] [CrossRef]
- Koski, M.; Naukkarinen, H. The Relationship between Stress and Severe Obesity: A Case-Control Study. Biomed. Hub. 2017, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary Habits and Their Relations to Insulin Resistance and Postprandial Lipemia in Nonalcoholic Steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Picklo, M.J.; Long, E.K.; Vomhof-DeKrey, E.E. Glutathionyl Systems and Metabolic Dysfunction in Obesity. Nutr. Rev. 2015, 73, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Jalili, V.; Poorahmadi, Z.; Hasanpour Ardekanizadeh, N.; Gholamalizadeh, M.; Ajami, M.; Houshiarrad, A.; Hajipour, A.; Shafie, F.; Alizadeh, A.; Mokhtari, Z.; et al. The Association between Obesity with Serum Levels of Liver Enzymes, Alanine Aminotransferase, Aspartate Aminotransferase, Alkaline Phosphatase and Gamma-glutamyl Transferase in Adult Women. Endocrinol. Diabetes Metab. 2022, 5, e367. [Google Scholar] [CrossRef]
- Samblas, M.; Milagro, F.I.; Martínez, A. DNA Methylation Markers in Obesity, Metabolic Syndrome, and Weight Loss. Epigenetics 2019, 14, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Uhley, V.E.; Jen, K.L. Changes in Feeding Efficiency and Carcass Composition in Rats on Repeated High-Fat Feedings. Int. J. Obes. 1989, 13, 849–856. [Google Scholar]
- Hill, J.O.; Fried, S.K.; DiGirolamo, M. Effects of a High-Fat Diet on Energy Intake and Expenditure in Rats. Life Sci. 1983, 33, 141–149. [Google Scholar] [CrossRef]
- Ledikwe, J.H.; Blanck, H.M.; Kettel Khan, L.; Serdula, M.K.; Seymour, J.D.; Tohill, B.C.; Rolls, B.J. Dietary Energy Density Is Associated with Energy Intake and Weight Status in US Adults. Am. J. Clin. Nutr. 2006, 83, 1362–1368. [Google Scholar] [CrossRef]
- Kasper, J.M.; Johnson, S.B.; Hommel, J.D. Fat Preference: A Novel Model of Eating Behavior in Rats. J. Vis. Exp. 2014, 88, 51575. [Google Scholar] [CrossRef]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky Gut: Effect of Dietary Fiber and Fats on Microbiome and Intestinal Barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Lu, L.-G. Nonalcoholic Fatty Liver Disease: Dyslipidemia, Risk for Cardiovascular Complications, and Treatment Strategy. J. Clin. Transl. Hepatol. 2015, 3, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zavaroni, I.; Chen, Y.D.; Reaven, G.M. Studies of the Mechanism of Fructose-Induced Hypertriglyceridemia in the Rat. Metabolism 1982, 31, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Lanaspa, M.A.; Rivard, C.J.; Roncal-Jimenez, C.A.; Orlicky, D.J.; Cicerchi, C.; McMahan, R.H.; Abdelmalek, M.F.; Rosen, H.R.; Jackman, M.R.; et al. High Fat and High Sucrose (Western) Diet Induce Steatohepatitis That Is Dependent on Fructokinase. Hepatology 2013, 58, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Importance of the Omega-6/Omega-3 Balance in Health and Disease: Evolutionary Aspects of Diet. In Healthy Agriculture, Healthy Nutrition, Healthy People; Karger Publishers: Basel, Switzerland, 2011; Volume 102, pp. 10–21. [Google Scholar] [CrossRef]
- Araya, J.; Rodrigo, R.; Videla, L.A.; Thielemann, L.; Orellana, M.; Pettinelli, P.; Poniachik, J. Increase in Long-Chain Polyunsaturated Fatty Acid n-6/n-3 Ratio in Relation to Hepatic Steatosis in Patients with Non-Alcoholic Fatty Liver Disease. Clin. Sci. 2004, 106, 635–643. [Google Scholar] [CrossRef]
- Castro, G.S.d.; Calder, P.C. Non-Alcoholic Fatty Liver Disease and Its Treatment with n-3 Polyunsaturated Fatty Acids. Clin. Nutr. 2018, 37, 37–55. [Google Scholar] [CrossRef]
- Rosqvist, F.; Kullberg, J.; Ståhlman, M.; Cedernaes, J.; Heurling, K.; Johansson, H.-E.; Iggman, D.; Wilking, H.; Larsson, A.; Eriksson, O.; et al. Overeating Saturated Fat Promotes Fatty Liver and Ceramides Compared With Polyunsaturated Fat: A Randomized Trial. J. Clin. Endocrinol. Metab. 2019, 104, 6207–6219. [Google Scholar] [CrossRef]
- Toshimitsu, K.; Matsuura, B.; Ohkubo, I.; Niiya, T.; Furukawa, S.; Hiasa, Y.; Kawamura, M.; Ebihara, K.; Onji, M. Dietary Habits and Nutrient Intake in Non-Alcoholic Steatohepatitis. Nutrition 2007, 23, 46–52. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Pacini, G.; De Michieli, F.; Cassader, M. Prolonged Saturated Fat–Induced, Glucose-Dependent Insulinotropic Polypeptide Elevation Is Associated with Adipokine Imbalance and Liver Injury in Nonalcoholic Steatohepatitis: Dysregulated Enteroadipocyte Axis as a Novel Feature of Fatty Liver. Am. J. Clin. Nutr. 2009, 89, 558–567. [Google Scholar] [CrossRef]
- Manco, M. Insulin Resistance and NAFLD: A Dangerous Liaison beyond the Genetics. Children 2017, 4, 74. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef]
- Ronis, M.J.J.; Baumgardner, J.N.; Sharma, N.; Vantrease, J.; Ferguson, M.; Tong, Y.; Wu, X.; Cleves, M.A.; Badger, T.M. Medium Chain Triglycerides Dose-Dependently Prevent Liver Pathology in a Rat Model of Non-Alcoholic Fatty Liver Disease. Exp. Biol. Med. 2013, 238, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Ströher, D.J.; de Oliveira, M.F.; Martinez-Oliveira, P.; Pilar, B.C.; Cattelan, M.D.P.; Rodrigues, E.; Bertolin, K.; Gonçalves, P.B.D.; Piccoli, J.d.C.E.; Manfredini, V. Virgin Coconut Oil Associated with High-Fat Diet Induces Metabolic Dysfunctions, Adipose Inflammation, and Hepatic Lipid Accumulation. J. Med. Food 2020, 23, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekara, C.S.; Nelson, A.; Spradley, M.; Wynn, A.; Robohm-Leavitt, C.; Shen, C.-L.; Kahathuduwa, C.N. Effects of Consumption of Coconut Oil or Coconut on Glycemic Control and Insulin Sensitivity: A Systematic Review and Meta-Analysis of Interventional Trials. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Barreto, L.P.; de Oliveira Silva, R.Y.; de Almeida, B.B.; Ovídio, P.P.; Jordao, A.A. Metabolic Effects of Coconut Oil on Fatty Liver and Oxidative Stress Induced by a High-Fat Diet in Rats. Gene Expr. 2023, 22, 10–18. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Zadeh, S.H.; Mozaffari-Khosravi, H.; Hosseinzadeh, M. Effect of Mediterranean Diet on Liver Enzymes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2022, 128, 1231–1239. [Google Scholar] [CrossRef]
- Turner, R.; Etienne, N.; Alonso, M.G.; de Pascual-Teresa, S.; Minihane, A.M.; Weinberg, P.D.; Rimbach, G. Antioxidant and Anti-Atherogenic Activities of Olive Oil Phenolics. Int. J. Vitam. Nutr. Res. 2005, 75, 61–70. [Google Scholar] [CrossRef]
- Pan, D.A.; Lillioja, S.; Milner, M.R.; Kriketos, A.D.; Baur, L.A.; Bogardus, C.; Storlien, L.H. Skeletal Muscle Membrane Lipid Composition Is Related to Adiposity and Insulin Action. J. Clin. Investig. 1995, 96, 2802–2808. [Google Scholar] [CrossRef]
- Álvarez-Amor, L.; Sierra, A.L.; Cárdenas, A.; López-Bermudo, L.; López-Beas, J.; Andújar, E.; Pérez-Alegre, M.; Gallego-Durán, R.; Varela, L.M.; Martin-Montalvo, A.; et al. Extra Virgin Olive Oil Improved Body Weight and Insulin Sensitivity in High Fat Diet-Induced Obese LDLr−/−.Leiden Mice without Attenuation of Steatohepatitis. Sci. Rep. 2021, 11, 8250. [Google Scholar] [CrossRef]
- Prieto, P.G.; Cancelas, J.; Villanueva-Peñacarrillo, M.L.; Valverde, I.; Malaisse, W.J. Effects of an Olive Oil-Enriched Diet on Plasma GLP-1 Concentration and Intestinal Content, Plasma Insulin Concentration, and Glucose Tolerance in Normal Rats. Endocrine 2005, 26, 107–115. [Google Scholar] [CrossRef]
- Garg, A.; Bantle, J.P.; Henry, R.R.; Coulston, A.M.; Griver, K.A.; Raatz, S.K.; Brinkley, L.; Chen, Y.D.; Grundy, S.M.; Huet, B.A. Effects of Varying Carbohydrate Content of Diet in Patients with Non-Insulin-Dependent Diabetes Mellitus. JAMA 1994, 271, 1421–1428. [Google Scholar] [CrossRef]
- Thomsen, C.; Storm, H.; Holst, J.J.; Hermansen, K. Differential Effects of Saturated and Monounsaturated Fats on Postprandial Lipemia and Glucagon-like Peptide 1 Responses in Patients with Type 2 Diabetes. Am. J. Clin. Nutr. 2003, 77, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Akhlaghi, M.; Sasani, M.R.; Barati Boldaji, R. Olive Oil Lessened Fatty Liver Severity Independent of Cardiometabolic Correction in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Nutrition 2019, 57, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Otrante, A.; Trigui, A.; Walha, R.; Berrougui, H.; Fulop, T.; Khalil, A. Extra Virgin Olive Oil Prevents the Age-Related Shifts of the Distribution of HDL Subclasses and Improves Their Functionality. Nutrients 2021, 13, 2235. [Google Scholar] [CrossRef] [PubMed]
- Brockman, D.A.; Chen, X.; Gallaher, D.D. High-Viscosity Dietary Fibers Reduce Adiposity and Decrease Hepatic Steatosis in Rats Fed a High-Fat Diet. J. Nutr. 2014, 144, 1415–1422. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Weight Loss during Oligofructose Supplementation Is Associated with Decreased Ghrelin and Increased Peptide YY in Overweight and Obese Adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef]
- Chang, H.-C.; Huang, C.-N.; Yeh, D.-M.; Wang, S.-J.; Peng, C.-H.; Wang, C.-J. Oat Prevents Obesity and Abdominal Fat Distribution, and Improves Liver Function in Humans. Plant. Foods Hum. Nutr. 2013, 68, 18–23. [Google Scholar] [CrossRef]
- Nornberg, F.R.; Liberali, R.; Coutinho, V.F. Effect of [beta]-glucan from oats on lipid profile in vivo/Efeito da [beta]-glucana da aveia sobre o perfil lipidico in vivo. Rev. Bras. Obes. Nutr. Emagrecimento 2013, 7, 93–105. [Google Scholar]
- Yu, J.; Xia, J.; Yang, C.; Pan, D.; Xu, D.; Sun, G.; Xia, H. Effects of Oat Beta-Glucan Intake on Lipid Profiles in Hypercholesterolemic Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 2043. [Google Scholar] [CrossRef]
- Amerizadeh, A.; Ghaheh, H.S.; Vaseghi, G.; Farajzadegan, Z.; Asgary, S. Effect of Oat (Avena sativa L.) Consumption on Lipid Profile With Focus on Triglycerides and High-Density Lipoprotein Cholesterol (HDL-C): An Updated Systematic Review. Curr. Probl. Cardiol. 2022, 48, 101153. [Google Scholar] [CrossRef]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Mejia, S.B.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The Effect of Oat β-Glucan on LDL-Cholesterol, Non-HDL-Cholesterol and ApoB for CVD Risk Reduction: A Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef]
- Cortez-Pinto, H.; Jesus, L.; Barros, H.; Lopes, C.; Moura, M.C.; Camilo, M.E. How Different Is the Dietary Pattern in Non-Alcoholic Steatohepatitis Patients? Clin. Nutr. 2006, 25, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, K.; Chen, Y.; Li, Y.; Li, Y.; Fu, K.; Feng, R. Associations between Dietary Nutrient Intakes and Hepatic Lipid Contents in NAFLD Patients Quantified by 1H-MRS and Dual-Echo MRI. Nutrients 2016, 8, 527. [Google Scholar] [CrossRef] [PubMed]
- Wehmeyer, M.H.; Zyriax, B.-C.; Jagemann, B.; Roth, E.; Windler, E.; Schulze zur Wiesch, J.; Lohse, A.W.; Kluwe, J. Nonalcoholic Fatty Liver Disease Is Associated with Excessive Calorie Intake Rather than a Distinctive Dietary Pattern. Medicine 2016, 95, e3887. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Godos, J.; Loguercio, C.; Salomone, F. Targeting Gut-Liver Axis for the Treatment of Nonalcoholic Steatohepatitis: Translational and Clinical Evidence. Transl. Res. 2016, 167, 116–124. [Google Scholar] [CrossRef]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montes de Oca, A.; Julián, M.T.; Ramos, A.; Puig-Domingo, M.; Alonso, N. Microbiota, Fiber, and NAFLD: Is There Any Connection? Nutrients 2020, 12, 3100. [Google Scholar] [CrossRef]
- Schnabl, B.; Brenner, D.A. Interactions Between the Intestinal Microbiome and Liver Diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef]
- Zurbau, A.; Noronha, J.C.; Khan, T.A.; Sievenpiper, J.L.; Wolever, T.M.S. The Effect of Oat β-Glucan on Postprandial Blood Glucose and Insulin Responses: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2021, 75, 1540–1554. [Google Scholar] [CrossRef]
- Hou, Q.; Li, Y.; Li, L.; Cheng, G.; Sun, X.; Li, S.; Tian, H. The Metabolic Effects of Oats Intake in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 10369–10387. [Google Scholar] [CrossRef]
- Poppitt, S.D.; van Drunen, J.D.E.; McGill, A.-T.; Mulvey, T.B.; Leahy, F.E. Supplementation of a High-Carbohydrate Breakfast with Barley Beta-Glucan Improves Postprandial Glycaemic Response for Meals but Not Beverages. Asia Pac. J. Clin. Nutr. 2007, 16, 16–24. [Google Scholar]
- Reyna, N.Y.; Cano, C.; Bermúdez, V.J.; Medina, M.T.; Souki, A.J.; Ambard, M.; Nuñez, M.; Ferrer, M.A.; Inglett, G.E. Sweeteners and Beta-Glucans Improve Metabolic and Anthropometrics Variables in Well Controlled Type 2 Diabetic Patients. Am. J. Ther. 2003, 10, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Cruz, M.; Tovar, A.R.; Aguilar-Salinas, C.A.; Medina-Vera, I.; Gil-Zenteno, L.; Hernández-Viveros, I.; López-Romero, P.; Ordaz-Nava, G.; Canizales-Quinteros, S.; Guillen Pineda, L.E.; et al. A Dietary Pattern Including Nopal, Chia Seed, Soy Protein, and Oat Reduces Serum Triglycerides and Glucose Intolerance in Patients with Metabolic Syndrome. J. Nutr. 2012, 142, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, Y.; Nie, C.; Xie, X.; Yuan, X.; Ma, Q.; Zhang, M.; Chen, Z.; Hu, X.; Li, J. Highland Barley β-Glucan Alleviated Western Diet-Induced Non-Alcoholic Fatty Liver Disease via Increasing Energy Expenditure and Regulating Bile Acid Metabolism in Mice. Food Funct. 2022, 13, 11664–11675. [Google Scholar] [CrossRef] [PubMed]

| Group 1 Control | Group 2 HCD-LFD | Group 3 HSF-LCD | Group 4 HMUSF | Group 5 HMCF | Group 6 HCHF | |
|---|---|---|---|---|---|---|
| Protein | 20% | 20% | 20% | 20% | 20% | 20% |
| Carbohydrate | 65% | 35% | 35% | 35% | ||
| Sucrose | 60% | |||||
| Oat | 64% | |||||
| Fat | 15% | 20% | 16% | |||
| Animal fat | 45% | |||||
| Olive oil | 45% | |||||
| Coconut oil | 45% | |||||
| Vitamin & Mineral mix | 0.30% | 0.30% | 0.30% | 0.30% | 0.30% | 0.30% |
| Energy density | 3.81 Kcal/g | 3.82 Kcal/g | 4.7 Kcal/g | 4.7 Kcal/g | 4.7 Kcal/g | 3.76 Kcal/g |
| Groups | Control | HCD-LFD | HSF-LCD | HMUSF | HMCF | HCHF |
|---|---|---|---|---|---|---|
| Glucose (mg/dL) | 73.86 ± 5.18 c,e | 93.57 ± 5.19 c | 181.00 ± 35.67 a,b,d,e,f | 86.33 ± 4.55 c | 100.57 ± 13.35 a,c,f | 72.86 ± 6.87 c,e |
| Insulin (mIU/mL) | 0.08 ± 0.03 b,c,e | 0.72 ± 0.12 a,c,d,e,f | 1.96 ± 0.19 a,b,d,e,f | 0.14 ± 0.02 b,c,e | 1.38 ± 0.13 a,b,c,d,f | 0.08 ± 0.04 b,c,e |
| HOMA-IR | 0.0150 ± 0.0062 b,c,e | 0.1666 ± 0.0241 a,c,e,f | 0.8774 ± 0.2019 a,b,d,e,f | 0.0303 ± 0.0039 c,e | 0.3417 ± 0.0530 a,b,c,d,f | 0.0140 ± 0.0071 b,c,e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljahdali, B.A.; Bajaber, A.S.; Al-Nouri, D.M.; Al-Khalifah, A.S.; Arzoo, S.; Alasmari, A.A. The Development of Nonalcoholic Fatty Liver Disease and Metabolic Syndromes in Diet-Induced Rodent Models. Life 2023, 13, 1336. https://doi.org/10.3390/life13061336
Aljahdali BA, Bajaber AS, Al-Nouri DM, Al-Khalifah AS, Arzoo S, Alasmari AA. The Development of Nonalcoholic Fatty Liver Disease and Metabolic Syndromes in Diet-Induced Rodent Models. Life. 2023; 13(6):1336. https://doi.org/10.3390/life13061336
Chicago/Turabian StyleAljahdali, Bayan Abdulhafid, Adnan Salem Bajaber, Doha M. Al-Nouri, Abdulrahman Saleh Al-Khalifah, Shaista Arzoo, and Abeer Abdullah Alasmari. 2023. "The Development of Nonalcoholic Fatty Liver Disease and Metabolic Syndromes in Diet-Induced Rodent Models" Life 13, no. 6: 1336. https://doi.org/10.3390/life13061336
APA StyleAljahdali, B. A., Bajaber, A. S., Al-Nouri, D. M., Al-Khalifah, A. S., Arzoo, S., & Alasmari, A. A. (2023). The Development of Nonalcoholic Fatty Liver Disease and Metabolic Syndromes in Diet-Induced Rodent Models. Life, 13(6), 1336. https://doi.org/10.3390/life13061336





