Changes in the AGE/Macrophage/TNF-α Pathway Affect Skin Dryness during KK-Ay/Tajcl Mice Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Measurement of Transepidermal Water Loss (TEWL) and Capacitance of the Dorsal Skin
2.3. Preparation and Staining of the Dorsal Skin
2.4. Analysis of Skin Prostaglandin E2 (PGE2), Tumor Necrosis Factor (TNF)-α, Interleukin (IL)-1β, and Cyclooxygenase (COX)-2 Levels via Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Statistical Analysis
3. Results
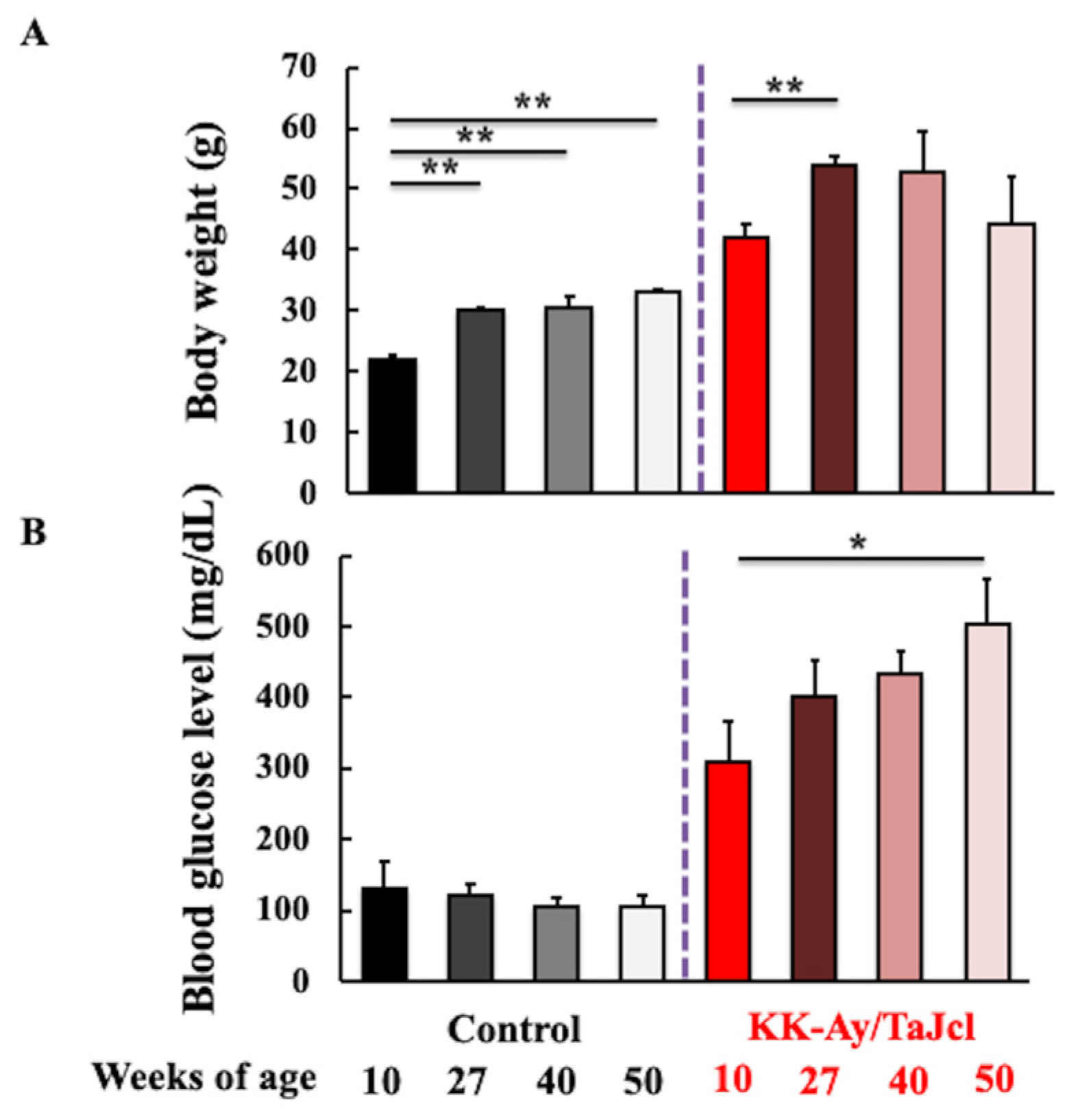
3.1. Effect of Aging on Body Weight and Blood Glucose Level of KK-Ay/Tajcl Mice
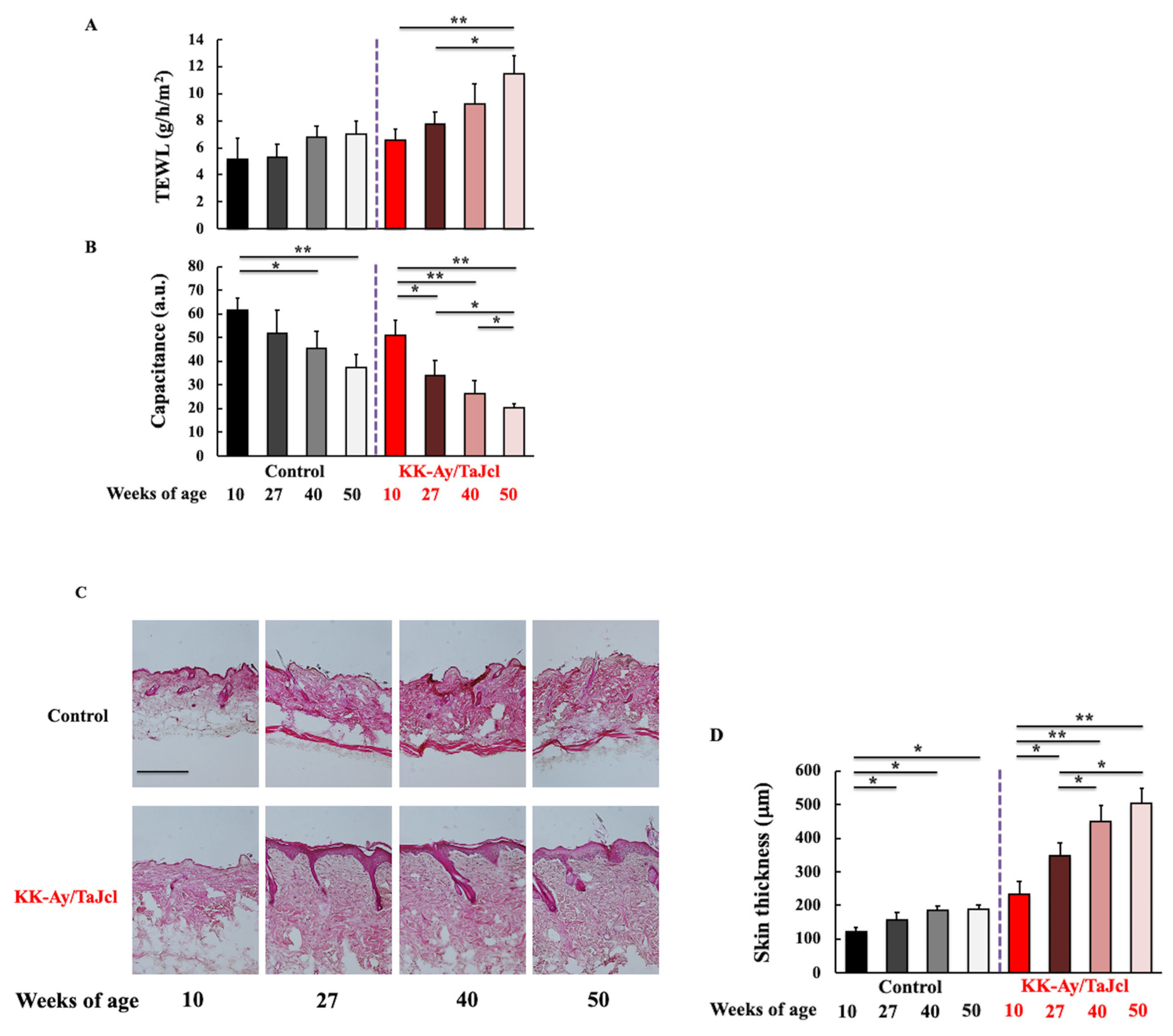
3.2. Effect of Aging on TEWL, Skin Hydration and Thickness, and the Expression of Collagen in KK-Ay/TaJcl Mice
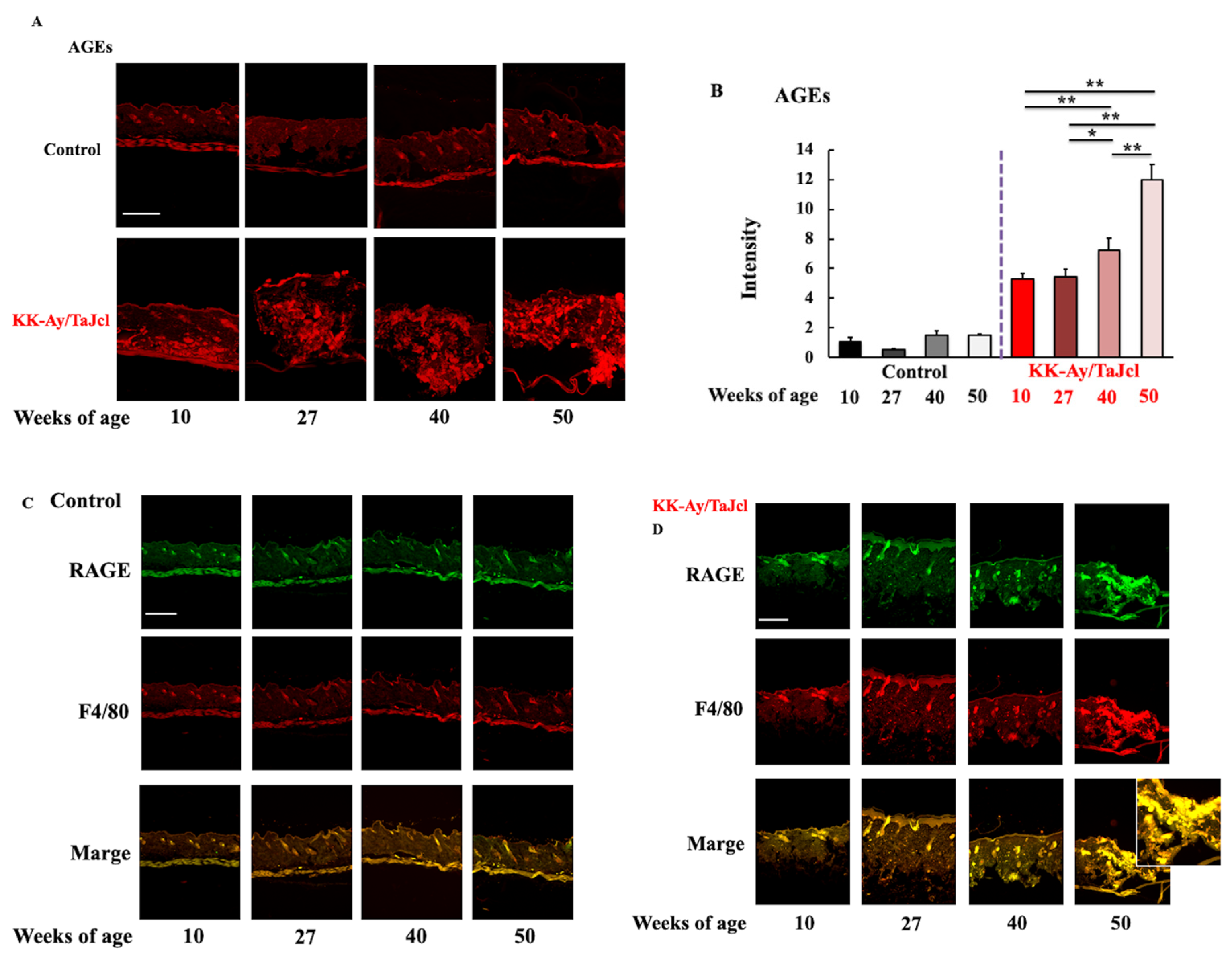
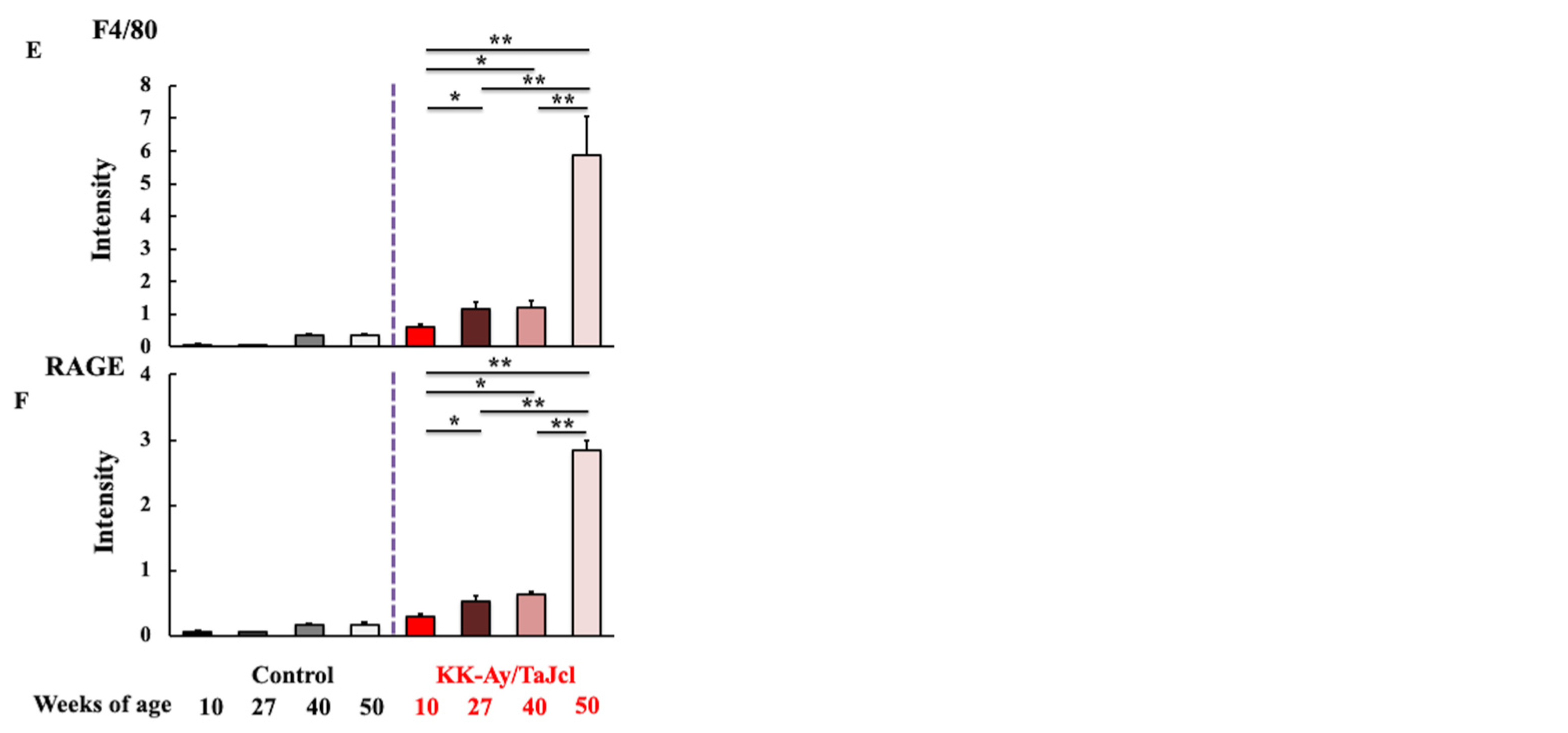
3.3. Effect of Aging on Skin Levels of AGEs, F4/80, and RAGE in KK-Ay/TaJcl Mice
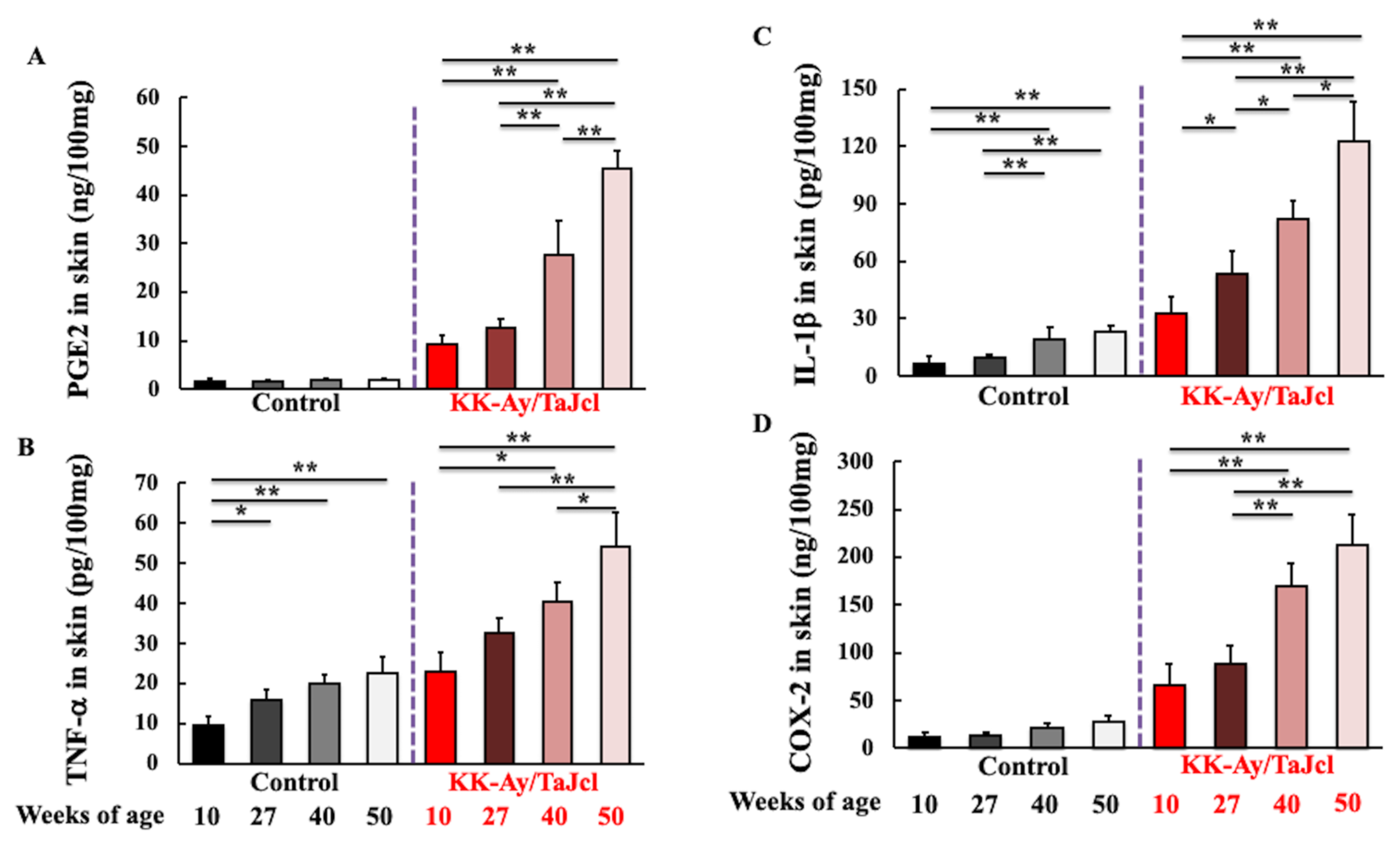
3.4. Effect of Aging on PGE2, TNF-α, IL-1β, and COX-2 Skin Levels in KK-Ay/TaJcl Mice
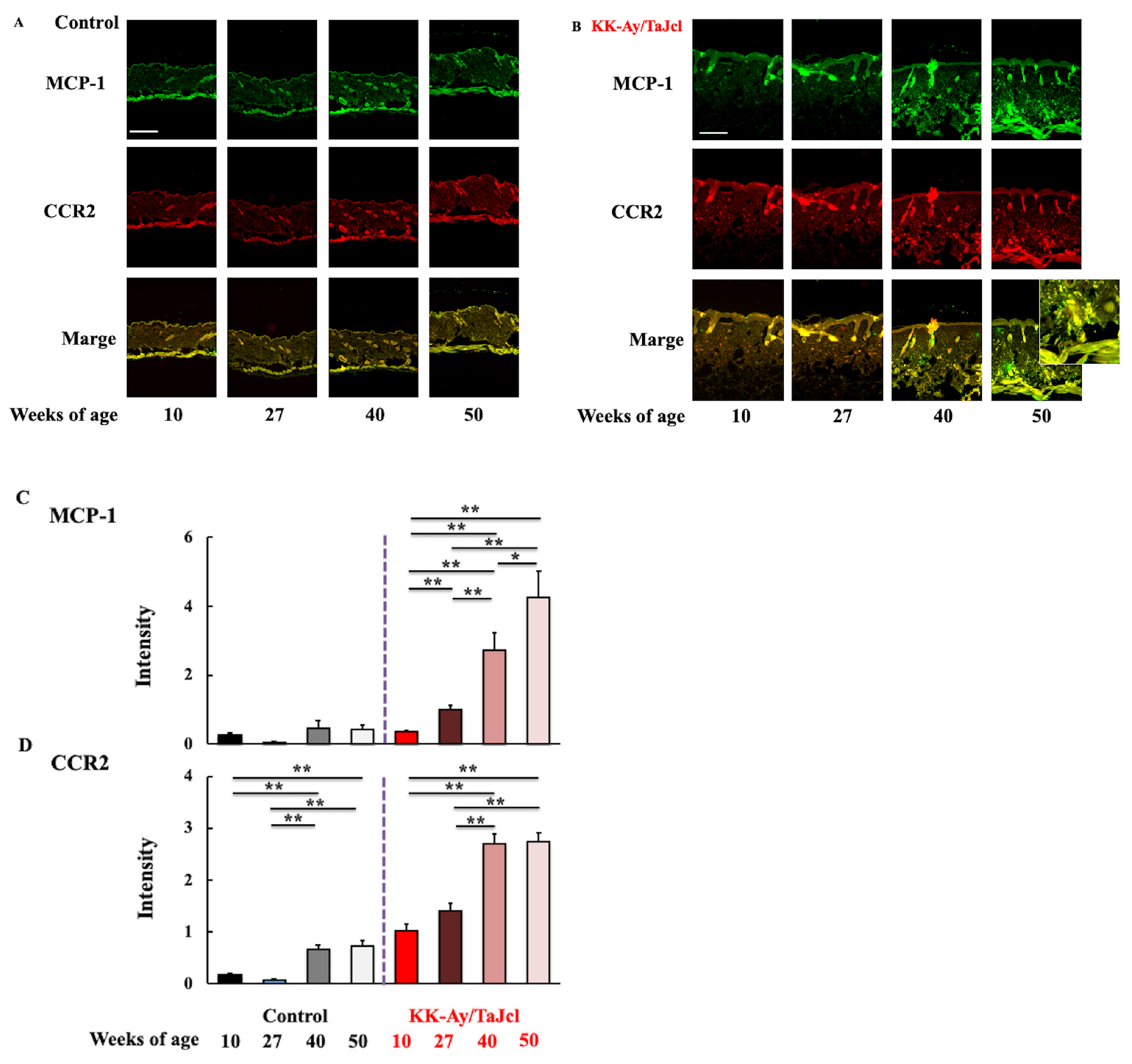
3.5. Effect of Aging on MCP-1 and CCR2 Skin Expression in KK-Ay/TaJcl Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyagaki, T. Skin infections complicated with diabetes mellitus and diabetic foot. Jpn. J. Environ. Infect. 2017, 32, 337–343. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- Ponirakis, G.; Petropoulos, I.N.; Fadavi, H.; Alam, U.; Asghar, O.; Marshall, A.; Tavakoli, M.; Malik, R.A. The diagnostic accuracy of Neuropad for assessing large and small fibre diabetic neuropathy. Diabet. Med. 2014, 31, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.W.; Jun, M.S.; Yang, H.K.; Lee, B.C. Cellular and molecular mechanisms and effects of berberine on obesity-induced inflammation. Biomedicines 2022, 10, 1739. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Goto, K.; Tanaka, S.; Horikawa, T.; Ooi, K. Skin, liver, and kidney interactions contribute to skin dryness in aging KK-Ay/Tajcl mice. Biomedicines 2022, 10, 2648. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M.; Kohn, R.R.; Cerami, A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc. Natl. Acad. Sci. USA 1984, 81, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Bucala, R.; Striker, L. Pathogenic effects of advanced glycosylation: Biochemical, biologic, and clinical implications for diabetes and aging. Lab. Investig. 1994, 70, 138–151. [Google Scholar]
- Shoji, T.; Koyama, H.; Morioka, T.; Tanaka, S.; Kizu, A.; Motoyama, K.; Mori, K.; Fukumoto, S.; Shioi, A.; Shimogaito, N.; et al. Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes. Diabetes 2006, 55, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Nagai, R.; Ishibashi, T.; Inomuta, H.; Ikeda, K.; Horiuchi, S. The relationship between accumulation of advanced glycation end products and expression of vascular endothelial growth factor in human diabetic retinas. Diabetologia 1997, 40, 764–769. [Google Scholar] [CrossRef]
- Kume, S.; Takeya, M.; Mori, T.; Araki, N.; Suzuki, H.; Horiuchi, S.; Kodama, T.; Miyauchi, Y.; Takahashi, K. Immunohistochemical and ultrastructural detection of advanced glycation end products in atherosclerotic lesions of human aorta with a novel specific monoclonal antibody. Am. J. Pathol. 1995, 147, 654–667. [Google Scholar]
- Dyer, D.G.; Dunn, J.A.; Thorpe, S.R.; Bailie, K.E.; Lyons, T.J.; McCance, D.R.; Baynes, J.W. Accumulation of maillard reaction products in skin collagen in diabetes and aging. J. Clin. Investig. 1993, 91, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, K.L.; Harris, C.S.; Saleem, A.; Beaulieu, L.P.; Ta, C.A.; Haddad, P.S.; Arnason, J.T. Seasonal phytochemical variation of anti-glycation principles in lowbush blueberry (Vaccinium angustifolium). Planta Med. 2009, 75, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Barel, A.O.; Clarys, P. Study of the stratum corneum barrier function by. transept water loss measurements: Comparison between two commercial instruments: Evaporimeter and tewameter. Skin Pharmacol. 1995, 8, 186–195. [Google Scholar] [CrossRef]
- Berardesca, E. European group for efficacy measurements on cosmetics and other topical products (EEMCO). Skin Res. Technol. 1997, 3, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Bekeredjian, R.; Walton, C.B.; MacCannell, K.A.; Ecker, J.; Kruse, F.; Outten, J.T.; Sutcliffe, D.; Gerard, R.D.; Bruick, R.K.; Shohet, R.V. Conditional HIF-1alpha expression produces a reversible cardiomyopathy. PLoS ONE 2010, 5, e11693. [Google Scholar] [CrossRef]
- Yokoyama, S.; Hiramoto, K.; Koyama, M.; Ooi, K. Skin disruption is associated with indomethacin-induced small intestinal injury in mice. Exp. Dermatol. 2014, 23, 659–663. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Ramasamy, R.; Vannucci, S.J.; Yan, S.S.; Herold, K.; Yan, S.F.; Schmidt, A.M. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005, 15, 16R–28R. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Naka, Y.; Schmidt, A.M. Glycation, inflammation, and RAGE: A scaffold for the macrovascular complications of diabetes and beyond. Circ. Res. 2003, 93, 1159–1169. [Google Scholar] [CrossRef]
- Bucciarelli, L.G.; Wendt, T.; Qu, W.; Lu, Y.; Lalla, E.; Rong, L.L.; Goova, M.T.; Moser, B.; Kislinger, T.; Lee, D.C.; et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation 2002, 106, 2827–2835. [Google Scholar] [CrossRef]
- Yagi, M.; Yonei, Y. Glycation stress and anti-aging 5. Glycative stress and receptors for AGEs as ligands. Glycative Stress Res. 2017, 4, 212–216. [Google Scholar]
- Molina-Holgado, E.; Ortiz, S.; Molina-Holgado, F.; Guaza, C. Induction of COX-2 and PGE2 biosynthesis by IL-1β is mediated by PKC and mitogen-activated protein kinases in murine astrocytes. Br. J. Pharmacol. 2000, 131, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, T.; Hiramoto, K.; Goto, K.; Sekijima, H.; Ooi, K. Differences in the mechanism of type 1 and type 2 diaberes-induced skin dryness by using model mice. Int. J. Med. Sci. 2021, 18, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Yonei, Y. Glycation stress and anti-aging: 14. Regulation of glycative stress. 2. Inhibition of the AGE production and accumulation. Glycative Stress Res. 2019, 6, 212–218. [Google Scholar]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M.; et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar] [CrossRef]
- Shimizu, I.; Yoshida, Y.; Moriya, J.; Nojima, A.; Uemura, A.; Kobayashi, Y.; Minamino, T. Semaphorin3E-induced inflammation contributes to insulin resistance in dietary obesity. Cell Metab. 2013, 18, 491–504. [Google Scholar] [CrossRef]
- Ando, Y.; Shinozawa, Y.; Iijima, Y.; Yu, B.C.; Sone, M.; Ooi, Y.; Watanaka, Y.; Chida, K.; Hakuno, F.; Takahashi, S.I. Tumor necrosis factor (TNF)-α-induced repression of GKAP42 protein levels through cGMP-dependent kinase (cGK)-Iα causes insulin resistance in 3T3-L1 adipocytes. J. Biol. Chem. 2015, 290, 5881–5892. [Google Scholar] [CrossRef]
- Guarneri, F.; Custurone, P.; Papaianni, V.; Gangemi, S. Involvement of RAGE and. oxidative stress in inflammatory and infections skin diseases. Antioxidants 2021, 10, 82. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiramoto, K.; Imai, M.; Tanaka, S.; Ooi, K. Changes in the AGE/Macrophage/TNF-α Pathway Affect Skin Dryness during KK-Ay/Tajcl Mice Aging. Life 2023, 13, 1339. https://doi.org/10.3390/life13061339
Hiramoto K, Imai M, Tanaka S, Ooi K. Changes in the AGE/Macrophage/TNF-α Pathway Affect Skin Dryness during KK-Ay/Tajcl Mice Aging. Life. 2023; 13(6):1339. https://doi.org/10.3390/life13061339
Chicago/Turabian StyleHiramoto, Keiichi, Masashi Imai, Shota Tanaka, and Kazuya Ooi. 2023. "Changes in the AGE/Macrophage/TNF-α Pathway Affect Skin Dryness during KK-Ay/Tajcl Mice Aging" Life 13, no. 6: 1339. https://doi.org/10.3390/life13061339
APA StyleHiramoto, K., Imai, M., Tanaka, S., & Ooi, K. (2023). Changes in the AGE/Macrophage/TNF-α Pathway Affect Skin Dryness during KK-Ay/Tajcl Mice Aging. Life, 13(6), 1339. https://doi.org/10.3390/life13061339




