Acute Myocardial Infarction in Patients with Hereditary Thrombophilia—A Focus on Factor V Leiden and Prothrombin G20210A
Abstract
:1. Introduction
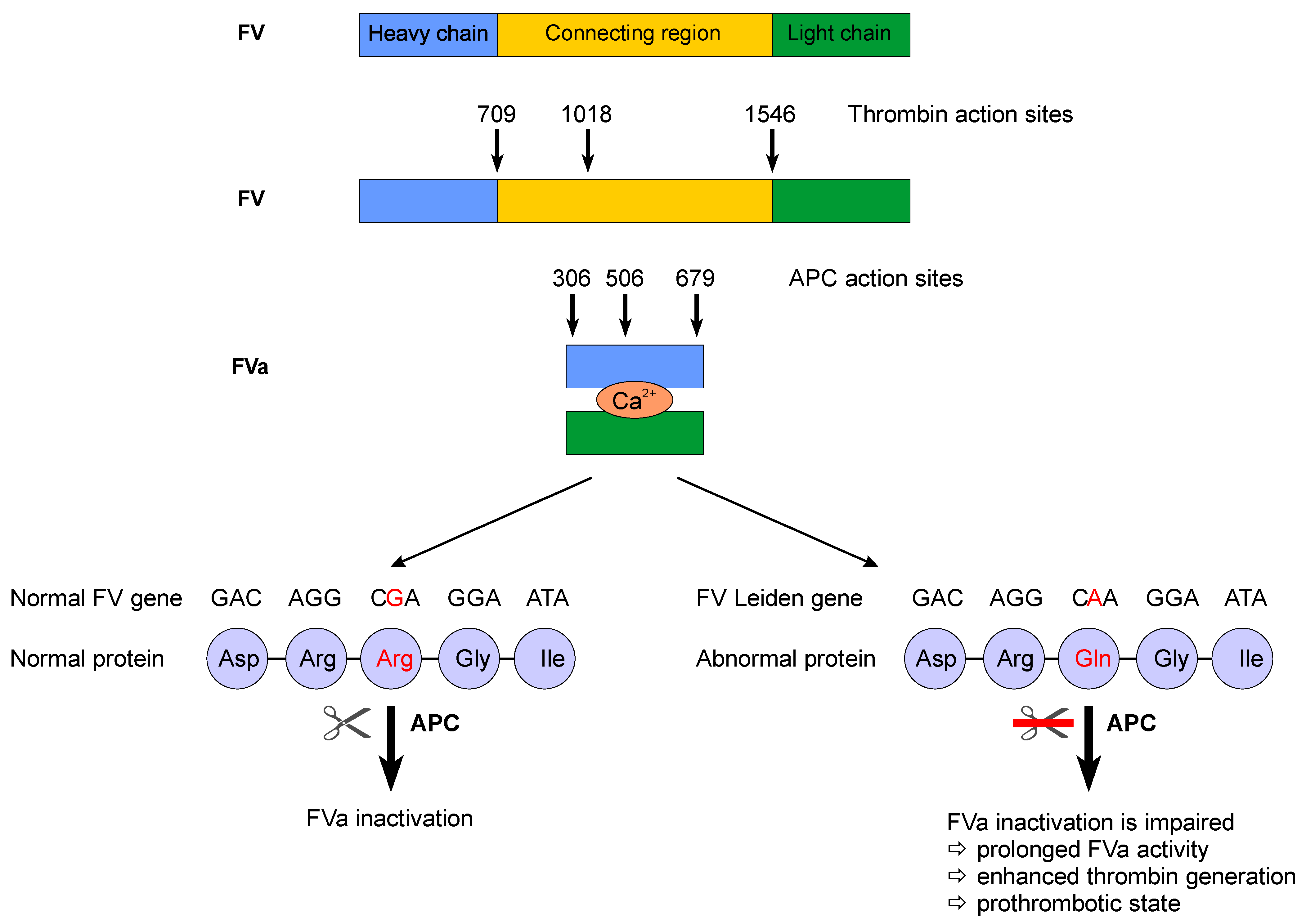
2. Factor V Leiden
2.1. Myocardial Infarction in FV Leiden Carriers
2.2. The Risk of Recurrence of MI
3. Prothrombin G20210A
3.1. Myocardial Infarction in Prothrombin G20210A Carriers
3.2. The Risk of Recurrence of MI
| Author, Year | Country | Cases | FV Leiden Prevalence of the Mutation/OR for the Association with MI | Prothrombin G20210A Prevalence of the Mutation/OR for the Association with MI | |
|---|---|---|---|---|---|
| No. | Age (Mean)% Sex | ||||
| Angeline et al., 2005 [65] | India | 52 MI | NR | Prevalence = 5.8% No association with MI | Prevalence = 0% No association with MI |
| Zee et al., 2006 [66] | USA | 523 MI | 58.3 y M | OR = 1.23 (95% CI 0.81–1.86) | OR = 1.05 (95% CI 0.65–1.72) |
| Hindorff et al., 2006 [60] | USA | 273 MI | 67.5 y F | NR | Prevalence = 2.8% OR = 2.2 (95% CI 1.1–4.7) |
| Mannuci et al., 2006 [67] | Italy | 520 ACS 54.2% MI | 67 y 72.1% M | Prevalence = 3.8% for heterozygotes p = 0.99 for association with ACS | Prevalence = 4% for heterozygotes p = 0.87 for association with ACS |
| Smith et al., 2007 [5] | USA | 856 MI | 65.3 y 57% F | OR = 1.0 (95% CI 0.7–1.5) | OR = 1.4 (95% CI 0.9–2.1) |
| Morgan et al., 2007 [68] | USA | 811 ACS 73% MI | 60.7 y—M 63.1 y—F 68% M | OR = 0.93 (95% CI 0.58–1.50) | OR = 0.92 (95% CI 0.52–1.64) |
| Celik et al., 2008 [69] | Turkey | 129 MI | ≤45 y | Prevalence = 7.8% No association with MI | Prevalence = 1.1% No association with MI |
| Settin et al., 2008 [24] | Egypt | 44 MI | 52.2 y 81.8% M | Prevalence = 43.0% OR = 4.45 (95% CI 2.17–9.13) for heterozygotes Prevalence = 9% OR = 8.19 (95% CI 1.91–35.21) for homozygotes | NR |
| Martinelli et al., 2008, [26] | Italy | 489 CAD (307 MI) | 60.3 y 83.6% M | Prevalence = 2.9% for heterozygotes Prevalence = 0.3% for homozygotes p = 0.725 for association with MI | Prevalence = 5.9% for heterozygotes p = 0.204 Prevalence = 0% for homozygotes |
| Pestana et al., 2009 [70] | Venezuela | 175 MI | 57.87 y 75.5% M | Prevalence = 2.29% for heterozygotes OR = 0.76 (95% CI 0.16–3.69) Prevalence = 0% for homozygotes | NR |
| Weischer et al., 2009 [71] | Denmark | 720 MI a 1123 MI b | ≥20 y | NR | HR = 1.7 (95% CI 1.1–2.7) for heterozygotes in general population studies OR = 2 (95% CI 1–3.8) for heterozygotes in case–control studies |
| Mannucci et al., 2010 [31] | Italy | 1880 MI | 39.6 y 89% M | OR = 1.66 (95% CI 1.15–2.38)—all MI patients OR = 1.00 (95% CI 0.58–1.72)—normal cholesterol OR = 3.58 (95% CI 2.19–5.86)—hypercholesterolemia | OR = 1.28 (95% CI 0.91–1.79) |
| Forte et al., 2011 [28] | Italy | 164 MI | 67 y 89% M | Prevalence = 5.4% for heterozygotes Prevalence = 0% for homozygotes No association with MI | Prevalence = 9.3% for heterozygotes Prevalence = 0.7% for homozygotes OR = 4.00 (95% CI 1.51–10.56)—all carriers |
| Dogra et al., 2012 [72] | India | 184 MI | 36.4 y 96.2% M | Prevalence = 7.1% OR = 3.7 (95% CI 1.5–9.5) | NR |
| Himabindu et al., 2012 [73] | India | 51 ACS 86% MI | ≤50 y 86% M | Prevalence = 0% | NR |
| Tomaiuolo et al., 2012 [27] | Italy | 955 MI and 698 MI | 38.7 y 62% M 69.5 y 65% M | Prevalence = 8.4% in W Prevalence = 3.5% in M and Prevalence = 3.5% in W Prevalence = 2.5% in M | Prevalence = 9% in W Prevalence = 4.2% in M and Prevalence = 3.4% in W Prevalence = 2.8% in M |
| Ben Slama et al., 2012 [32] | Tunis | 100 MI | 46.92 y 84% M | Prevalence = 9% OR = 1.55 (95% CI 0.58–4.12) Prevalence = 0% for homozygotes | Prevalence = 3% OR = 1.21 (95% CI 0.22–5.94) Prevalence = 0% for homozygotes |
| Onrat et al., 2012 [58] | Turkey | 140 MI | 42.04 y 57.19 y 60.7% M | Prevalence = 0.20% p = 0.293 (MI vs. healty controls) p = 0.741 (MI ≤ 45 y vs. MI > 45 y) Prevalence = 0% for homozygotes | Prevalence = 14.29% p = 0.469 (MI vs. healty controls) p = 0.535 (MI ≤ 45 y vs. MI > 45 y) Prevalence = 0% for homozygotes |
| Gonchar et al., 2012 | Belarus | 175 MI | NR | Prevalence = 6.9% OR = 2.41 (95% CI 0.96–6.02) p = 0.05 for heterozygotes Prevalence = 0% for homozygotes | Prevalence = 2.3% OR = 2.08 (95% CI 0.46–9.42) p = 0.56 for heterozygotes Prevalence = 0% for homozygotes |
| Sode et al., 2013 [18] | Denmark | 2708 MI | ≥20 y | OR = 1.0 (95% CI 0.9–1.2) for heterozygous OR = 0.9 (95% CI 0.4–2.1) for homozygous | OR = 1.3 (95% CI 1.0–1.7) for heterozygous No event among homozygous |
| Vaccarino et al., 2013 [74] | Italy | 60 MI | ≤46 y 100% M | NR | OR = 1.26 (95% CI 0.35–4.46) for heterozygous OR = 1.09 (95% CI 0.10–12.21) for homozygous OR = 1.22 (95% CI 0.39–3.82) for all carriers |
| Ezzat et al., 2014 [75] | Egypt | 30 MI | 45.5 y 80% M | Prevalence = 26.7% for heterozygous Prevalence = 3.3% for homozygous p < 0.05 for association with MI | NR |
| Kaur et al., 2014 [76] | India | 184 MI 166 MI | 36.4 y 96.2% M 64.1 y 74.7% M | Prevalence = 7.1% Prevalence = 3% OR = 2.4 (95% CI 0.9–7.0) | NR |
| Alkhiary et al., 2015 [61] | Egypt | 31 MI | 34.16 y 90.3% M | Prevalence = 51.6% for heterozygous OR = 6.48 (95% CI 1.56–26.84) Prevalence = 3.2% for homozygous p = 0.469 for association with MI | Prevalence = 45.2% for heterozygous OR = 4.6 (95% CI 1.13–19.24) Prevalence = 0% for homozygous |
| Hmimech et al., 2016 [54] | Morocco | 100 MI | 58.6 y 70% M | NR | Prevalence = 59% for heterozygous OR = 32.73 (95% CI 15.11–69.71) p < 0.001 for association with MI Prevalence = 10% for homozygous OR = 115 (95% CI 1.75–7332) p = 0.003 for association with MI |
| Rallidis et al., 2017 [57] | Greece | 255 MI | <36 y 87.8% M | Prevalence = 7.8% for heterozygous p = 0.512 for association with MI Prevalence = 0% for homozygous | Prevalence = 7.4% for heterozygous OR = 2.24 (95% CI 1.10–4.25) Prevalence = 0% for homozygous |
| Milgrom et al., 2017 [77] | USA | 153 79% MI | ≤45 y 73.8% M | Prevalence = 7% p = 0.88 for association with MI | Prevalence = 3% No association with MI |
| El-Fatah et al., 2018 [78] | Egypt | 120 MI | 57.5 y 51.7% M | NR | Prevalence = 3.3% for heterozygous OR = 4.67 (95% CI 0.24–88.30) p = 0.303 for association with MI Prevalence = 0% for homozygous |
| Mohammed et al., 2018 [79] | Iraq | 56 ACS | <50 y 96.4% M | Prevalence = 1.8% | Prevalence = 1.8% |
| Amara et al., 2018 [45] | Tunis | 200 51% MI | 62.71 y 61.5% M | Prevalence = 17.6% OR = 0.92 (95% CI 0.42–1.99), p = 0.86 (CAD + MI vs. CAD-MI) OR = 4.23 (95% CI 1.29–13.8), p = 0.02 (CAD + MI vs. control) | NR |
| Stepien et al., 2019 [36] | Poland | 84 MINOCA | 45.5 y 60.7% M | Prevalence = 14.3% | Prevalence = 4.8% |
| Msalati et al., 2021 [80] | Libya | 69 MI | 53.70 y NR | Prevalence = 3.27% p = 0.5 for association with MI | Prevalence = 2.5% p = 0.36 for association with MI |
| Golestani et al., 2022 [81] | Iran | 150 MI | 51.78 y 51.3% M | OR = 5.17 (95% CI 0.59–44.8) for heterozygous, p = 0.13 for association with MI OR = 1.6 (95% CI 0.00) for homozygous, p = 0.99 for association with MI | NR |
| Author, Year | Country | Cases | Outcome, Follow-Up Duration (Mean) | FV Leiden in Patients with Recurrent Events | Prothrombin G20210A in Patients with Recurrent Events | |
|---|---|---|---|---|---|---|
| No. | Age (Mean) % Sex | |||||
| Mannuci et al., 2006 [67] | Italy | 520 ACS 54.2% MI | 67 y 72.1% M | MACE—death, MI, or target lesion revascularisation 22.2 months | Prevalence = 3.7% p = 0.8 for association with MACE | Prevalence = 3.7% p = 0.8 for association with MACE |
| Van der Krabben et al., 2008 [43] | Netherlands | 542 MI | 56 y M | MACE—MI, UA, PTCA, CABG surgery and death due to coronary events 11 years | RR = 1.0 (95% CI 0.6–1.6) | RR = 1.8 (95% CI 0.8–4.1) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montagnana, M.; Lippi, G.; Danese, E. An Overview of Thrombophilia and Associated Laboratory Testing. Methods Mol. Biol. 2017, 1646, 113–135. [Google Scholar] [CrossRef]
- Ornstein, D.L.; Cushman, M. Cardiology patient page. Factor V Leiden. Circulation 2003, 107, e94–e97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eppenberger, D.; Nilius, H.; Anagnostelis, B.; Huber, C.A.; Nagler, M. Current Knowledge on Factor V Leiden Mutation as a Risk Factor for Recurrent Venous Thromboembolism: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 883986. [Google Scholar] [CrossRef] [PubMed]
- Elkattawy, S.; Alyacoub, R.; Singh, K.S.; Fichadiya, H.; Kessler, W. Prothrombin G20210A Gene Mutation-Induced Recurrent Deep Vein Thrombosis and Pulmonary Embolism: Case Report and Literature Review. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096211058486. [Google Scholar] [CrossRef]
- Smith, N.L.; Bis, J.C.; Biagiotti, S.; Rice, K.; Lumley, T.; Kooperberg, C.; Wiggins, K.L.; Heckbert, S.R.; Psaty, B.M. Variation in 24 hemostatic genes and associations with non-fatal myocardial infarction and ischemic stroke. J. Thromb. Haemost. 2008, 6, 45–53. [Google Scholar] [CrossRef]
- Casas, J.P.; Hingorani, A.D.; Bautista, L.E.; Sharma, P. Meta-analysis of genetic studies in ischemic stroke: Thirty-two genes involving approximately 18,000 cases and 58,000 controls. Arch. Neurol. 2004, 61, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- De Paula Sabino, A.; Ribeiro, D.D.; Carvalho, M.; Cardoso, J.; Dusse, L.M.; Fernandes, A.P. Factor V Leiden and increased risk for arterial thrombotic disease in young Brazilian patients. Blood Coagul. Fibrinolysis 2006, 17, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Hamedani, A.G.; Cole, J.W.; Mitchell, B.D.; Kittner, S.J. Meta-analysis of factor V Leiden and ischemic stroke in young adults: The importance of case ascertainment. Stroke 2010, 41, 1599–1603. [Google Scholar] [CrossRef] [Green Version]
- Alhazzani, A.A.; Kumar, A.; Selim, M. Association between Factor V Gene Polymorphism and Risk of Ischemic Stroke: An Updated Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2018, 27, 1252–1261. [Google Scholar] [CrossRef]
- Juul, K.; Tybjaerg-Hansen, A.; Steffensen, R.; Kofoed, S.; Jensen, G.; Nordestgaard, B.G. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood 2002, 100, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Hennekens, C.H.; Lindpaintner, K.; Stampfer, M.J.; Eisenberg, P.R.; Miletich, J.P. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N. Engl. J. Med. 1995, 332, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Zeb, M.; Kondapaneni, V.; Gutlapalli, S.D.; Choudhari, J.; Sodiya, O.T.; Toulassi, I.A.; Cancarevic, I. Association of G20210A Prothrombin Gene Mutation and Cerebral Ischemic Stroke in Young Patients. Cureus 2020, 12, e11984. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Miletich, J.P. G20210A mutation in prothrombin gene and risk of myocardial infarction, stroke, and venous thrombosis in a large cohort of US men. Circulation 1999, 99, 999–1004. [Google Scholar] [CrossRef] [Green Version]
- Doggen, C.J.; Cats, V.M.; Bertina, R.M.; Rosendaal, F.R. Interaction of coagulation defects and cardiovascular risk factors: Increased risk of myocardial infarction associated with factor V Leiden or prothrombin 20210A. Circulation 1998, 97, 1037–1041. [Google Scholar] [CrossRef]
- Van de Water, N.S.; French, J.K.; Lund, M.; Hyde, T.A.; White, H.D.; Browett, P.J. Prevalence of factor V Leiden and prothrombin variant G20210A in patients age <50 years with no significant stenoses at angiography three to four weeks after myocardial infarction. J. Am. Coll. Cardiol. 2000, 36, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Gowda, M.S.; Zucker, M.L.; Vacek, J.L.; Carriger, W.L.; Van Laeys, D.L.; Rachel, J.M.; Strope, B.D. Incidence of factor V Leiden in patients with acute myocardial infarction. J. Thromb. Thrombolysis 2000, 9, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Grassi, M.; Assanelli, D.; Mozzini, C.; Albertini, F.; Salvadori, G.; Archetti, S.; Negrini, R.; Galeazzi, G.; Pezzini, A. Modeling premature occurrence of acute coronary syndrome with atherogenic and thrombogenic risk factors and gene markers in extended families. J. Thromb. Haemost. 2005, 3, 2238–2244. [Google Scholar] [CrossRef]
- Sode, B.F.; Allin, K.H.; Dahl, M.; Gyntelberg, F.; Nordestgaard, B.G. Risk of venous thromboembolism and myocardial infarction associated with factor V Leiden and prothrombin mutations and blood type. CMAJ 2013, 185, E229–E237. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.; Liu, E.H.; Higgins, J.P.; Keavney, B.D.; Lowe, G.D.; Collins, R.; Danesh, J. Seven haemostatic gene polymorphisms in coronary disease: Meta-analysis of 66,155 cases and 91,307 controls. Lancet 2006, 367, 651–658. [Google Scholar] [CrossRef]
- Hoffman, M.; Monroe, D.M. Coagulation 2006: A modern view of hemostasis. Hematol. Oncol. Clin. N. Am. 2007, 21, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Dahlback, B. Novel insights into the regulation of coagulation by factor V isoforms, tissue factor pathway inhibitoralpha, and protein S. J. Thromb. Haemost. 2017, 15, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Dickerman, J.D. Hereditary thrombophilia. Thromb. J. 2006, 4, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozmen, F.; Ozmen, M.M.; Ozalp, N.; Akar, N. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil. Cerrahi. Derg. 2009, 15, 113–119. [Google Scholar] [PubMed]
- Settin, A.; Dowaidar, M.; El-Baz, R.; Abd-Al-Samad, A.; El-Sayed, I.; Nasr, M. Frequency of factor V Leiden mutation in Egyptian cases with myocardial infarction. Hematology 2008, 13, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; Rosendaal, F.R.; Psaty, B.M.; Cook, E.F.; Valliere, J.; Kuller, L.H.; Tracy, R.P. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: Results from the Cardiovascular Health Study. Thromb. Haemost. 1998, 79, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, N.; Trabetti, E.; Pinotti, M.; Olivieri, O.; Sandri, M.; Friso, S.; Pizzolo, F.; Bozzini, C.; Caruso, P.P.; Cavallari, U.; et al. Combined effect of hemostatic gene polymorphisms and the risk of myocardial infarction in patients with advanced coronary atherosclerosis. PLoS ONE 2008, 3, e1523. [Google Scholar] [CrossRef] [Green Version]
- Tomaiuolo, R.; Bellia, C.; Caruso, A.; Di Fiore, R.; Quaranta, S.; Noto, D.; Cefalu, A.B.; Di Micco, P.; Zarrilli, F.; Castaldo, G.; et al. Prothrombotic gene variants as risk factors of acute myocardial infarction in young women. J. Transl. Med. 2012, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Forte, G.I.; Vaccarino, L.; Palmeri, M.; Branzi, A.; Caldarera, C.M.; Scola, L.; Caruso, C.; Licastro, F.; Lio, D. Analysis of polymorphisms Leiden Factor V G1691A and prothrombin G20210A as risk factors for acute myocardial infarction. Biogerontology 2011, 12, 485–490. [Google Scholar] [CrossRef] [Green Version]
- Rallidis, L.S.; Xenogiannis, I.; Brilakis, E.S.; Bhatt, D.L. Causes, Angiographic Characteristics, and Management of Premature Myocardial Infarction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 2431–2449. [Google Scholar] [CrossRef]
- Rosendaal, F.R.; Siscovick, D.S.; Schwartz, S.M.; Beverly, R.K.; Psaty, B.M.; Longstreth, W.T., Jr.; Raghunathan, T.E.; Koepsell, T.D.; Reitsma, P.H. Factor V Leiden (resistance to activated protein C) increases the risk of myocardial infarction in young women. Blood 1997, 89, 2817–2821. [Google Scholar] [CrossRef] [Green Version]
- Mannucci, P.M.; Asselta, R.; Duga, S.; Guella, I.; Spreafico, M.; Lotta, L.; Merlini, P.A.; Peyvandi, F.; Kathiresan, S.; Ardissino, D. The association of factor V Leiden with myocardial infarction is replicated in 1880 patients with premature disease. J. Thromb. Haemost. 2010, 8, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Berredjeb Ben Slama, D.; Fekih-Mrissa, N.; Haggui, A.; Nsiri, B.; Baraket, N.; Haouala, H.; Gritli, N. Lack of association between factor V Leiden and prothrombin G20210A polymorphisms in Tunisian subjects with a history of myocardial infarction. Cardiovasc. Pathol. 2013, 22, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, B.K.; Eriksson, N.; Vos, G.J.A.; Meijer, K.; Siegbahn, A.; James, S.; Wallentin, L.; Ten Berg, J.M. Factor V Leiden Does Not Modify the Phenotype of Acute Coronary Syndrome or the Extent of Myocardial Necrosis. J. Am. Heart Assoc. 2021, 10, e020025. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, D. Myocardial Infarction with Nonobstructive Coronary Arteries: A Call for Individualized Treatment. J. Am. Heart Assoc. 2019, 8, e013361. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.; Nowak, K.; Wypasek, E.; Zalewski, J.; Undas, A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: Comparison with cryptogenic stroke. Int. J. Cardiol. 2019, 290, 1–6. [Google Scholar] [CrossRef]
- Da Costa, A.; Tardy, B.; Haouchette, K.; Mismetti, P.; Cerisier, A.; Lamaud, M.; Guyotat, D.; Isaaz, K. Long term prognosis of patients with myocardial infarction and normal coronary angiography: Impact of inherited coagulation disorders. Thromb. Haemost. 2004, 91, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, A.; Isaaz, K.; Faure, E.; Mourot, S.; Cerisier, A.; Lamaud, M. Clinical characteristics, aetiological factors and long-term prognosis of myocardial infarction with an absolutely normal coronary angiogram; a 3-year follow-up study of 91 patients. Eur. Heart J. 2001, 22, 1459–1465. [Google Scholar] [CrossRef]
- Dacosta, A.; Tardy-Poncet, B.; Isaaz, K.; Cerisier, A.; Mismetti, P.; Simitsidis, S.; Reynaud, J.; Tardy, B.; Piot, M.; Decousus, H.; et al. Prevalence of factor V Leiden (APCR) and other inherited thrombophilias in young patients with myocardial infarction and normal coronary arteries. Heart 1998, 80, 338–340. [Google Scholar] [CrossRef]
- Merlo, A.C.; Troccolo, A.; Piredda, E.; Porto, I.; Gil Ad, V. Myocardial Infarction with Non-obstructive Coronary Arteries: Risk Factors and Associated Comorbidities. Front. Cardiovasc. Med. 2022, 9, 895053. [Google Scholar] [CrossRef]
- Mansourati, J.; Da Costa, A.; Munier, S.; Mercier, B.; Tardy, B.; Ferec, C.; Isaaz, K.; Blanc, J.J. Prevalence of factor V Leiden in patients with myocardial infarction and normal coronary angiography. Thromb. Haemost. 2000, 83, 822–825. [Google Scholar] [CrossRef]
- Vallus, G.; Dlustus, B.; Acsady, G.; Papp, Z.; Skopal, J.; Nagy, Z.; Prohaszka, Z.; Romics, L.; Karadi, I.; Nagy, B. Factor V Leiden and apolipoprotein E genotypes in severe femoropopliteal atherosclerosis with restenosis. Clin. Chim. Acta 2007, 377, 256–260. [Google Scholar] [CrossRef]
- Van der Krabben, M.D.; Rosendaal, F.R.; van der Bom, J.G.; Doggen, C.J. Polymorphisms in coagulation factors and the risk of recurrent cardiovascular events in men after a first myocardial infarction. J. Thromb. Haemost. 2008, 6, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodi, B.K.; Tragante, V.; Kleber, M.E.; Holmes, M.V.; Schmidt, A.F.; McCubrey, R.O.; Howe, L.J.; Direk, K.; Allayee, H.; Baranova, E.V.; et al. Association of Factor V Leiden with Subsequent Atherothrombotic Events: A GENIUS-CHD Study of Individual Participant Data. Circulation 2020, 142, 546–555. [Google Scholar] [CrossRef]
- Amara, A.; Mrad, M.; Sayeh, A.; Haggui, A.; Lahideb, D.; Fekih-Mrissa, N.; Haouala, H.; Nsiri, B. Association of FV G1691A Polymorphism but not A4070G With Coronary Artery Disease. Clin. Appl. Thromb. Hemost. 2018, 24, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K.; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, M.; Li, R.; Chen, N.; Pang, N.; Li, Y.; Deng, X.; Wang, L.; Luo, M.; Liu, Y.; Hao, H.; et al. Platelet-Derived Factor V Is a Critical Mediator of Arterial Thrombosis. J. Am. Heart Assoc. 2017, 6, e006345. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, B.K.; Eriksson, N.; Ross, S.; Claassens, D.M.F.; Asselbergs, F.W.; Meijer, K.; Siegbahn, A.; James, S.; Pare, G.; Wallentin, L.; et al. Factor V Leiden and the Risk of Bleeding in Patients with Acute Coronary Syndromes Treated with Antiplatelet Therapy: Pooled Analysis of 3 Randomized Clinical Trials. J. Am. Heart Assoc. 2021, 10, e021115. [Google Scholar] [CrossRef] [PubMed]
- Al-Amer, O.M. The role of thrombin in haemostasis. Blood Coagul. Fibrinolysis 2022, 33, 145–148. [Google Scholar] [CrossRef]
- Dziadosz, M.; Baxi, L.V. Global prevalence of prothrombin gene mutation G20210A and implications in women’s health: A systematic review. Blood Coagul. Fibrinolysis 2016, 27, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Burzotta, F.; Paciaroni, K.; De Stefano, V.; Crea, F.; Maseri, A.; Leone, G.; Andreotti, F. G20210A prothrombin gene polymorphism and coronary ischaemic syndromes: A phenotype-specific meta-analysis of 12 034 subjects. Heart 2004, 90, 82–86. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Bijsterveld, N.R.; Moons, A.H.; Levi, M.; Buller, H.R.; Peters, R.J. Genetic variation in coagulation and fibrinolytic proteins and their relation with acute myocardial infarction: A systematic review. Circulation 2001, 104, 3063–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hmimech, W.; Idrissi, H.H.; Diakite, B.; Baghdadi, D.; Korchi, F.; Habbal, R.; Nadifi, S. Association of C677T MTHFR and G20210A FII prothrombin polymorphisms with susceptibility to myocardial infarction. BioMed. Rep. 2016, 5, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Ren, H.; Chen, H.; Song, J.; Li, S.; Lee, C.; Liu, J.; Cui, Y. Prothrombin G20210A (rs1799963) polymorphism increases myocardial infarction risk in an age-related manner: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 13550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burzotta, F.; Paciaroni, K.; De Stefano, V.; Chiusolo, P.; Manzoli, A.; Casorelli, I.; Leone, A.M.; Rossi, E.; Leone, G.; Maseri, A.; et al. Increased prevalence of the G20210A prothrombin gene variant in acute coronary syndromes without metabolic or acquired risk factors or with limited extent of disease. Eur. Heart J. 2002, 23, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Rallidis, L.S.; Gialeraki, A.; Tsirebolos, G.; Tsalavoutas, S.; Rallidi, M.; Iliodromitis, E. Prothrombotic genetic risk factors in patients with very early ST-segment elevation myocardial infarction. J. Thromb. Thrombolysis 2017, 44, 267–273. [Google Scholar] [CrossRef]
- Onrat, S.T.; Akci, O.; Soylemez, Z.; Onrat, E.; Avsar, A. Prevalence of myocardial infarction polymorphisms in Afyonkarahisar, Western Turkey. Mol. Biol. Rep. 2012, 39, 9257–9264. [Google Scholar] [CrossRef]
- Rosendaal, F.R.; Siscovick, D.S.; Schwartz, S.M.; Psaty, B.M.; Raghunathan, T.E.; Vos, H.L. A common prothrombin variant (20210 G to A) increases the risk of myocardial infarction in young women. Blood 1997, 90, 1747–1750. [Google Scholar] [CrossRef] [Green Version]
- Hindorff, L.A.; Psaty, B.M.; Carlson, C.S.; Heckbert, S.R.; Lumley, T.; Smith, N.L.; Lemaitre, R.N.; Rieder, M.J.; Nickerson, D.A.; Reiner, A.P. Common genetic variation in the prothrombin gene, hormone therapy, and incident nonfatal myocardial infarction in postmenopausal women. Am. J. Epidemiol. 2006, 163, 600–607. [Google Scholar] [CrossRef] [Green Version]
- Alkhiary, W.; Azzam, H.; Yossof, M.M.; Aref, S.; Othman, M.; El-Sharawy, S. Association of Hemostatic Gene Polymorphisms with Early-Onset Ischemic Heart Disease in Egyptian Patients. Clin. Appl. Thromb. Hemost. 2016, 22, 535–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roach, R.E.; Roshani, S.; Meijer, K.; Hamulyak, K.; Lijfering, W.M.; Prins, M.H.; Buller, H.R.; Middeldorp, S. Risk of cardiovascular disease in double heterozygous carriers and homozygous carriers of F5 R506Q (factor V Leiden) and F2 (prothrombin) G20210A: A retrospective family cohort study. Br. J. Haematol. 2011, 153, 134–136. [Google Scholar] [CrossRef]
- Burzotta, F.; Leone, A.M.; Paciaroni, K.; De Stefano, V.; Rossi, E.; Testa, L.; Giannico, F.; Leone, G.; Maseri, A.; Crea, F.; et al. G20210A prothrombin gene variant and clinical outcome in patients with a first acute coronary syndrome. Haematologica 2004, 89, 1134–1138. [Google Scholar]
- Emiroglu, O.; Durdu, S.; Egin, Y.; Akar, A.R.; Alakoc, Y.D.; Zaim, C.; Ozyurda, U.; Akar, N. Thrombotic gene polymorphisms and postoperative outcome after coronary artery bypass graft surgery. J. Cardiothorac. Surg. 2011, 6, 120. [Google Scholar] [CrossRef] [Green Version]
- Angeline, T.; Bentley, H.A.; Hawk, A.B.; Manners, R.J.; Mokashi, H.A.; Jeyaraj, N.; Tsongalis, G.J. Prevalence of the Factor V G1691A and the Factor II/prothrombin G20210A gene polymorphisms among Tamilians. Exp. Mol. Pathol. 2005, 79, 9–13. [Google Scholar] [CrossRef]
- Zee, R.Y.; Cook, N.R.; Cheng, S.; Erlich, H.A.; Lindpaintner, K.; Ridker, P.M. Multi-locus candidate gene polymorphisms and risk of myocardial infarction: A population-based, prospective genetic analysis. J. Thromb. Haemost. 2006, 4, 341–348. [Google Scholar] [CrossRef]
- Marcucci, R.; Brogi, D.; Sofi, F.; Giglioli, C.; Valente, S.; Liotta, A.A.; Lenti, M.; Gori, A.M.; Prisco, D.; Abbate, R.; et al. PAI-1 and homocysteine, but not lipoprotein (a) and thrombophilic polymorphisms, are independently associated with the occurrence of major adverse cardiac events after successful coronary stenting. Heart 2006, 92, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Morgan, T.M.; Krumholz, H.M.; Lifton, R.P.; Spertus, J.A. Nonvalidation of reported genetic risk factors for acute coronary syndrome in a large-scale replication study. JAMA 2007, 297, 1551–1561. [Google Scholar] [CrossRef] [Green Version]
- Celik, M.; Altintas, A.; Celik, Y.; Karabulut, A.; Ayyildiz, O. Thrombophilia in young patients with acute myocardial infarction. Saudi Med. J. 2008, 29, 48–54. [Google Scholar]
- Pestana, C.I.; Torres, A.; Blanco, S.; Rojas, M.J.; Mendez, C.; Lopez, J.L.; de Bosch, N.B.; Porco, A. Factor V Leiden and the risk of venous thrombosis, myocardial infarction, and stroke: A case-control study in Venezuela. Genet. Test. Mol. Biomark. 2009, 13, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Weischer, M.; Juul, K.; Zacho, J.; Jensen, G.B.; Steffensen, R.; Schroeder, T.V.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Prothrombin and risk of venous thromboembolism, ischemic heart disease and ischemic cerebrovascular disease in the general population. Atherosclerosis 2010, 208, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Dogra, R.K.; Das, R.; Ahluwalia, J.; Kumar, R.M.; Talwar, K.K. Prothrombotic gene polymorphisms and plasma factors in young North Indian survivors of acute myocardial infarction. J. Thromb. Thrombolysis 2012, 34, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Himabindu, G.; Rajasekhar, D.; Latheef, K.; Sarma, P.V.; Vanajakshamma, V.; Chaudhury, A.; Bitla, A.R. Factor V Leiden mutation is not a predisposing factor for acute coronary syndromes. Indian Heart J. 2012, 64, 570–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaccarino, L.; Vitale, S.; Caruso, M.; Palmeri, M.; Scola, L.; Bova, M.; Caruso, C.; Massenti, M.F.; Vitale, F.; Novo, S.; et al. Myocardial infarction marker levels are influenced by prothrombin and tumor necrosis factor-alpha gene polymorphisms in young patients. Cytokine 2013, 61, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Ezzat, H.; Attia, F.A.; Mokhtar, A.; El-Tokhy, H.M.; Alalfy, M.N.; Elkhouly, N.Y. Prevalence of thrombophilic gene polymorphisms (FVL G1691A and MTHFR C677T) in patients with myocardial infarction. Egypt. J. Med. Hum. Genet. 2014, 15, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Das, R.; Ahluwalia, J.; Kumar, R.M.; Talwar, K.K. Genetic polymorphisms, Biochemical Factors, and Conventional Risk Factors in Young and Elderly North Indian Patients with Acute Myocardial Infarction. Clin. Appl. Thromb. Hemost. 2016, 22, 178–183. [Google Scholar] [CrossRef]
- Milgrom, A.; Lee, K.; Rothschild, M.; Makadia, F.; Duhon, G.; Min, S.; Wang, P.; Glueck, C.J. Thrombophilia in 153 Patients with Premature Cardiovascular Disease </=Age 45. Clin. Appl. Thromb. Hemost. 2018, 24, 295–302. [Google Scholar] [CrossRef] [Green Version]
- El-Fattah, A.A.A.; Sadik, N.A.H.; Sedrak, H.; Battah, A.; Nabil, M. Association of genetic variants of hemostatic genes with myocardial infarction in Egyptian patients. Gene 2018, 641, 212–219. [Google Scholar] [CrossRef]
- Mohammed, W.J.; Al-Musawi, B.M.S.; Oberkanins, C.; Puhringer, H. Molecular assessment of some cardiovascular genetic risk factors among Iraqi patients with ischemic heart diseases. Int. J. Health Sci. 2018, 12, 44–50. [Google Scholar]
- Msalati, A.; Bashein, A.; Ghrew, M.; Khalil, I.; Sedaa, K.; Ali, A.; Zaid, A. Association of venous thromboembolism and myocardial infarction with Factor V Leiden and Factor II gene mutations among Libyan patients. Libyan J. Med. 2021, 16, 1857525. [Google Scholar] [CrossRef]
- Golestani, A.; Rahimi, A.; Moridi, N.; Anani-Sarab, G.; Salmani, F.; Dastjerdi, K.; Azdaki, N.; Sajjadi, S.M. Association of factor V Leiden R506Q, FXIIIVal34Leu, and MTHFR C677T polymorphisms with acute myocardial infarction. Egypt. J. Med. Hum. Genet. 2022, 23, 118. [Google Scholar] [CrossRef]
- Guella, I.; Duga, S.; Ardissino, D.; Merlini, P.A.; Peyvandi, F.; Mannucci, P.M.; Asselta, R. Common variants in the haemostatic gene pathway contribute to risk of early-onset myocardial infarction in the Italian population. Thromb. Haemost. 2011, 106, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Dostalova, G.; Belohlavek, J.; Hlubocka, Z.; Bayerova, K.; Bobcikova, P.; Kvasnicka, T.; Kvasnicka, J.; Linhart, A.; Karetova, D. Multiple thrombophilia mutations as a possible cause of premature myocardial infarction. Wien. Klin. Wochenschr. 2017, 129, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Tutun, U.; Aksoyek, A.; Ulus, A.T.; Misirlioglu, M.; Cicekcioglu, F.; Ozisik, K.; Ihsan Parlar, A.; Baran Budak, A.; Gedik, S.; Katircioglu, S.F. Gene polymorphisms in patients below 35 years of age who underwent coronary artery bypass surgery. Coron. Artery Dis. 2006, 17, 35–39. [Google Scholar] [CrossRef]
- Abou-Ismail, M.Y.; Citla Sridhar, D.; Nayak, L. Estrogen and thrombosis: A bench to bedside review. Thromb. Res. 2020, 192, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Lidegaard, O.; Lokkegaard, E.; Jensen, A.; Skovlund, C.W.; Keiding, N. Thrombotic stroke and myocardial infarction with hormonal contraception. N. Engl. J. Med. 2012, 366, 2257–2266. [Google Scholar] [CrossRef] [Green Version]
- Tanis, B.C.; van den Bosch, M.A.; Kemmeren, J.M.; Cats, V.M.; Helmerhorst, F.M.; Algra, A.; van der Graaf, Y.; Rosendaal, F.R. Oral contraceptives and the risk of myocardial infarction. N. Engl. J. Med. 2001, 345, 1787–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosendaal, F.R.; Van Hylckama Vlieg, A.; Tanis, B.C.; Helmerhorst, F.M. Estrogens, progestogens and thrombosis. J. Thromb. Haemost. 2003, 1, 1371–1380. [Google Scholar] [CrossRef]
- Psaty, B.M.; Smith, N.L.; Lemaitre, R.N.; Vos, H.L.; Heckbert, S.R.; LaCroix, A.Z.; Rosendaal, F.R. Hormone replacement therapy, prothrombotic mutations, and the risk of incident nonfatal myocardial infarction in postmenopausal women. JAMA 2001, 285, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Kolitz, T.; Shiber, S.; Sharabi, I.; Winder, A.; Zandman-Goddard, G. Cardiac Manifestations of Antiphospholipid Syndrome with Focus on Its Primary Form. Front. Immunol. 2019, 10, 941. [Google Scholar] [CrossRef]
- Gotsman, I.; Mosseri, M. Acute myocardial infarction in a young women with normal coronary arteries and a combined thrombophilia. Int. J. Cardiol. 2005, 99, 483–484. [Google Scholar] [CrossRef]
- Stammers, A.H.; Dorion, R.P.; Trowbridge, C.; Yen, B.; Klayman, M.; Murdock, J.D.; Woods, E.; Gilbert, C. Coagulation management of a patient with factor V Leiden mutation, lupus anticoagulant, and activated protein C resistance: A case report. Perfusion 2005, 20, 115–120. [Google Scholar] [CrossRef]
- Urbanus, R.T.; Siegerink, B.; Roest, M.; Rosendaal, F.R.; de Groot, P.G.; Algra, A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: A case-control study. Lancet Neurol. 2009, 8, 998–1005. [Google Scholar] [CrossRef]
- Agosti, P.; Mancini, I.; Sadeghian, S.; Pagliari, M.T.; Abbasi, S.H.; Pourhosseini, H.; Boroumand, M.; Lotfi-Tokaldany, M.; Pappalardo, E.; Maino, A.; et al. Factor V Leiden but not the factor II 20210G>A mutation is a risk factor for premature coronary artery disease: A case-control study in Iran. Res. Pract. Thromb. Haemost. 2023, 7, 100048. [Google Scholar] [CrossRef]
- Mahmoodi, B.K.; Veeger, N.J.; Middeldorp, S.; Lijfering, W.M.; Brouwer, J.L.; Ten Berg, J.; Hamulyak, K.; Meijer, K. Interaction of Hereditary Thrombophilia and Traditional Cardiovascular Risk Factors on the Risk of Arterial Thromboembolism: Pooled Analysis of Four Family Cohort Studies. Circ. Cardiovasc. Genet. 2016, 9, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Kaykcoglu, M.; Hasdemir, C.; Eroglu, Z.; Kosova, B.; Can, L.H.; Ildizli, M.; Yavuzgil, O.; Payzin, S.; Turkoglu, C. Homozygous factor V Leiden mutation in two siblings presenting with acute myocardial infarction: A rare cause of myocardial infarction in the young. Blood Coagul. Fibrinolysis 2005, 16, 281–286. [Google Scholar] [CrossRef]
- Kim, R.J.; Becker, R.C. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: A meta-analysis of published studies. Am. Heart J. 2003, 146, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Dowaidar, M.; Settin, A. Risk of myocardial infarction related to factor V Leiden mutation: A meta-analysis. Genet. Test. Mol. Biomarkers 2010, 14, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Middendorf, K.; Gohring, P.; Huehns, T.Y.; Seidel, D.; Steinbeck, G.; Nikol, S. Prevalence of resistance against activated protein C resulting from factor V Leiden is significantly increased in myocardial infarction: Investigation of 507 patients with myocardial infarction. Am. Heart J. 2004, 147, 897–904. [Google Scholar] [CrossRef]
- Morton, N.E. Analysis of family resemblance. I. Introduction. Am. J. Hum. Genet. 1974, 26, 318–330. [Google Scholar] [PubMed]
- Jin, B.; Li, Y.; Ge-Shang, Q.Z.; Ni, H.C.; Shi, H.M.; Shen, W. Varied association of prothrombin G20210A polymorphism with coronary artery disease susceptibility in different ethnic groups: Evidence from 15,041 cases and 21,507 controls. Mol. Biol. Rep. 2011, 38, 2371–2376. [Google Scholar] [CrossRef]
- Pelliccia, F.; Pepine, C.J.; Berry, C.; Camici, P.G. The role of a comprehensive two-step diagnostic evaluation to unravel the pathophysiology of MINOCA: A review. Int. J. Cardiol. 2021, 336, 1–7. [Google Scholar] [CrossRef]
- Habash, F.; Vallurupalli, S. Challenges in management of left ventricular thrombus. Ther. Adv. Cardiovasc. Dis. 2017, 11, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Ucar, F.; Celik, S.; Ovali, E.; Karti, S.S.; Pakdemir, A.; Yilmaz, M.; Onder, E. Coexistence of prothrombic risk factors and its relation to left ventricular thrombus in acute myocardial infarction. Acta Cardiol. 2004, 59, 33–39. [Google Scholar] [CrossRef]
- Celik, S.; Ovali, E.; Baykan, M.; Ucar, F.; Erdol, C.; Durmus, I.; Kaplan, S. Factor V Leiden and its relation to left ventricular thrombus in acute myocardial infarction. Acta Cardiol. 2001, 56, 1–6. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Zhang, S.; Taylor, A.K.; Huang, X.; Luo, B.; Spector, E.B.; Fang, P.; Richards, C.S.; Committee, A.L.Q.A. Venous thromboembolism laboratory testing (factor V Leiden and factor II c.*97G>A), 2018 update: A technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2018, 20, 1489–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreotti, F.; Becker, R.C. Atherothrombotic disorders: New insights from hematology. Circulation 2005, 111, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Kujovich, J.L. Factor V Leiden thrombophilia. Genet. Med. 2011, 13, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Krawczak, M.; Nikolaus, S.; von Eberstein, H.; Croucher, P.J.; El Mokhtari, N.E.; Schreiber, S. PopGen: Population-based recruitment of patients and controls for the analysis of complex genotype-phenotype relationships. Community Genet. 2006, 9, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Satra, M.; Samara, M.; Wozniak, G.; Tzavara, C.; Kontos, A.; Valotassiou, V.; Vamvakopoulos, N.K.; Tsougos, I.; Aleporou-Marinou, V.; Patrinos, G.P.; et al. Sequence variations in the FII, FV, F13A1, FGB and PAI-1 genes are associated with differences in myocardial perfusion. Pharmacogenomics 2011, 12, 195–203. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badescu, M.C.; Butnariu, L.I.; Costache, A.D.; Gheorghe, L.; Seritean Isac, P.N.; Chetran, A.; Leancă, S.A.; Afrăsânie, I.; Duca, Ș.-T.; Gorduza, E.V.; et al. Acute Myocardial Infarction in Patients with Hereditary Thrombophilia—A Focus on Factor V Leiden and Prothrombin G20210A. Life 2023, 13, 1371. https://doi.org/10.3390/life13061371
Badescu MC, Butnariu LI, Costache AD, Gheorghe L, Seritean Isac PN, Chetran A, Leancă SA, Afrăsânie I, Duca Ș-T, Gorduza EV, et al. Acute Myocardial Infarction in Patients with Hereditary Thrombophilia—A Focus on Factor V Leiden and Prothrombin G20210A. Life. 2023; 13(6):1371. https://doi.org/10.3390/life13061371
Chicago/Turabian StyleBadescu, Minerva Codruta, Lăcrămioara Ionela Butnariu, Alexandru Dan Costache, Liliana Gheorghe, Petronela Nicoleta Seritean Isac, Adriana Chetran, Sabina Andreea Leancă, Irina Afrăsânie, Ștefania-Teodora Duca, Eusebiu Vlad Gorduza, and et al. 2023. "Acute Myocardial Infarction in Patients with Hereditary Thrombophilia—A Focus on Factor V Leiden and Prothrombin G20210A" Life 13, no. 6: 1371. https://doi.org/10.3390/life13061371
APA StyleBadescu, M. C., Butnariu, L. I., Costache, A. D., Gheorghe, L., Seritean Isac, P. N., Chetran, A., Leancă, S. A., Afrăsânie, I., Duca, Ș.-T., Gorduza, E. V., Costache, I. I., & Rezus, C. (2023). Acute Myocardial Infarction in Patients with Hereditary Thrombophilia—A Focus on Factor V Leiden and Prothrombin G20210A. Life, 13(6), 1371. https://doi.org/10.3390/life13061371











