Current Research on the Use of the Omental Flap in Breast Reconstruction and Post-Mastectomy Lymphedema: A Focus on Omental-Vascularized Lymph Node Transfer
Abstract
:1. The History of the Omental-Based Breast Reconstruction
2. Autologous Breast Reconstruction versus Implant-Based Breast Reconstruction
3. Current Practices and Methods of Omental-Based Breast Reconstruction
3.1. Omental-Based Reconstruction as an Alternative Method in Autologous Reconstruction
3.2. Omental Fat-Augmented Free Flap Reconstruction
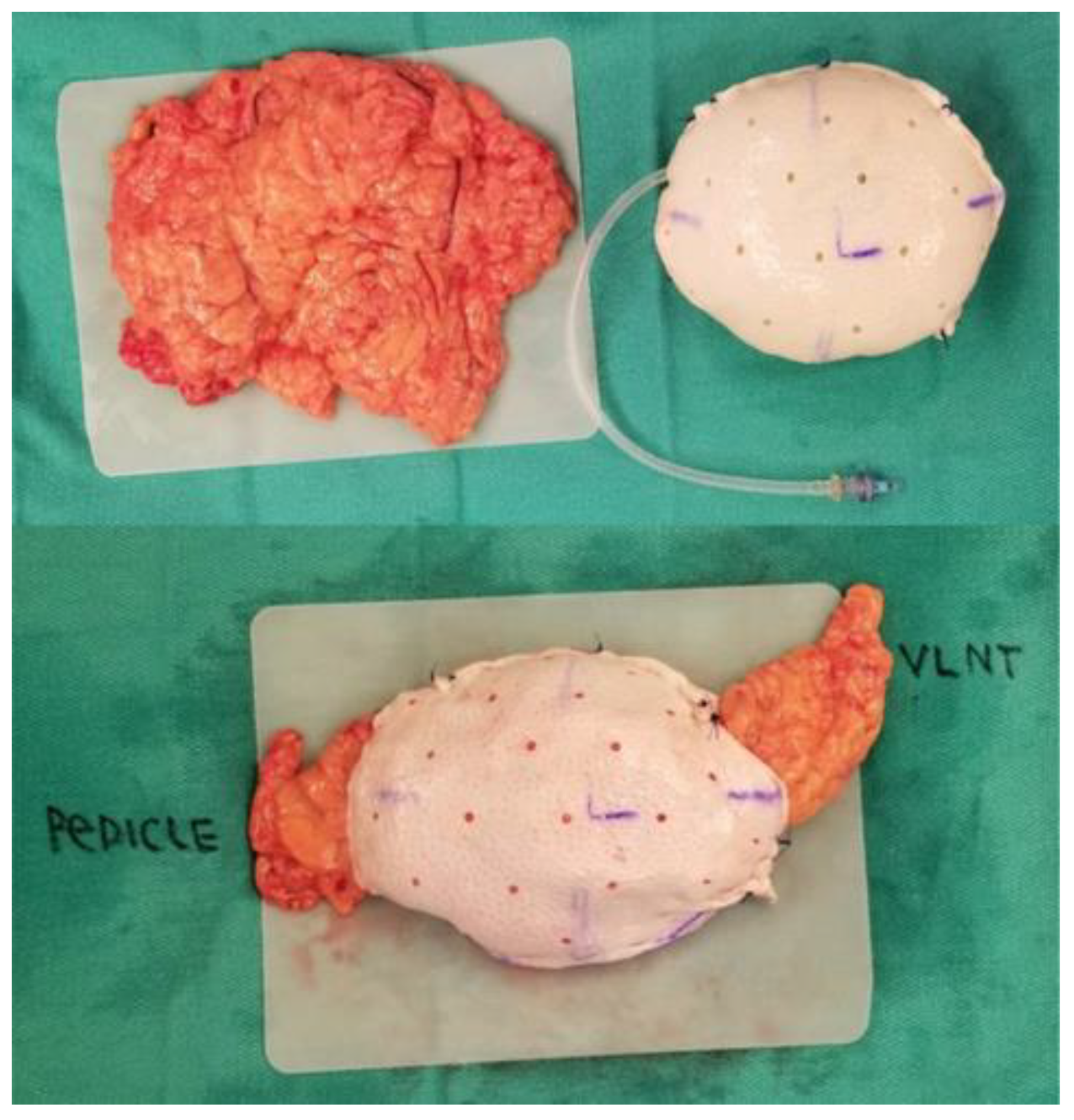
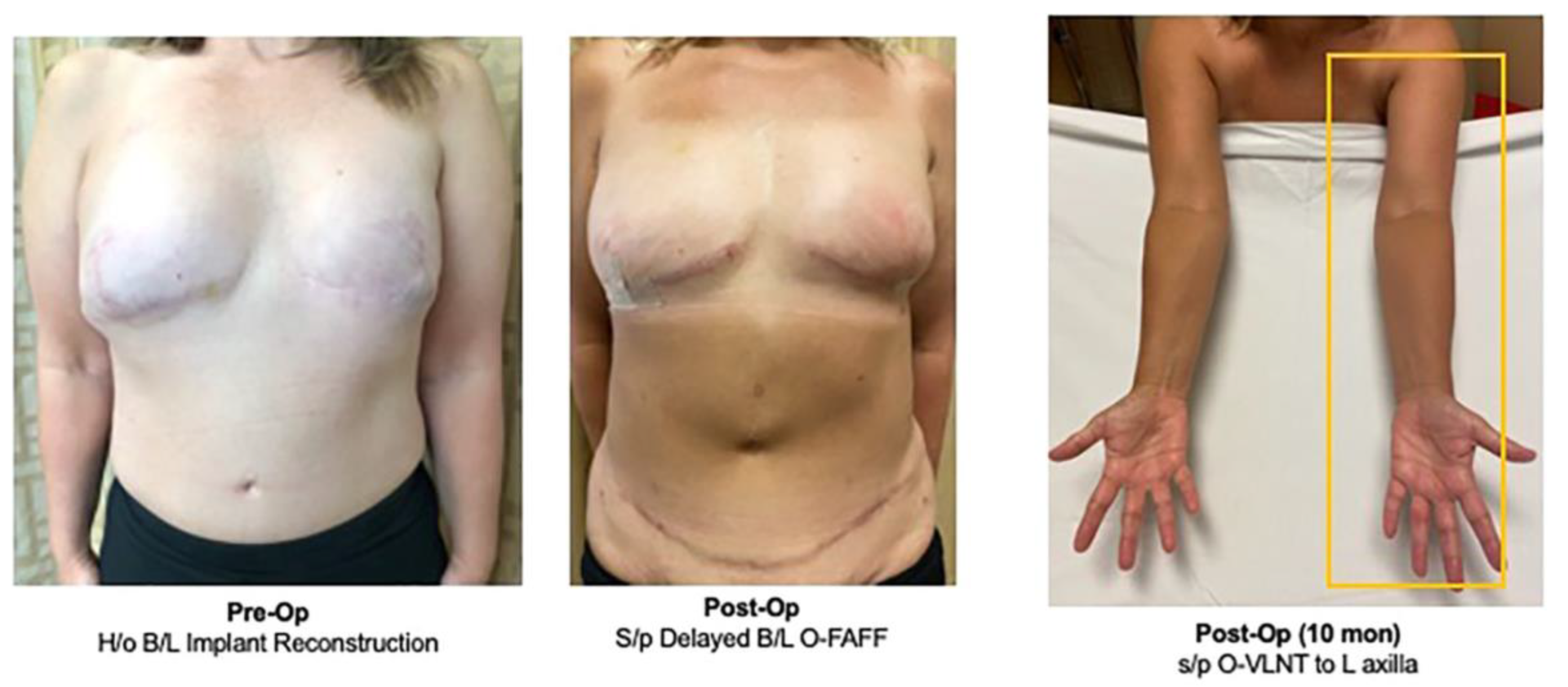
3.3. Concurrent Treatment for Lymphedema: Omental Vascularized Lymph Node Transfer
4. Current Challenges in Omental-Based Breast Reconstruction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Frey, J.D.; Yu, J.W.; Cohen, S.M.; Zhao, L.C.; Choi, M.; Levine, J.P. Robotically Assisted Omentum Flap Harvest: A Novel, Minimally Invasive Approach for Vascularized Lymph Node Transfer. Plast. Reconstr. Surg. Glob. Open. 2020, 8, e2505. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, J.; Yao, X.; Zhao, G.; Sun, F.; Sheng, W.; Lu, F.; Zhan, H.; Liu, C. Laparoscopic omental flap for the treatment of thoracic aortic graft infection: Report of two cases and review of the literature. J. Cardiothorac. Surg. 2020, 15, 120. [Google Scholar] [CrossRef]
- Settembre, N.; D’oria, M.; Saba, C.; Bouziane, Z.; Malikov, S. Negative Pressure Wound Therapy to Promote Fixation and Remodeling of Omental Flap in Patients with Revascularized Limbs: A Case Series. Ann. Vasc. Surg. 2018, 52, 313.e5–313.e8. [Google Scholar] [CrossRef]
- Shahzad, F.; Vaynrub, M.; Nelson, J.; Bott, M.; Mehrara, B. Omentum as a Vascular Carrier for Salvage Thoracic Spine Osseous Reconstruction. Plast. Reconstr. Surg. 2022, 150, 1364e–1366e. [Google Scholar] [CrossRef] [PubMed]
- Bruce, W.J.; Olla, D.R.; Feimster, J.W.; Woldanski, L.M.; Hart, K.D.; Reid, A.M.; Schwartz, B.D.; Berry, N.N. Transretroperitoneal Pedicled Omental Flap for Coverage of Traumatic Sacral Defect: A Case Report. Plast. Reconstr. Surg. Glob. Open. 2022, 10, e4298. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, V. Omentum a powerful biological source in regenerative surgery. Regen. Ther. 2019, 11, 182–191. [Google Scholar] [CrossRef]
- Liebermann-Meffert, D. The greater omentum: Anatomy, embryology, and surgical applications. Surg. Clin. N. Am. 2000, 80, 275–293. [Google Scholar] [CrossRef]
- Ma, I.T.; Deptual, P.; Nguyen, D.H. Evolution in breast reconstruction using the omentum. Ann. Breast Surg. 2021, 5, 18. [Google Scholar] [CrossRef]
- Kiricuta, I. The use of the great omentum in the surgery of breast cancer. Presse Med. 1963, 71, 15–17. [Google Scholar]
- Ni, C.; Zhu, Z.; Xin, Y.; Xie, Q.; Yuan, H.; Zhong, M.; Xia, W.; Zhu, X.; Lv, Z.; Song, X. Oncoplastic breast reconstruction with omental flap: A retrospective study and systematic review. J. Cancer 2018, 9, 1782–1790. [Google Scholar] [CrossRef] [Green Version]
- Kokosis, G.; Vorstenbosch, J.M.; Lombardi, A.B.; Shamsunder, M.G.M.; Mehrara, B.; Hespe, G.E.; Wang, L.; Brennan, C.W.; Ganly, I.M.; Matros, E.M. Use of the Omental Free Flap for Treatment of Chronic Anterior Skull Base Infections. Plast. Reconstr. Surg. Glob. Open. 2020, 8, e2988. [Google Scholar] [CrossRef] [PubMed]
- Nahabedian, M.Y.; Patel, K. Autologous flap breast reconstruction: Surgical algorithm and patient selection. J. Surg. Oncol. 2016, 113, 865–874. [Google Scholar] [CrossRef]
- Mohan, A.T.; Zhu, L.; Vijayasekaran, A.; Saint-Cyr, M. Autologous breast reconstruction in low body mass index patients. Clin. Plast. Surg. 2020, 47, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Namnoum, J.D. Indications and Controversies for Implant-Only Based Breast Reconstruction. Clin. Plast. Surg. 2018, 45, 47–54. [Google Scholar] [CrossRef] [PubMed]
- American Society of Plastic Surgery. Plastic Surgery Statistics Report: ASPS National Clearinghouse of Plastic Surgery Procedural Statistics. ASPS, 2020. Available online: https://www.plasticsurgery.org/news/plastic-surgery-statistics (accessed on 1 May 2023).
- Garvey, P.B.; Villa, M.T.; Rozanski, A.T.; Liu, J.; Robb, G.L.; Beahm, E.K. The advantages of free abdominal based flaps over implants for breast reconstruction in obese patients. Plast. Reconstr. Surg. 2012, 130, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Toyserkani, N.M.; Jørgensen, M.G.; Tabatabaeifar, S.; Damsgaard, T.; Sørensen, J.A. Autologous versus implant-based breast reconstruction: A systematic review and meta-analysis of Breast-Q patient-reported outcomes. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 278–285. [Google Scholar] [CrossRef]
- von Glinski, M.; Holler, N.; Kümmel, S.; Reinisch, M.; Wallner, C.; Wagner, J.M.; Dadras, M.; Sogorski, A.; Lehnhardt, M.; Behr, B. Autologous vs. implant-based breast reconstruction after skin- and nipple-sparing mastectomy-A deeper insight considering surgical and patient-reported outcomes. Front. Surg. 2022, 9, 903734. [Google Scholar] [CrossRef]
- Bennett, K.G.; Qi, J.; Kim, H.M.; Hamill, J.B.; Pusic, A.L.; Wilkins, E.G. Comparison of 2-Year Complication Rates Among Common Techniques for Postmastectomy Breast Reconstruction. JAMA Surg. 2018, 153, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Stefura, T.; Rusinek, J.; Wątor, J.; Zagórski, A.; Zając, M.; Libondi, G.; Wysocki, W.M.; Koziej, M. Implant vs. autologous tissue-based breast reconstruction: A systematic review and meta-analysis of the studies comparing surgical approaches in 55,455 patients. J. Plast. Reconstr. Aesthet. Surg. 2022, 77, 346–358. [Google Scholar] [CrossRef]
- Costanzo, D.; Klinger, M.; Lisa, A.; Maione, L.; Battistini, A.; Vinci, V. The evolution of autologous breast reconstruction. Breast J. 2020, 26, 2223–2225. [Google Scholar] [CrossRef]
- Claro, F., Jr.; Sarian, L.O.; Pinto-Neto, A.M. Omentum for Mammary Disorders: A 30-Year Systematic Review. Ann. Surg. Oncol. 2015, 22, 2540–2550. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Ma, I.T.; Choi, Y.K.; Zak, Y.; Dua, M.M.; Wapnir, I.L. Creating a Biological Breast Implant with an Omental Fat-Augmented Free Flap. Plast. Reconstr. Surg. 2022, 149, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Cothier-Savey, I.; Tamtawi, B.; Dohnt, F.; Raulo, Y.; Baruch, J. Immediate Breast Reconstruction Using a Laparoscopically Harvested Omental Flap. Plastic and Reconstructive Surgery. Plast. Reconstr. Surg. 2001, 107, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- van Alphen, T.C.; Fechner, M.R.; Smit, J.M.; Slooter, G.D.; Broekhuysen, C.L. The laparoscopically harvested omentum as a free flap for autologous breast reconstruction. Microsurgery 2017, 37, 539–545. [Google Scholar] [CrossRef]
- Zaha, H.; Inamine, S.; Naito, T.; Nomura, H. Laparoscopically harvested omental flap for immediate breast reconstruction. Am. J. Surg. 2006, 192, 556–558. [Google Scholar] [CrossRef]
- Kahter, A.; Ghazy, H.; Setit, A.; Shams, N.; Gohar, O.; Abdelwahab, K.; Eldamshety, O.; Fathi, A. Laparoscopically Harvested Omental Flap for Immediate Total Breast Reconstruction; Lessons Learnt Through Ten-Year Experience in a Tertiary Oncology Center. Surg. Innov. 2022, 15533506221120149. [Google Scholar] [CrossRef]
- Kim, E.K.; Chae, S.; Ahn, S.H. Single-port laparoscopically harvested omental flap for immediate breast reconstruction. Breast Cancer Res. Treat. 2020, 184, 375–384. [Google Scholar] [CrossRef]
- Fabrizio, T.; Guarro, G.; Filippini, A.; La Torre, G.; Grieco, M.P. Indications for Limitations Of The Omental Pedicle Flap In Immediate Breast Reconstruction-Surgical Results Evaluation And Breast-Q© 2.0 Survey. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 1352–1359. [Google Scholar] [CrossRef]
- Zaha, H.; Abe, N.; Sagawa, N.; Unesoko, M. Oncoplastic surgery with omental flap reconstruction: A study of 200 cases. Breast Cancer Res. Treat. 2017, 162, 267–274. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Rochlin, D.H.; Deptula, P.L.; Zak, Y.; Dua, M.; Wapnir, I.L. A Novel Fat-Augmented Omentum-Based Construct for Unilateral and Bilateral Free-Flap Breast Reconstruction in Underweight and Normal Weight Women Receiving Nipple or Skin-Sparing Mastectomies. Ann. Surg. Oncol. 2022, 30, 3048–3057. [Google Scholar] [CrossRef]
- Crowley, J.S.; Liu, F.C.; Rizk, N.M.; Nguyen, D. Concurrent management of lymphedema and breast reconstruction with single-stage omental vascularized lymph node transfer and autologous breast reconstruction: A case series. Microsurgery 2023. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Torii, S.; Hasegawa, T.; Nishizeki, O. Endoscopic omental harvest. Plast. Reconstr. Surg. 1998, 102, 2450–2453. [Google Scholar] [CrossRef]
- Goldsmith, H.S.; De los Santos, R. Omental transposition for the treatment of chronic lymphedema. Rev. Surg. 1966, 23, 303–304. [Google Scholar] [PubMed]
- Goldsmith, H.S. Long term evaluation of omental transposition for chronic lymphedema. Ann. Surg. 1974, 180, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Shesol, B.F.; Nakashima, R.; Alavi, A.; Hamilton, R.W. Successful lymph node transplantation in rats, with restoration of lymphatic function. Plast. Reconstr. Surg. 1979, 63, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Egorov, Y.S.; Abalmasov, K.G.; Ivanov, V.V.; Abramov, Y.A.; Gainulin, R.M.; Chatterjee, S.S.; Khussainov, B.E. Autotransplantation of the greater omentum in the treatment of chronic lymphedema. Lymphology 1994, 27, 137–143. [Google Scholar]
- Nguyen, A.T.; Suami, H. Laparoscopic Free Omental Lymphatic Flap for the Treatment of Lymphedema. Plast. Reconstr. Surg. 2015, 136, 114–118. [Google Scholar] [CrossRef]
- Shash, H.; Al-Halabi, B.; Aldekhayel, S.; Dionisopoulos, T. Laparoscopic Harvesting of Omental Flaps for Breast Reconstruction-A Review of the Literature and Outcome Analysis. Plast. Surg. Oakv. 2018, 26, 126–133. [Google Scholar] [CrossRef]
- Wang, Z.H.; Xin, P.; Qu, X.; Zhang, Z.T. Breast reconstruction using a laparoscopically harvested pedicled omental flap after endoscopic mastectomy for patients with breast cancer: An observational study of a minimally invasive method. Gland. Surg. 2020, 9, 676–688. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.C.; Thawanyarat, K.; Navarro, Y.; Nguyen, D.H. Current Research on the Use of the Omental Flap in Breast Reconstruction and Post-Mastectomy Lymphedema: A Focus on Omental-Vascularized Lymph Node Transfer. Life 2023, 13, 1380. https://doi.org/10.3390/life13061380
Liu FC, Thawanyarat K, Navarro Y, Nguyen DH. Current Research on the Use of the Omental Flap in Breast Reconstruction and Post-Mastectomy Lymphedema: A Focus on Omental-Vascularized Lymph Node Transfer. Life. 2023; 13(6):1380. https://doi.org/10.3390/life13061380
Chicago/Turabian StyleLiu, Farrah C., Kometh Thawanyarat, Yelissa Navarro, and Dung H. Nguyen. 2023. "Current Research on the Use of the Omental Flap in Breast Reconstruction and Post-Mastectomy Lymphedema: A Focus on Omental-Vascularized Lymph Node Transfer" Life 13, no. 6: 1380. https://doi.org/10.3390/life13061380





