Role of Exercise Stress Echocardiography in Pulmonary Hypertension
Abstract
:1. Introduction
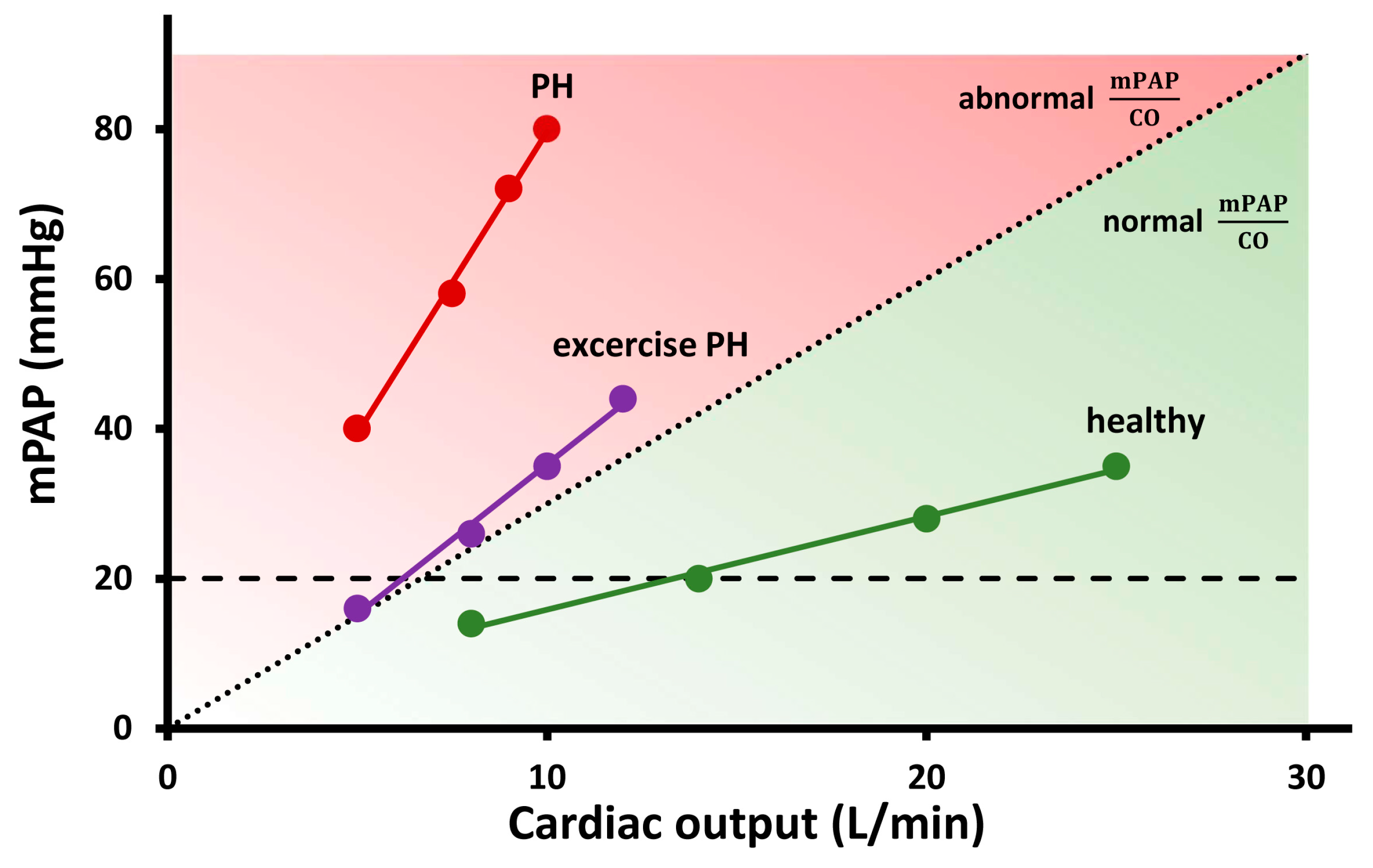
2. How Does Pulmonary Circulation Response to Exercise?
3. Methods for Assessing Pulmonary Haemodynamics
3.1. RHC
3.2. Echocardiography
4. Clinical Application of Exercise Stress Echocardiography
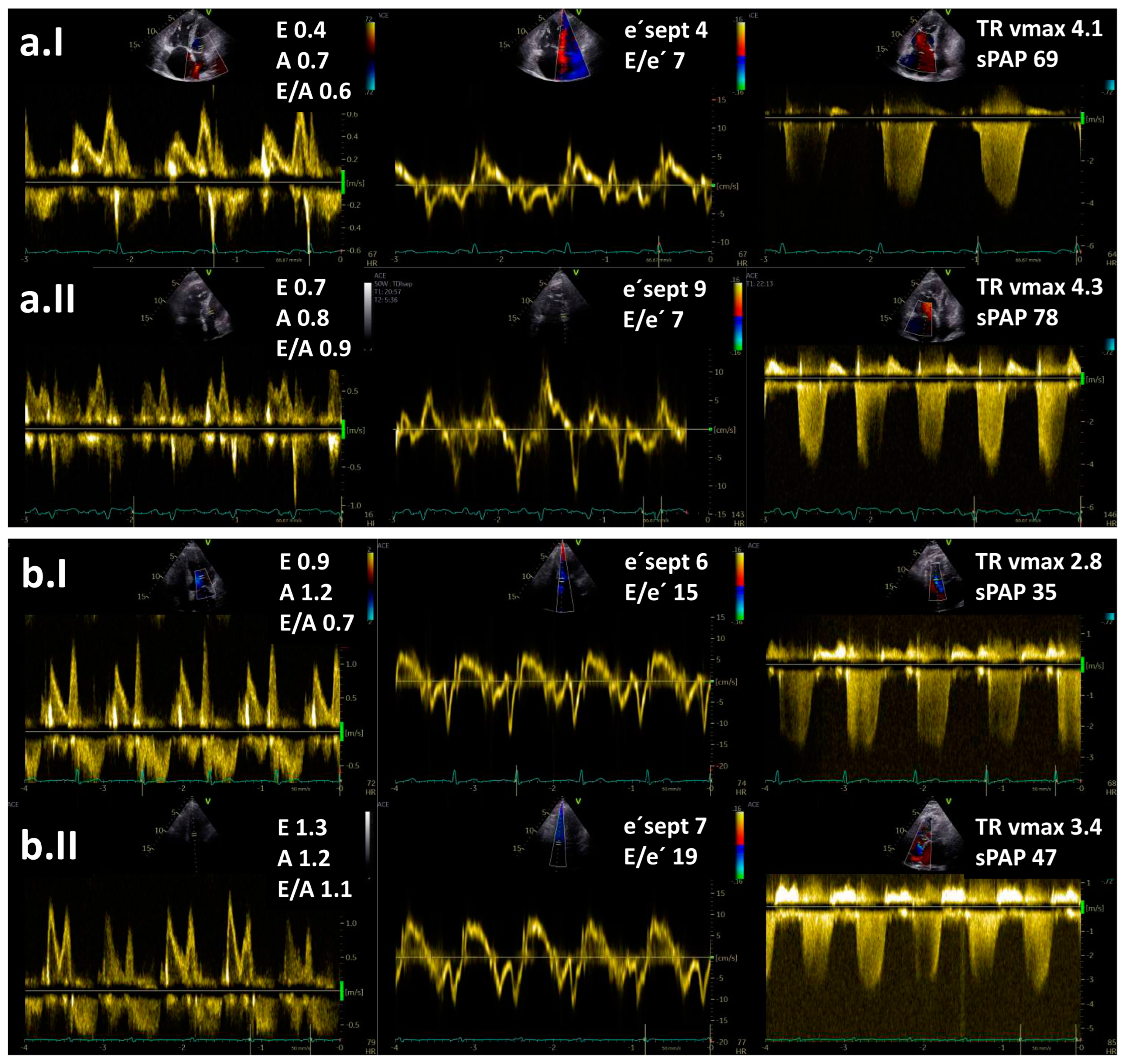
4.1. Diagnostic Role
4.2. Prognostic Role
| Author | Subjects (n) | Echocardiographic Parameters | Most Relevant Findings |
|---|---|---|---|
| Grünig, 2013 [70] | 124 PH patients (PAH, CTEPH) and impaired RV systolic function | ∆sPAP | Exercise-induced sPAP increase ≤ 30 mmHg was related to the worst outcome (HR 2.84, 95% CI 1.92–6.78; p = 0.018). |
| Almeida, 2014 [73] | 14 subjects (7 controls, 7 patients with PH) | ∆S’, ∆TAPSE and ∆FAC | The magnitude of increase in ∆S’, ∆TAPSE and ∆FAC in healthy controls was higher than in patients (all p < 0.05. |
| Guo, 2019 [72] | 46 subjects (31 patients with pre-capillary PH, 15 controls) | ∆S’, ∆TAPSE and ∆FAC | Significant increase in ∆S’ (p = 0.002), ∆TAPSE (p < 0.001) and ∆FAC (p < 0.001) was noted only in healthy controls. |
| Ireland, 2021 [62] | 35 subjects with known or suspected PH | Exercise RVEF | Exercise RVEF can detect occult RV dysfunction (AUC = 0.81, cut off of exercise RVEF = 38%). Patients with exercise RVEF < 38% had an increased propensity for clinical worsening over 4 years compared to patients with RVEF > 38% (p = 0.014). |
| Saito, 2023 [71] | 345 patients (1666 HFpEF, 179 controls) | mPAP/CO slope | Patients with mPAP/CO slope > 5.2 mmHg/L/min had a higher rate of adverse events (all-cause mortality, HF events) compared to those with mPAP/CO slope < 5.2 mmHg/L/min (p = 0.0002). |
4.3. Practical Approach to Exercise Stress Echocardiography
- In the case of a step protocol, haemodynamic measurements are performed towards the end of each exercise level when a steady state in oxygen consumption on a given exercise level is achieved (usually in 3–5 min). For practical reasons, shorter time intervals can be chosen (e.g., 2 min steps aiming for a duration of the exercise time of∼10 min), which appear to be a good compromise [3].
- The feasibility of obtaining diagnostic-quality measurements of TR velocity decreases with increasing exercise load, with 54% at low exercise (20 W) and 49% at peak exercise [10]. The administration of agitated colloids enhances a continuous Doppler tricuspid regurgitation signal and allows reliable estimation of sPAP during exercise [30].
- At higher heart rates, the fusion of the mitral E and A waves prevents the estimation of the LV filling pressures. It was reported that E/e′ could not be measured in about 10% of subjects during submaximal exercise (20 W) and in about 25% of patients during peak exercise [29]. Therefore, the acquisition of images during the submaximal phase before the fusion of E and A waves is advised (heart rate 100–110 bpm) [75].
- Acquisition during early recovery is not optimal, as haemodynamics change very rapidly after cessation of exercise, and previous invasive studies demonstrated that PAWP returned to the baseline levels already 1 min post-exercise [76].
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802148. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, G.; Herve, P.; Barbera, J.A.; Chaouat, A.; Chemla, D.; Condliffe, R.; Garcia, G.; Grünig, E.; Howard, L.; Humbert, M.; et al. An official European Respiratory Society statement: Pulmonary haemodynamics during exercise. Eur. Respir. J. 2017, 50, 1700578. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, G.; Berghold, A.; Scheidl, S.; Olschewski, H. Pulmonary arterial pressure during rest and exercise in healthy subjects: A systematic review. Eur. Respir. J. 2009, 34, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Zeder, K.; Banfi, C.; Steinrisser-Allex, G.; Maron, B.A.; Humbert, M.; Lewis, G.D.; Berghold, A.; Olschewski, H.; Kovacs, G. Diagnostic, prognostic and differential-diagnostic relevance of pulmonary haemodynamic parameters during exercise: A systematic review. Eur. Respir. J. 2022, 60, 2103181. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Bossone, E.; Naeije, R.; Grünig, E.; Saggar, R.; Lancellotti, P.; Ghio, S.; Varga, J.; Rajagopalan, S.; Oudiz, R.J.; et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013, 128, 1470–1479. [Google Scholar] [CrossRef]
- Naeije, R.; Vanderpool, R.; Dhakal, B.P.; Saggar, R.; Saggar, R.; Vachiery, J.-L.; Lewis, G.D. Exercise-induced pulmonary hypertension: Physiological basis and methodological concerns. Am. J. Respir. Crit. Care Med. 2013, 178, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Wolsk, E.; Bakkestrøm, R.; Thomsen, J.H.; Balling, L.; Andersen, M.J.; Dahl, J.S.; Hassager, C.; Møller, J.E.; Gustafsson, F. The Influence of Age on Hemodynamic Parameters During Rest and Exercise in Healthy Individuals. JACC Heart Fail. 2017, 5, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, A.R.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [Green Version]
- Obokata, M.; Kane, G.C.; Reddy, Y.N.V.; Olson, T.P.; Melenovsky, V.; Borlaug, B.A. Role of Diastolic Stress Testing in the Evaluation for Heart Failure with Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation 2017, 135, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claeys, M.; Claessen, G.; La Gerche, A.; Petit, T.; Belge, C.; Meyns, B.; Bogaert, J.; Willems, R.; Claus, P.; Delcroix, M. Impaired Cardiac Reserve and Abnormal Vascular Load Limit Exercise Capacity in Chronic Thromboembolic Disease. JACC Cardiovasc. Imaging 2019, 12, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Zern, E.K.; Lau, E.S.; Wooster, L.; Bailey, C.S.; Cunningham, T.; Eisman, A.S.; Hardin, K.M.; Farrell, R.; Sbarbaro, J.A.; et al. Exercise Pulmonary Hypertension Predicts Clinical Outcomes in Patients With Dyspnea on Effort. J. Am. Coll. Cardiol. 2020, 75, 17–26. [Google Scholar] [CrossRef]
- Stamm, A.; Saxer, S.; Lichtblau, M.; Hasler, E.D.; Jordan, S.; Huber, L.C.; Bloch, K.E.; Distler, O.; Ulrich, S. Exercise pulmonary haemodynamics predict outcome in patients with systemic sclerosis. Eur. Respir. J. 2016, 48, 1658–1667. [Google Scholar] [CrossRef] [Green Version]
- Zeder, K.; Avian, A.; Bachmaier, G.; Douschan, P.; Foris, V.; Sassmann, T.; Moazedi-Fuerst, F.C.; Graninger, W.B.; Hafner, F.; Brodmann, M.; et al. Exercise Pulmonary Resistances Predict Long-Term Survival in Systemic Sclerosis. Chest 2021, 159, 781–790. [Google Scholar] [CrossRef]
- Lewis, G.D.; Murphy, R.M.; Shah, R.V.; Pappagianopoulos, P.P.; Malhotra, R.; Bloch, K.D.; Systrom, D.M.; Semigran, M.J. Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ. Heart Fail. 2011, 4, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Hasler, E.D.; Müller-Mottet, S.; Furian, M.; Saxer, S.; Huber, L.C.; Maggiorini, M.; Speich, R.; Bloch, K.E.; Ulrich, S. Pressure-Flow During Exercise Catheterization Predicts Survival in Pulmonary Hypertension. Chest 2016, 150, 57–67. [Google Scholar] [CrossRef]
- Bentley, R.F.; Barker, M.; Esfandiari, S.; Wright, S.P.; Valle, F.H.; Granton, J.T.; Mak, S. Normal and abnormal relationships of pulmonary artery to wedge pressure during exercise. J. Am. Heart Assoc. 2020, 9, e016339. [Google Scholar] [CrossRef]
- Eisman, A.S.; Shah, R.V.; Dhakal, B.P.; Pappagianopoulos, P.P.; Wooster, L.; Bailey, C.; Cunningham, T.F.; Hardin, K.M.; Baggish, A.L.; Ho, J.E.; et al. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ. Heart Fail. 2018, 11, e004750. [Google Scholar] [CrossRef]
- Janda, S.; Shahidi, N.; Gin, K.; Swiston, J. Diagnostic accuracy of echocardiography for pulmonary hypertension: A systematic review and meta-analysis. Heart 2011, 97, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.-R.; Yan, P.-J.; Liu, S.-D.; Hu, Y.; Yang, K.-H.; Song, B.; Lei, J.-Q. Diagnostic accuracy of transthoracic echocardiography for pulmonary hypertension: A systematic review and meta-analysis. BMJ Open 2019, 9, e033084. [Google Scholar] [CrossRef] [Green Version]
- Erdei, T.; Smiseth, O.A.; Marino, P.; Fraser, A.G. A systematic review of diastolic stress tests in heart failure with preserved ejection fraction, with proposals from the EU-FP7 MEDIA study group. Eur. J. Heart Fail. 2014, 16, 1345–1361. [Google Scholar] [CrossRef]
- Motoji, Y.; Forton, K.; Pezzuto, B.; Faoro, V.; Naeije, R. Resistive or dynamic exercise stress testing of the pulmonary circulation and the right heart. Eur. Respir. J. 2017, 50, 1700151. [Google Scholar] [CrossRef]
- Ha, J.W.; Andersen, O.S.; Smiseth, O.A. Diastolic Stress Test: Invasive and Noninvasive Testing. JACC Cardiovasc. Imaging 2020, 13, 272–282. [Google Scholar] [CrossRef]
- Mottram, P.M.; Haluska, B.A.; Marwick, T.H. Response of B-type natriuretic peptide to exercise in hypertensive patients with suspected diastolic heart failure: Correlation with cardiac function, hemodynamics, and workload. Am. Heart J. 2004, 148, 365–370. [Google Scholar] [CrossRef]
- Samuel, T.J.; Beaudry, R.; Haykowsky, M.J.; Sarma, S.; Park, S.; Dombrowsky, T.; Bhella, P.S.; Nelson, M.D. Isometric handgrip echocardiography: A noninvasive stress test to assess left ventricular diastolic function. Clin. Cardiol. 2017, 40, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Pongpaopattanakul, P.; Imerbtham, T.; Kitimala, J.; Phatthanawayu, S.; Kaewkong, P. Effects of a dynamic handgrip exercise on left ventricular diastolic functions in diabetes mellitus patients: A preliminary clinical data. Chula. Med. J. 2022, 66, 283–291. [Google Scholar] [CrossRef]
- Effects of a dynamic handgrip exercise on left ventricular diastolic functions in diabetes mellitus patients: A preliminary clinical data Utility of E/e′ Ratio During Low-Level Exercise to Diagnose Heart Failure With Preserved. JACC Cardiovasc. Imaging 2023, 16, 145–155. [CrossRef] [PubMed]
- Claessen, G.; La Gerche, A.; Voigt, J.-U.; Dymarkowski, S.; Schnell, F.; Petit, T.; Willems, R.; Claus, P.; Delcroix, M.; Heidbuchel, H. Accuracy of Echocardiography to Evaluate Pulmonary Vascular and RV Function During Exercise. JACC Cardiovasc. Imaging 2016, 9, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Chang, S.M.; Nabi, F.; Shah, D.J.; Estep, J.D. Cardiac imaging in patients with heart failure and preserved ejection fraction. Circ Cardiovasc. Imaging 2017, 10, e006547. [Google Scholar] [CrossRef] [Green Version]
- Talreja, D.R.; Nishimura, R.A.; Oh, J.K. Estimation of Left Ventricular Filling Pressure with Exercise by Doppler Echocardiography in Patients with Normal Systolic Function: A Simultaneous Echocardiographic-Cardiac Catheterization Study. J. Am. Soc. Echocardiogr. 2007, 20, 477–479. [Google Scholar] [CrossRef]
- Grünig, E.; Weissmann, S.; Ehlken, N.; Fijalkowska, A.; Fischer, C.; Fourme, T.; Galié, N.; Ghofrani, A.; Harrison, R.E.; Huez, S.; et al. Stress doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension results of a multicenter european analysis of pulmonary artery pressure response to exercise and hypoxia. Circulation 2009, 119, 1747–1757. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, F.; Anklesaria, Z.; Shagroni, T.; Saggar, R.; Gargani, L.; Bossone, E.; Ryan, M.; Channick, R.; Saggar, R. A review of exercise pulmonary hypertension in systemic sclerosis. J. Scleroderma Relat. Disord. 2019, 4, 225–237. [Google Scholar] [CrossRef]
- Steen, V.; Chou, M.; Shanmugam, V.; Mathias, M.; Kuru, T.; Morrissey, R. Exercise-induced pulmonary arterial hypertension in patients with systemic sclerosis. Chest 2008, 134, 146–151. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Papangelopoulou, K.; Anthi, A.; Tsangaris, I.; Varounis, C.; Makavos, G.; Konstantonis, D.; Vlachoyiannopoulos, P.; Orfanos, S.E.; Iliodromitis, E.K. The role of exercise doppler echocardiography to unmask pulmonary arterial hypertension in selected patients with systemic sclerosis and equivocal baseline echocardiographic values for pulmonary hypertension. Diagnostics 2021, 11, 1200. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.-C.; Gibbs, J.S.R. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef]
- Tolle, J.J.; Waxman, A.B.; Van Horn, T.L.; Pappagianopoulos, P.P.; Systrom, D.M. Exercise-induced pulmonary arterial hypertension. Circulation 2008, 118, 2183–2189. [Google Scholar] [CrossRef] [Green Version]
- Shim, C.Y.; Kim, S.-A.; Choi, D.; Yang, W.-I.; Kim, J.-M.; Moon, S.-H.; Lee, H.-J.; Park, S.; Choi, E.-Y.; Chung, N.; et al. Clinical outcomes of exercise-induced pulmonary hypertension in subjects with preserved left ventricular ejection fraction: Implication of an increase in left ventricular filling pressure during exercise. Heart 2011, 97, 1417–1424. [Google Scholar] [CrossRef]
- Ghio, S.; Klersy, C.; Magrini, G.; D’Armini, A.M.; Scelsi, L.; Raineri, C.; Pasotti, M.; Serio, A.; Campana, C.; Viganò, M. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int. J. Cardiol. 2010, 140, 272–278. [Google Scholar] [CrossRef]
- Vonk-Noordegraaf, A.; Haddad, F.; Chin, K.M.; Forfia, P.R.; Kawut, S.M.; Lumens, J.; Naeije, R.; Newman, J.; Oudiz, R.J.; Provencher, S.; et al. Right Heart Adaptation to Pulmonary Arterial Hypertension. J Am Coll Cardiol. 2013, 62, D22–D33. [Google Scholar] [CrossRef] [PubMed]
- Vonk Noordegraaf, A.; Westerhof, B.E.; Westerhof, N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J. Am. Coll Cardiol. 2017, 69, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; McLaughlin, V.V.; Archer, S.L.; Badesch, D.B.; Barst, R.J.; Farber, H.W.; Lindner, J.R.; Mathier, M.A.; McGoon, M.D.; Park, M.H.; et al. A report of the american college of cardiology foundation task force on expert consensus documents and the american heart association. Circulation 2009, 119, 2250–2294. [Google Scholar] [CrossRef] [Green Version]
- D’Alonzo, G.E. Survival in Patients with Primary Pulmonary Hypertension. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Dalmer, A.; Axmann, J.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Seeger, W.; Sommer, N.; Wilhelm, J.; et al. Reserve of Right Ventricular-Arterial Coupling in the Setting of Chronic Overload. Circ. Heart Fail. 2019, 12, e005512. [Google Scholar] [CrossRef] [PubMed]
- Forfia, P.R.; Fisher, M.R.; Mathai, S.C.; Housten-Harris, T.; Hemnes, A.R.; Borlaug, B.A.; Chamera, E.; Corretti, M.C.; Champion, H.C.; Abraham, T.P.; et al. Tricuspid Annular Displacement Predicts Survival in Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2006, 174, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Burke, M.A.; Katz, D.H.; Beussink, L.; Selvaraj, S.; Gupta, D.K.; Fox, J.; Chakrabarti, S.; Sauer, A.J.; Rich, J.D.; Freed, B.H.; et al. Prognostic Importance of Pathophysiologic Markers in Patients With Heart Failure and Preserved Ejection Fraction. Circ. Heart Fail. 2014, 7, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, S.F.; Hussain, I.; AbouEzzeddine, O.F.; Takahama, H.; Kwon, S.H.; Forfia, P.; Roger, V.L.; Redfield, M.M. Right Ventricular Function in Heart Failure With Preserved Ejection Fraction. Circulation 2014, 130, 2310–2320. [Google Scholar] [CrossRef] [Green Version]
- Mauritz, G.-J.; Kind, T.; Marcus, J.T.; Bogaard, H.-J.; van de Veerdonk, M.; Postmus, P.E.; Boonstra, A.; Westerhof, N.; Vonk-Noordegraaf, A. Progressive Changes in Right Ventricular Geometric Shortening and Long-term Survival in Pulmonary Arterial Hypertension. Chest 2012, 141, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Melenovsky, V.; Hwang, S.J.; Lin, G.; Redfield, M.M.; Borlaug, B.A. Right heart dysfunction in heart failure with preserved ejection fraction. Eur. Heart J. 2014, 35, 3452–3462. [Google Scholar] [CrossRef] [Green Version]
- Champion, H.C.; Michelakis, E.D.; Hassoun, P.M. Comprehensive Invasive and Noninvasive Approach to the Right Ventricle–Pulmonary Circulation Unit. Circulation 2009, 120, 992–1007. [Google Scholar] [CrossRef] [Green Version]
- Yeo, T.C.; Dujardin, K.S.; Tei, C.; Mahoney, D.W.; McGoon, M.D.; Seward, J.B. Value of a Doppler-Derived Index Combining Systolic and Diastolic Time Intervals in Predicting Outcome in Primary Pulmonary Hypertension. Am. J. Cardiol. 1998, 81, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Conroy, J.; Narula, J. Imaging of the Right Ventricle. Cardiol Clin. 2012, 30, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Addetia, K.; Bhave, N.M.; Tabit, C.E.; Gomberg-Maitland, M.; Freed, B.H.; Dill, K.E.; Lang, R.M.; Mor-Avi, V.; Patel, A.R. Sample Size and Cost Analysis for Pulmonary Arterial Hypertension Drug Trials Using Various Imaging Modalities to Assess Right Ventricular Size and Function End Points. Circ. Cardiovasc. Imaging 2014, 7, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryo, K.; Goda, A.; Onishi, T.; Delgado-Montero, A.; Tayal, B.; Champion, H.C.; Simon, M.A.; Mathier, M.A.; Gladwin, M.T.; Gorcsan, J., 3rd. Characterization of Right Ventricular Remodeling in Pulmonary Hypertension Associated With Patient Outcomes by 3-Dimensional Wall Motion Tracking Echocardiography. Circ. Cardiovasc. Imaging 2015, 8, e003176. [Google Scholar] [CrossRef] [Green Version]
- Richter, M.J.; Peters, D.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Sommer, N.; Gall, H.; Grimminger, F.; Seeger, W.; Tello, K. Evaluation and prognostic relevance of right ventricular-arterial coupling in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2020, 201, 116–119. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Alunni, G.; Murrone, A.; Zuchi, C.; Coiro, S.; Riccini, C.; Mengoni, A.; D’antonio, A.; Ambrosio, G. Prognostic value of right ventricular dysfunction in heart failure with reduced ejection fraction: Superiority of longitudinal strain over tricuspid annular plane systolic excursion. Circ. Cardiovasc. Imaging 2018, 11, e006894. [Google Scholar] [CrossRef] [Green Version]
- Butcher, S.C.; Fortuni, F.; Montero-Cabezas, J.M.; Abou, R.; El Mahdiui, M.; Van Der Bijl, P.; Van Der Velde, E.T.; Marsan, N.A.; Bax, J.J.; Delgado, V. Right ventricular myocardial work: Proof-of-concept for non-invasive assessment of right ventricular function. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 142–152. [Google Scholar] [CrossRef]
- Ünlü, S.; Bézy, S.; Cvijic, M.; Duchenne, J.; Delcroix, M.; Voigt, J.-U. Right ventricular strain related to pulmonary artery pressure predicts clinical outcome in patients with pulmonary arterial hypertension. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 635–642. [Google Scholar] [CrossRef]
- Haeck, M.L.; Scherptong, R.W.; Marsan, N.A.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Vliegen, H.W.; Delgado, V. Prognostic Value of Right Ventricular Longitudinal Peak Systolic Strain in Patients With Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2012, 5, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Fine, N.M.; Chen, L.; Bastiansen, P.M.; Frantz, R.P.; Pellikka, P.A.; Oh, J.K.; Kane, G.C. Outcome Prediction by Quantitative Right Ventricular Function Assessment in 575 Subjects Evaluated for Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2013, 6, 711–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ireland, C.G.; Damico, R.L.; Kolb, T.M.; Mathai, S.C.; Mukherjee, M.; Zimmerman, S.L.; Shah, A.A.; Wigley, F.M.; Houston, B.A.; Hassoun, P.M.; et al. Exercise right ventricular ejection fraction predicts right ventricular contractile reserve. J. Heart Lung. Transplant. 2021, 40, 504–512. [Google Scholar] [CrossRef]
- Hsu, S.; Houston, B.A.; Tampakakis, E.; Bacher, A.C.; Rhodes, P.S.; Mathai, S.C.; Damico, R.L.; Kolb, T.M.; Hummers, L.K.; Shah, A.A.; et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation 2016, 133, 2413–2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, S.; Kokkonen-Simon, K.M.; Kirk, J.A.; Kolb, T.M.; Damico, R.L.; Mathai, S.C.; Mukherjee, M.; Shah, A.A.; Wigley, F.M.; Margulies, K.B.; et al. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension. Circulation 2018, 137, 2360–2370. [Google Scholar] [CrossRef]
- Tedford, R.J.; Mudd, J.O.; Girgis, R.E.; Mathai, S.C.; Zaiman, A.L.; Housten-Harris, T.; Boyce, D.; Kelemen, B.W.; Bacher, A.C.; Shah, A.A.; et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ. Heart Fail. 2013, 6, 953–963. [Google Scholar] [CrossRef] [Green Version]
- Rommel, K.-P.; von Roeder, M.; Oberueck, C.; Latuscynski, K.; Besler, C.; Blazek, S.; Stiermaier, T.; Fengler, K.; Adams, V.; Sandri, M.; et al. Load-independent systolic and diastolic right ventricular function in heart failure with preserved ejection fraction as assessed by resting and handgrip exercise pressure–volume loops. Circ. Heart Fail. 2018, 11, e004121. [Google Scholar] [CrossRef]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure With Preserved Ejection Fraction. JACC Cardiovasc. Imaging 2017, 10, 1211–1221. [Google Scholar] [CrossRef]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef]
- Huston, J.H.; Maron, B.A.; French, J.; Huang, S.; Thayer, T.; Farber-Eger, E.H.; Wells, Q.S.; Choudhary, G.; Hemnes, A.R.; Brittain, E.L. Association of Mild Echocardiographic Pulmonary Hypertension With Mortality and Right Ventricular Function. JAMA Cardiol. 2019, 4, 1112–1121. [Google Scholar] [CrossRef]
- Grünig, E.; Tiede, H.; Enyimayew, E.O.; Ehlken, N.; Seyfarth, H.-J.; Bossone, E.; D’andrea, A.; Naeije, R.; Olschewski, H.; Ulrich, S.; et al. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation 2013, 128, 2005–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Obokata, M.; Harada, T.; Kagami, K.; Sorimachi, H.; Yuasa, N.; Kato, T.; Wada, N.; Okumura, Y.; Ishii, H. Disproportionate exercise-induced pulmonary hypertension in relation to cardiac output in heart failure with preserved ejection fraction: A non-invasive echocardiographic study. Eur. J. Heart Fail. 2023, Online ahead of print. [CrossRef] [PubMed]
- Guo, D.-C.; Li, Y.-D.; Yang, Y.-H.; Zhu, W.-W.; Sun, L.-L.; Jiang, W.; Ye, X.; Cai, Q.-Z.; Lu, X.-Z.; Ye, X.-G. Influence of impaired right ventricular contractile reserve on exercise capacity in patients with precapillary pulmonary hypertension: A study with exercise stress echocardiography. Echocardiography 2019, 36, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.R.; Loureiro, M.J.; Lopes, L.; Cotrim, C.; Lopes, L.; Repolho, D.; Pereira, H. Echocardiographic assessment of right ventricular contractile reserve in patients with pulmonary hypertension. Rev. Port. Cardiol. 2014, 33, 155–163. [Google Scholar] [CrossRef]
- Suzuki, K.; Hirano, Y.; Yamada, H.; Murata, M.; Daimon, M.; Takeuchi, M.; Seo, Y.; Izumi, C.; Akaishi, M. Practical guidance for the implementation of stress echocardiography. J. Echocardiogr. 2018, 16, 105–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Nishimura, R.A.; Sorajja, P.; Lam, C.S.; Redfield, M.M. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ. Heart Fail. 2010, 3, 588–595. [Google Scholar] [CrossRef] [Green Version]
| Author | Type | Protocol |
|---|---|---|
| Ha, 2020 [25] | semi-supine bicycle | stepped protocol: cycling at a cadence of 60 r.p.m. starting at 25 W and increasing in increments of 25 W every 3 min |
| Erdei, 2014 [23] | semi-supine bicycle | ramp protocol: cycling at a cadence of 60 r.p.m. starting at 15 W with 5 W increments every minute |
| Motram, 2004 [26] | treadmill | Bruce protocol |
| Jake Samuel, 2017 [27] | isometric handgrip | holding the dynamometer at 40% of MVC for 3 min |
| Pongpaopattanakul, 2022 [28] | dynamic handgrip | squeezing the dynamometer at 2 kg at a cadence of 30 r.p.m. for 3 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škafar, M.; Ambrožič, J.; Toplišek, J.; Cvijić, M. Role of Exercise Stress Echocardiography in Pulmonary Hypertension. Life 2023, 13, 1385. https://doi.org/10.3390/life13061385
Škafar M, Ambrožič J, Toplišek J, Cvijić M. Role of Exercise Stress Echocardiography in Pulmonary Hypertension. Life. 2023; 13(6):1385. https://doi.org/10.3390/life13061385
Chicago/Turabian StyleŠkafar, Mojca, Jana Ambrožič, Janez Toplišek, and Marta Cvijić. 2023. "Role of Exercise Stress Echocardiography in Pulmonary Hypertension" Life 13, no. 6: 1385. https://doi.org/10.3390/life13061385
APA StyleŠkafar, M., Ambrožič, J., Toplišek, J., & Cvijić, M. (2023). Role of Exercise Stress Echocardiography in Pulmonary Hypertension. Life, 13(6), 1385. https://doi.org/10.3390/life13061385






