An Overview of Cefiderocol’s Therapeutic Potential and Underlying Resistance Mechanisms
Abstract
1. Introduction
2. Top-Priority Pathogens and Current Last-Line Therapeutic Options
2.1. Carbapenem-Resistant Acinetobacter baumannii
2.2. Carbapenem-Resistant Pseudomonas aeruginosa
2.3. Carbapenem- and Third-Generation-Cephalosporins-Resistant Enterobacterales
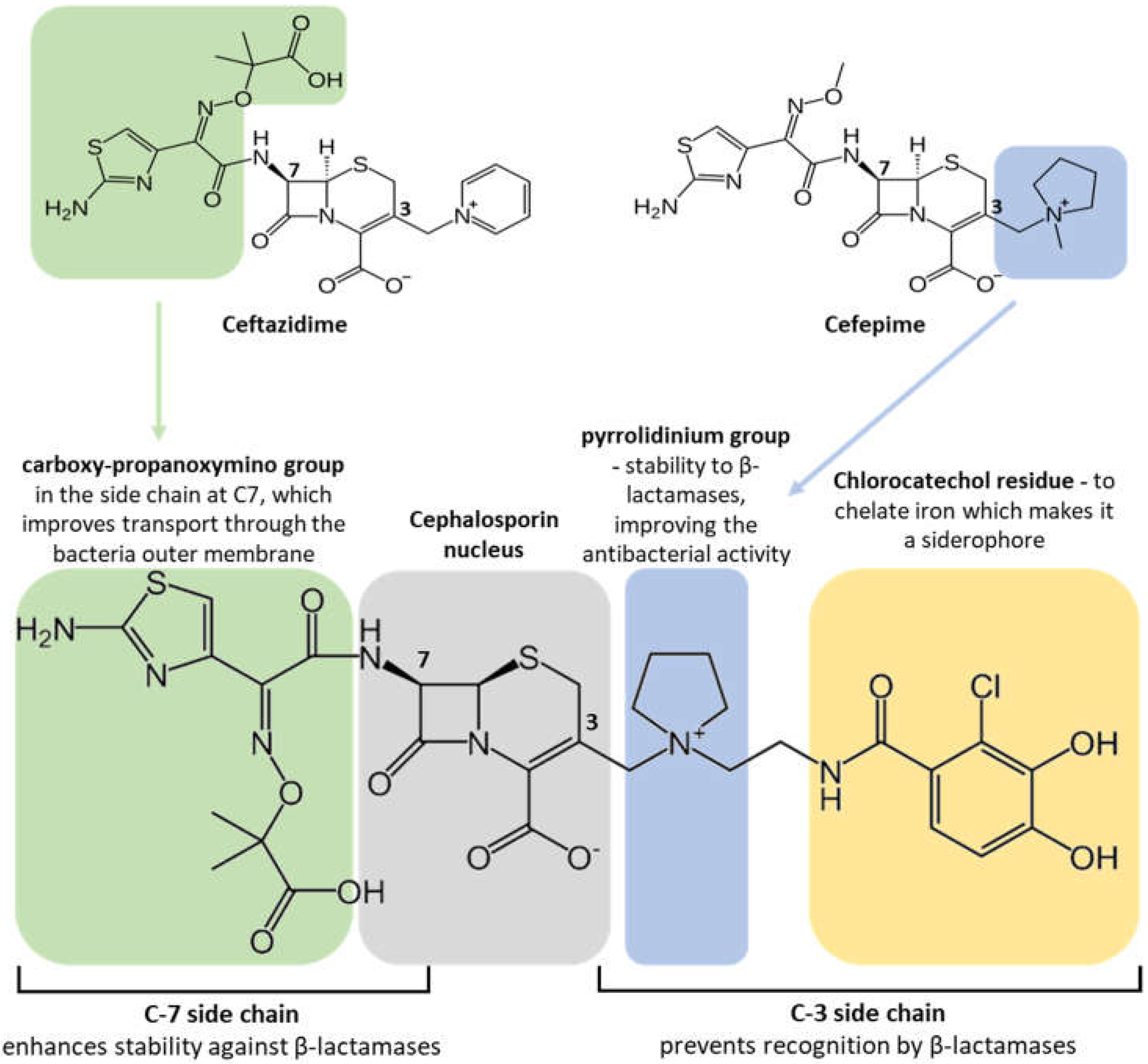
3. Cephalosporins
4. Siderophores: Biological Function
5. Cefiderocol
5.1. Mechanism of Action
5.2. Therapeutic Indications
5.3. Mechanisms of Resistance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walesch, S.; Birkelbach, J.; Jezequel, G.; Haeckl, F.P.J.; Hegemann, J.D.; Hesterkamp, T.; Hirsch, A.K.H.; Hammann, P.; Muller, R. Fighting antibiotic resistance-strategies and (pre)clinical developments to find new antibacterials. EMBO Rep. 2023, 24, e56033. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Carrara, E.; Retamar, P.; Tangden, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022, 28, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. Available online: https://wellcomecollection.org/works/rdpck35v (accessed on 5 April 2023).
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2020 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America guidance on the treatment of AmpC beta-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 74, 2089–2114. [Google Scholar] [CrossRef]
- Antunes, L.C.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef]
- Jawad, A.; Seifert, H.; Snelling, A.M.; Heritage, J.; Hawkey, P.M. Survival of Acinetobacter baumannii on dry surfaces: Comparison of outbreak and sporadic isolates. J. Clin. Microbiol. 1998, 36, 1938–1941. [Google Scholar] [CrossRef]
- Bonomo, R.A.; Szabo, D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 43 (Suppl. S2), S49–S56. [Google Scholar] [CrossRef]
- Da Silva, G.J.; Domingues, S. Insights on the horizontal gene transfer of carbapenemase determinants in the opportunistic pathogen Acinetobacter baumannii. Microorganisms 2016, 4, 29. [Google Scholar] [CrossRef]
- Quick, J.; Cumley, N.; Wearn, C.M.; Niebel, M.; Constantinidou, C.; Thomas, C.M.; Pallen, M.J.; Moiemen, N.S.; Bamford, A.; Oppenheim, B.; et al. Seeking the source of Pseudomonas aeruginosa infections in a recently opened hospital: An observational study using whole-genome sequencing. BMJ Open 2014, 4, e006278. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 guidance on the treatment of extended-spectrum beta-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 75, 187–212. [Google Scholar] [CrossRef]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Radhey, S.G. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar] [CrossRef]
- Walsh, T.R. A one-health approach to antimicrobial resistance. Nat. Microbiol. 2018, 3, 854–855. [Google Scholar] [CrossRef]
- Sheu, C.C.; Chang, Y.T.; Lin, S.Y.; Chen, Y.H.; Hsueh, P.R. Infections caused by carbapenem-resistant Enterobacteriaceae: An update on therapeutic options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, B. Study on genetic engineering of Acremonium chrysogenum, the cephalosporin C producer. Synth. Syst. Biotechnol. 2016, 1, 143–149. [Google Scholar] [CrossRef]
- Corcione, S.; Lupia, T.; De Rosa, F.G. Novel cephalosporins in septic subjects and severe infections: Present findings and future perspective. Front. Med. 2021, 8, 617378. [Google Scholar] [CrossRef]
- Hansen, G.T. Continuous evolution: Perspective on the epidemiology of carbapenemase resistance among Enterobacterales and other Gram-negative bacteria. Infect. Dis. Ther. 2021, 10, 75–92. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control; World Health Organization. Antimicrobial Resistance Surveillance in Europe 2023–2021 Data; European Centre for Disease Prevention and Control: Stockholm, Sweden; World Health Organization: Geneva, Switzerland, 2023.
- Lee, Y.L.; Chen, H.M.; Hii, I.M.; Hsueh, P.R. Carbapenemase-producing Enterobacterales infections: Recent advances in diagnosis and treatment. Int. J. Antimicrob. Agents 2022, 59, 106528. [Google Scholar] [CrossRef]
- Rodriguez-Bano, J.; Gutierrez-Gutierrez, B.; Machuca, I.; Pascual, A. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef]
- Tilahun, M.; Kassa, Y.; Gedefie, A.; Ashagire, M. Emerging carbapenem-resistant Enterobacteriaceae infection, its epidemiology and novel treatment options: A review. Infect. Drug Resist. 2021, 14, 4363–4374. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; Lopez, C.A.; Gnanakaran, S. Permeability barrier of Gram-negative cell envelopes and approaches to bypass it. ACS Infect. Dis. 2015, 1, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, C.; Wang, Q.; Wang, X.; Chen, H.; Li, H.; Zhang, F.; Wang, H. Evaluation of the in vitro activity of new polymyxin B analogue SPR206 against clinical MDR, colistin-resistant and tigecycline-resistant Gram-negative bacilli. J. Antimicrob. Chemother. 2020, 75, 2609–2615. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.Y.; Jamshidi, S.; Sutton, J.M.; Rahman, K.M. Current advances in developing inhibitors of bacterial multidrug efflux pumps. Curr. Med. Chem. 2016, 23, 1062–1081. [Google Scholar] [CrossRef]
- Fleeman, R.M.; Debevec, G.; Antonen, K.; Adams, J.L.; Santos, R.G.; Welmaker, G.S.; Houghten, R.A.; Giulianotti, M.A.; Shaw, L.N. Identification of a novel polyamine scaffold with potent efflux pump inhibition activity toward multi-drug resistant bacterial pathogens. Front. Microbiol. 2018, 9, 1301. [Google Scholar] [CrossRef]
- Isabella, V.M.; Campbell, A.J.; Manchester, J.; Sylvester, M.; Nayar, A.S.; Ferguson, K.E.; Tommasi, R.; Miller, A.A. Toward the rational design of carbapenem uptake in Pseudomonas aeruginosa. Chem. Biol. 2015, 22, 535–547. [Google Scholar] [CrossRef]
- Mollmann, U.; Heinisch, L.; Bauernfeind, A.; Kohler, T.; Ankel-Fuchs, D. Siderophores as drug delivery agents: Application of the “Trojan Horse” strategy. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2009, 22, 615–624. [Google Scholar] [CrossRef]
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef]
- Page, M.G.P. The role of iron and siderophores in infection, and the development of siderophore antibiotics. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, S529–S537. [Google Scholar] [CrossRef]
- Kramer, J.; Ozkaya, O.; Kummerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Wu, J.Y.; Srinivas, P.; Pogue, J.M. Cefiderocol: A novel agent for the management of multidrug-resistant Gram-negative organisms. Infect. Dis. Ther. 2020, 9, 17–40. [Google Scholar] [CrossRef]
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, S538–S543. [Google Scholar] [CrossRef]
- Ito-Horiyama, T.; Ishii, Y.; Ito, A.; Sato, T.; Nakamura, R.; Fukuhara, N.; Tsuji, M.; Yamano, Y.; Yamaguchi, K.; Tateda, K. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob. Agents Chemother. 2016, 60, 4384–4386. [Google Scholar] [CrossRef]
- Sadek, M.; Bosch Duran, J.; Poirel, L.; Nordmann, P. Impact of minor carbapenemases on susceptibility to novel beta-lactam/beta-lactamase inhibitor combinations and cefiderocol in Enterobacterales. Antimicrob. Agents Chemother. 2023, 67, e0007823. [Google Scholar] [CrossRef]
- Food and Drug Administration. NDA 209445: Cefiderocol; Food and Drug Administration: Silver Spring, MD, USA, 2019.
- European Medicines Agency. Assessment Report-Fetcroja; European Medicines Agency: Amsterdam, The Netherlands, 2020.
- Syed, Y.Y. Cefiderocol: A review in serious Gram-negative bacterial infections. Drugs 2021, 81, 1559–1571. [Google Scholar] [CrossRef]
- Bonomo, R.A. Cefiderocol: A novel siderophore cephalosporin defeating carbapenem-resistant pathogens. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, S519–S520. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Golden, A.R.; Zelenitsky, S.; Wiebe, K.; Lawrence, C.K.; Adam, H.J.; Idowu, T.; Domalaon, R.; Schweizer, F.; Zhanel, M.A.; et al. Cefiderocol: A siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant Gram-negative bacilli. Drugs 2019, 79, 271–289. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Portsmouth, S.; van Veenhuyzen, D.; Echols, R.; Machida, M.; Ferreira, J.C.A.; Ariyasu, M.; Tenke, P.; Nagata, T.D. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: A phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect. Dis. 2018, 18, 1319–1328. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Gregori, D.; Sasset, L.; Trevenzoli, M.; Scaglione, V.; Lo Menzo, S.; Marinello, S.; Mengato, D.; Venturini, F.; Tiberio, I.; et al. Cefiderocol-based versus colistin-based regimens for severe carbapenem-resistant Acinetobacter baumannii infections: A propensity score-weighted, retrospective cohort study during the first two years of the COVID-19 pandemic. Microorganisms 2023, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Marchaim, D.; Thamlikitkul, V.; Carmeli, Y.; Chiu, C.-H.; Daikos, G.; Dhar, S.; Durante-Mangoni, E.; Gikas, A.; Kotanidou, A.; et al. Colistin Monotherapy versus Combination Therapy for Carbapenem-Resistant Organisms. NEJM Evid. 2023, 2, EVIDoa2200131. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, L.; Sun, S.; Yin, Y.; Wang, R.; Chen, F.; Wang, X.; Zhang, Y.; Hou, J.; Zhang, Y.; et al. Occurrence of high levels of cefiderocol resistance in carbapenem-resistant Escherichia coli before its approval in China: A report from China CRE-network. Microbiol. Spectr. 2022, 10, e0267021. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Lu, Y.; Chen, Z.; Wu, X.; Hua, X.; Jiang, Y.; Zhou, J.; Yu, Y. Emergence of high-level cefiderocol resistance in carbapenem-resistant Klebsiella pneumoniae from bloodstream infections in patients with hematologic malignancies in China. Microbiol. Spectr. 2022, 10, e0008422. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Clinical Breakpoints-Bacteria (v 10.0) (Jan 1, 2020); European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2020.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Isler, B.; Kidd, T.J.; Stewart, A.G.; Harris, P.; Paterson, D.L. Achromobacter Infections and Treatment Options. Antimicrob. Agents Chemother. 2020, 64, e01025-20. [Google Scholar] [CrossRef]
- Oueslati, S.; Bogaerts, P.; Dortet, L.; Bernabeu, S.; Ben Lakhal, H.; Longshaw, C.; Glupczynski, Y.; Naas, T. In vitro Activity of Cefiderocol and Comparators against Carbapenem-Resistant Gram-Negative Pathogens from France and Belgium. Antibiotics 2022, 11, 1352. [Google Scholar] [CrossRef]
- Rolston, K.V.I.; Gerges, B.; Shelburne, S.; Aitken, S.L.; Raad, I.; Prince, R.A. Activity of Cefiderocol and Comparators against Isolates from Cancer Patients. Antimicrob. Agents Chemother. 2020, 64, e01955-19. [Google Scholar] [CrossRef]
- Warner, N.C.; Bartelt, L.A.; Lachiewicz, A.M.; Tompkins, K.M.; Miller, M.B.; Alby, K.; Jones, M.B.; Carr, A.L.; Alexander, J.; Gainey, A.B.; et al. Cefiderocol for the Treatment of Adult and Pediatric Patients with Cystic Fibrosis and Achromobacter xylosoxidans Infections. Clin. Infect. Dis. 2021, 73, e1754–e1757. [Google Scholar] [CrossRef]
- Beauruelle, C.; Lamoureux, C.; Mashi, A.; Ramel, S.; Le Bihan, J.; Ropars, T.; Dirou, A.; Banerjee, A.; Tande, D.; Le Bars, H.; et al. In Vitro Activity of 22 Antibiotics against Achromobacter Isolates from People with Cystic Fibrosis. Are There New Therapeutic Options? Microorganisms 2021, 9, 2473. [Google Scholar] [CrossRef]
- Magallon, A.; Amoureux, L.; Garrigos, T.; Sonois, M.; Varin, V.; Neuwirth, C.; Bador, J. Role of AxyABM overexpression in acquired resistance in Achromobacter xylosoxidans. J. Antimicrob. Chemother. 2022, 77, 926–929. [Google Scholar] [CrossRef]
- Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Karlowsky, J.A.; Sahm, D.F. In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against a Recent Collection of Clinically Relevant Gram-Negative Bacilli from North America and Europe, Including Carbapenem-Nonsusceptible Isolates (SIDERO-WT-2014 Study). Antimicrob. Agents Chemother. 2017, 61, e00093-17. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Hackel, M.A.; Takemura, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In Vitro Susceptibility of Gram-Negative Pathogens to Cefiderocol in Five Consecutive Annual Multinational SIDERO-WT Surveillance Studies, 2014 to 2019. Antimicrob. Agents Chemother. 2022, 66, e0199021. [Google Scholar] [CrossRef]
- Yamano, Y.; Ishibashi, N.; Kuroiwa, M.; Takemura, M.; Sheng, W.H.; Hsueh, P.R. Characterisation of cefiderocol-non-susceptible Acinetobacter baumannii isolates from Taiwan. J. Glob. Antimicrob. Resist. 2022, 28, 120–124. [Google Scholar] [CrossRef]
- Poirel, L.; Sadek, M.; Nordmann, P. Contribution of PER-type and NDM-type beta-lactamases to cefiderocol resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e0087721. [Google Scholar] [CrossRef]
- Malik, S.; Kaminski, M.; Landman, D.; Quale, J. Cefiderocol resistance in Acinetobacter baumannii: Roles of beta-lactamases, siderophore receptors, and Penicillin Binding Protein 3. Antimicrob. Agents Chemother. 2020, 64, e01221-20. [Google Scholar] [CrossRef]
- Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Karlowsky, J.A.; Sahm, D.F. In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against Carbapenem-Nonsusceptible and Multidrug-Resistant Isolates of Gram-Negative Bacilli Collected Worldwide in 2014 to 2016. Antimicrob. Agents Chemother. 2018, 62, e01968-17. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, Against Gram-Negative Bacilli Isolated by Clinical Laboratories in North America and Europe in 2015–2016: SIDERO-WT-2015. Int. J. Antimicrob. Agents 2019, 53, 456–466. [Google Scholar] [CrossRef]
- Kohira, N.; West, J.; Ito, A.; Ito-Horiyama, T.; Nakamura, R.; Sato, T.; Rittenhouse, S.; Tsuji, M.; Yamano, Y. In Vitro Antimicrobial Activity of a Siderophore Cephalosporin, S-649266, against Enterobacteriaceae Clinical Isolates, Including Carbapenem-Resistant Strains. Antimicrob. Agents Chemother. 2016, 60, 729–734. [Google Scholar] [CrossRef]
- Alzahrani, O.M.; Uddin, F.; Mahmoud, S.F.; Alswat, A.S.; Sohail, M.; Youssef, M. Resistance to Some New Drugs and Prevalence of ESBL- and MBL-Producing Enterobacteriaceae Uropathogens Isolated from Diabetic Patients. Life 2022, 12, 2125. [Google Scholar] [CrossRef]
- Poirel, L.; Ortiz de la Rosa, J.M.; Sakaoglu, Z.; Kusaksizoglu, A.; Sadek, M.; Nordmann, P. NDM-35-producing ST167 Escherichia coli highly resistant to beta-lactams including cefiderocol. Antimicrob. Agents Chemother. 2022, 66, e0031122. [Google Scholar] [CrossRef]
- Lasarte-Monterrubio, C.; Guijarro-Sanchez, P.; Vazquez-Ucha, J.C.; Alonso-Garcia, I.; Alvarez-Fraga, L.; Outeda, M.; Martinez-Guitian, M.; Pena-Escolano, A.; Maceiras, R.; Lence, E.; et al. Antimicrobial Activity of Cefiderocol against the Carbapenemase-Producing Enterobacter cloacae Complex and Characterization of Reduced Susceptibility Associated with Metallo-beta-Lactamase VIM-1. Antimicrob. Agents Chemother. 2023, 67, e0150522. [Google Scholar] [CrossRef] [PubMed]
- Nurjadi, D.; Kocer, K.; Chanthalangsy, Q.; Klein, S.; Heeg, K.; Boutin, S. New Delhi metallo-beta-lactamase facilitates the emergence of cefiderocol resistance in Enterobacter cloacae. Antimicrob. Agents Chemother. 2022, 66, e02011-21. [Google Scholar] [CrossRef] [PubMed]
- Naas, T.; Lina, G.; Santerre Henriksen, A.; Longshaw, C.; Jehl, F. In vitro activity of cefiderocol and comparators against isolates of Gram-negative pathogens from a range of infection sources: SIDERO-WT-2014-2018 studies in France. JAC Antimicrob. Resist. 2021, 3, dlab081. [Google Scholar] [CrossRef] [PubMed]
- Stracquadanio, S.; Torti, E.; Longshaw, C.; Henriksen, A.S.; Stefani, S. In vitro activity of cefiderocol and comparators against isolates of Gram-negative pathogens from a range of infection sources: SIDERO-WT-2014-2018 studies in Italy. J. Glob. Antimicrob. Resist. 2021, 25, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Mc Gann, P.; Geringer, M.R.; Hall, L.R.; Lebreton, F.; Markelz, E.; Kwak, Y.I.; Johnson, S.; Ong, A.C.; Powell, A.; Tekle, T.; et al. Pan-drug resistant Providencia rettgeri contributing to a fatal case of COVID-19. J. Med. Microbiol. 2021, 70, 001406. [Google Scholar] [CrossRef] [PubMed]
- Padovani, M.; Bertelli, A.; Corbellini, S.; Piccinelli, G.; Gurrieri, F.; De Francesco, M.A. In vitro activity of cefiderocol on multiresistant bacterial strains and genomic analysis of two cefiderocol resistant strains. Antibiotics 2023, 12, 785. [Google Scholar] [CrossRef]
- Streling, A.P.; Al Obaidi, M.M.; Lainhart, W.D.; Zangeneh, T.; Khan, A.; Dinh, A.Q.; Hanson, B.; Arias, C.A.; Miller, W.R. Evolution of Cefiderocol Non-Susceptibility in Pseudomonas aeruginosa in a Patient without Previous Exposure to the Antibiotic. Clin. Infect. Dis. 2021, 73, e4472–e4474. [Google Scholar] [CrossRef]
- Sadek, M.; Le Guern, R.; Kipnis, E.; Gosset, P.; Poirel, L.; Dessein, R.; Nordmann, P. Progressive in vivo development of resistance to cefiderocol in Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2023, 42, 61–66. [Google Scholar] [CrossRef]
- Werth, B.J.; Ashford, N.K.; Penewit, K.; Waalkes, A.; Holmes, E.A.; Bryan, A.; Salipante, S.J. Evolution of cefiderocol resistance in Stenotrophomonas maltophilia using in vitro serial passage techniques. JAC-Antimicrob. Resist. 2022, 4, dlac011. [Google Scholar] [CrossRef]
- Ito, A.; Sato, T.; Ota, M.; Takemura, M.; Nishikawa, T.; Toba, S.; Kohira, N.; Miyagawa, S.; Ishibashi, N.; Matsumoto, S.; et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob. Agents Chemother. 2018, 62, e01454-17. [Google Scholar] [CrossRef]
- Poirel, L.; Sadek, M.; Kusaksizoglu, A.; Nordmann, P. Co-resistance to ceftazidime-avibactam and cefiderocol in clinical isolates producing KPC variants. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2022, 41, 677–680. [Google Scholar] [CrossRef]
- Poirel, L.; Ortiz de la Rosa, J.M.; Sadek, M.; Nordmann, P. Impact of acquired broad-spectrum beta-lactamases on susceptibility to cefiderocol and newly developed beta-lactam/beta-lactamase inhibitor combinations in Escherichia coli and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2022, 66, e0003922. [Google Scholar] [CrossRef]
- Coppi, M.; Antonelli, A.; Niccolai, C.; Bartolini, A.; Bartolini, L.; Grazzini, M.; Mantengoli, E.; Farese, A.; Pieralli, F.; Mechi, M.T.; et al. Nosocomial outbreak by NDM-1-producing Klebsiella pneumoniae highly resistant to cefiderocol, Florence, Italy, August 2021 to June 2022. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2022, 27, 2200795. [Google Scholar] [CrossRef]
- Abdul-Mutakabbir, J.C.; Nguyen, L.; Maassen, P.T.; Stamper, K.C.; Kebriaei, R.; Kaye, K.S.; Castanheira, M.; Rybak, M.J. In vitro antibacterial activity of cefiderocol against multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e0264620. [Google Scholar] [CrossRef]
- Hobson, C.A.; Cointe, A.; Jacquier, H.; Choudhury, A.; Magnan, M.; Courroux, C.; Tenaillon, O.; Bonacorsi, S.; Birgy, A. Cross-resistance to cefiderocol and ceftazidime-avibactam in KPC beta-lactamase mutants and the inoculum effect. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 1172.e7–1172.e10. [Google Scholar] [CrossRef]
- Shields, R.K.; Iovleva, A.; Kline, E.G.; Kawai, A.; McElheny, C.L.; Doi, Y. Clinical evolution of AmpC-mediated ceftazidime-avibactam and cefiderocol resistance in Enterobacter cloacae complex following exposure to cefepime. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 2713–2716. [Google Scholar] [CrossRef]
- Kawai, A.; McElheny, C.L.; Iovleva, A.; Kline, E.G.; Sluis-Cremer, N.; Shields, R.K.; Doi, Y. Structural basis of reduced susceptibility to ceftazidime-avibactam and cefiderocol in Enterobacter cloacae due to AmpC R2 loop deletion. Antimicrob. Agents Chemother. 2020, 64, e00198-20. [Google Scholar] [CrossRef]
- Sato, T.; Ito, A.; Ishioka, Y.; Matsumoto, S.; Rokushima, M.; Kazmierczak, K.M.; Hackel, M.; Sahm, D.F.; Yamano, Y. Escherichia coli strains possessing a four amino acid YRIN insertion in PBP3 identified as part of the SIDERO-WT-2014 surveillance study. JAC-Antimicrob. Resist. 2020, 2, dlaa081. [Google Scholar] [CrossRef]
- Nordmann, P.; Shields, R.K.; Doi, Y.; Takemura, M.; Echols, R.; Matsunaga, Y.; Yamano, Y. Mechanisms of reduced susceptibility to cefiderocol among isolates from the CREDIBLE-CR and APEKS-NP clinical trials. Microb. Drug Resist. 2022, 28, 398–407. [Google Scholar] [CrossRef]
- Takemura, M.; Yamano, Y.; Matsunaga, Y.; Ariyasu, M.; Echols, R.; Den Nagata, T. 1266. Characterization of shifts in minimum inhibitory concentrations during treatment with cefiderocol or comparators in the phase 3 CREDIBLE-CR and APEKS-NP studies. Open Forum Infect. Dis. 2020, 7, S649–S650. [Google Scholar] [CrossRef]
- Kocer, K.; Boudour-Halil, D.; Chanthalangsy, Q.; Sahr, A.; Heeg, K.; Boutin, S.; Nurjadi, D. Genomic modification of TonB andeEmergence of small-colony phenotype in VIM- and NDM-producing Escherichia coli following cefiderocol exposure in vitro. Antimicrob. Agents Chemother. 2023, 67, e0011823. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Boutin, S.; Kocer, K.; Fiedler, M.O.; Storzinger, D.; Weigand, M.A.; Tan, B.; Richter, D.; Rupp, C.; Mieth, M.; et al. Rapid development of cefiderocol resistance in carbapenem-resistant Enterobacter cloacae during therapy is associated with heterogeneous mutations in the catecholate siderophore receptor cirA. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 74, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Price, T.K.; Davar, K.; Contreras, D.; Ward, K.W.; Garner, O.B.; Simner, P.J.; Yang, S.; Chandrasekaran, S. Case report and genomic analysis of cefiderocol-resistant Escherichia coli clinical isolates. Am. J. Clin. Pathol. 2022, 157, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.M.; Somprasong, N.; Hagen, J.P.; Nottingham, R.; Sahl, J.W.; Webb, J.R.; Mayo, M.; Currie, B.J.; Podin, Y.; Wagner, D.M.; et al. Exploring cefiderocol resistance mechanisms in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 2023, 67, e0017123. [Google Scholar] [CrossRef] [PubMed]
- McElheny, C.L.; Fowler, E.L.; Iovleva, A.; Shields, R.K.; Doi, Y. In vitro evolution of cefiderocol resistance in an NDM-producing Klebsiella pneumoniae due to functional loss of CirA. Microbiol. Spectr. 2021, 9, e0177921. [Google Scholar] [CrossRef]
- Gupta, A.; Landman, D.; Quale, J. Relationship of TonB-dependent receptors with susceptibility to cefiderocol in clinical isolates of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2022, 77, 1282–1285. [Google Scholar] [CrossRef]
- Simner, P.J.; Mostafa, H.H.; Bergman, Y.; Ante, M.; Tekle, T.; Adebayo, A.; Beisken, S.; Dzintars, K.; Tamma, P.D. Progressive development of cefiderocol resistance in Escherichia coli during therapy is associated with an increase in blaNDM-5 copy number and gene expression. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 75, 47–54. [Google Scholar] [CrossRef]
- Smoke, S.M.; Brophy, A.; Reveron, S.; Iovleva, A.; Kline, E.G.; Marano, M.; Miller, L.P.; Shields, R.K. Evolution and Transmission of Cefiderocol-Resistant Acinetobacter baumannii During an Outbreak in the Burn Intensive Care Unit. Clin. Infect. Dis. 2023, 76, e1261–e1265. [Google Scholar] [CrossRef]
- Sansone, P.; Giaccari, L.G.; Coppolino, F.; Aurilio, C.; Barbarisi, A.; Passavanti, M.B.; Pota, V.; Pace, M.C. Cefiderocol for carbapenem-resistant bacteria: Handle with care! A review of the real-world evidence. Antibiotics 2022, 11, 904. [Google Scholar] [CrossRef]
- Simner, P.J.; Beisken, S.; Bergman, Y.; Ante, M.; Posch, A.E.; Tamma, P.D. Defining baseline mechanisms of cefiderocol resistance in the Enterobacterales. Microb. Drug Resist. 2022, 28, 161–170. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Ma, X.; Zhao, L.; Guan, J.; Zhao, J.; Yu, W.; Li, Y.; Ni, W.; Gao, Z. Resistance to cefiderocol involved expression of PER-1 beta-lactamase and downregulation of iron transporter system in carbapenem-resistant Acinetobacter baumannii. Infect. Drug Resist. 2022, 15, 7177–7187. [Google Scholar] [CrossRef]
- Choby, J.E.; Ozturk, T.; Satola, S.W.; Jacob, J.T.; Weiss, D.S. Widespread cefiderocol heteroresistance in carbapenem-resistant Gram-negative pathogens. Lancet Infect. Dis. 2021, 21, 597–598. [Google Scholar] [CrossRef]
- Choby, J.E.; Ozturk, T.; Satola, S.W.; Jacob, J.T.; Weiss, D.S. Does cefiderocol heteroresistance explain the discrepancy between the APEKS-NP and CREDIBLE-CR clinical trial results? Lancet Microbe 2021, 2, e648–e649. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Guidance Document on Broth Microdilution Testing of Cefiderocol; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2020. [Google Scholar]

| Bacteria | Susceptibility | Resistance | ||
|---|---|---|---|---|
| Achromobacter spp. | Cefiderocol appeared as a promising therapeutic alternative for managing Achromobacter infections in patients with cystic fibrosis. | [53,54,55,56,57] | Overexpression of AxyABM efflux pump in A. xylosoxidans was associated with a threefold higher cefiderocol MIC. | [58] |
| Acinetobacter baumannii Complex | SIDERO-WT study 2014 reports 100% of susceptibility of 158 A. baumannii complex isolates. SIDERO-WT studies from 2015 to 2019 report percentages of susceptibility to cefiderocol ranging 97.6% to 99.1%. | [59,60] | Twenty-one cefiderocol-non-susceptible carbapenem-resistant A. baumannii isolates were characterized, highlighting the contribution β-lactamases, including the presence of an ESBL (PER-1), and deficiency of the iron siderophore transporter PiuA in several isolates. In the second study, by investigating a series of A. baumannii clinical isolates with elevated MICs of cefiderocol, the authors showed that PER-like β-lactamases and, to a lesser extent, NDM-like β-lactamases, significantly contributed to reduced susceptibility to cefiderocol. Thirdly, cefiderocol resistance was associated with reduced expression of the siderophore receptor gene pirA in A. baumannii isolates. | [61,62,63] |
| Burkholderia cepacia Complex | Cefiderocol was more potent in vitro than cefepime, ceftazidime-avibactam, ceftolozane-tazobactam, ciprofloxacin, and colistin. | [54,59,64,65] | Only 1 of the 4, 1 of the 12, and 5 of the 89 isolates tested had a cefiderocol MICs of 16, 8, and ≥8 μg/mL, respectively. | [59,64,65] |
| Citrobacter freundii Complex | All the isolates tested in the SIDERO-WT study 2014 (n = 303) were susceptible to cefiderocol. | [59,66] | Only 1 of the 32 and 1 of the 252 isolates tested had a cefiderocol MIC of 8 μg/mL. | [64,65] |
| Citrobacter koseri | SIDERO-WT study 2014 involving 73 isolates of C. koseri with MICs ranging from 0.006 to 4 μg/mL. | [59] | Study involving 73 isolates of C. koseri with MICs ranging from 0.008 to 4 μg/mL. In the second study, 1 of 169 isolates had a MIC of 8 μg/mL. | [59,65] |
| Escherichia coli | SIDERO-WT study 2014 involving 1529 isolates of E. coli with MICs ranging from ≤0.002 to 4 μg/mL. The MIC90 value of cefiderocol against E. coli isolates was 0.5 and 1 μg/mL in the second and third study, respectively. | [59,65,66] | In total, 10 out of 142 E. coli isolates were resistant to cefiderocol. In 26 of 1158 E. coli isolates harboring NDM-5 high levels of cefiderocol resistance was reported, in the second study. In the third study, a multidrug-resistant ST167 Escherichia coli clinical isolate recovered from a patient hospitalized in Switzerland produced NDM-35 showing ca. 10-fold increased hydrolytic activity toward cefiderocol compared to NDM-1. | [49,67,68] |
| Enterobacter cloacae Complex | In SIDERO-WT-2014 study and in another study involving 514 and 103 isolates of E. cloacae, respectively, the MIC90 value was 1 μg/mL. | [59,66] | In the first study, the authors report 2 cefiderocol-resistant ECC isolates in a collection of 10 isolates collected from diabetic patients. In the second study, the potential role of the VIM-1 carbapenemase in cefiderocol resistance in the ECC was highlighted. This effect is probably enhanced due to combination with additional mechanisms, such as ESBL production and siderophore inactivation. The presence of the NDM β-lactamase facilitates the emergence of resistance via nonsynonymous mutations of the cirA catecholate siderophore receptor in the third study. | [67,69,70] |
| Klebsiella (Enterobacter) aerogenes | The MIC90 value of cefiderocol against E. aerogenes isolates in was 0.5 μg/mL in both studies with 238 and 100 isolates. | [59,66] | In this study, 1158 cefiderocol resistant isolates were identified, of which 20 (1.7%) were K. aerogenes. | [49] |
| Klebsiella pneumoniae | The MIC90 value of cefiderocol against 765 and 100 K. pneumoniae isolates was 0.5 and 0.125 μg/mL, in the first and in the second study, respectively. In the second study MIC values ranged between ≤0.063–2 μg/mL. | [59,66] | In the first study, 7 out of 91 K. pneumoniae were resistant to cefiderocol. In the second study, 1158 cefiderocol-resistant isolates were identified, of which 798 (68.9%) were K. pneumoniae. In the third study, the authors characterized four cefiderocol-non-susceptible K. pneumoniae strains (4/86, 4.7%). | [49,50,67] |
| Klebsiella oxytoca | In the SIDERO-WT-2014 (505 isolates) and 2015 (349 isolates) the MIC values of cefiderocol ranged between ≤0.002 and 2 μg/mL and MIC90 value was 0.25 and 0.5 μg/mL, respectively. | [59,65] | In this study, 1158 cefiderocol resistant isolates were identified, of which 23 (2%) were K. oxytoca. | [49] |
| Morganella morganii | All of 37 and 32 isolates tested were classified as susceptible to cefiderocol, respectively, in two studies. | [71,72] | Only 1 out of 1158 isolates of M. morganii was classified as cefiderocol-resistant in this study. | [49] |
| Proteus spp. | All of 89 isolates tested were classified as susceptible to cefiderocol. | [71] | Only 2 out of 52 isolates were resistant to cefiderocol. In a second study, 1 of 10 isolates resistant to carbapenems was resistant to cefiderocol. | [67,72] |
| Providencia rettgeri | Treatment of complicated urinary tract infections (cUTI), due to Gram-negative bacteria in patients with limited or no alternative treatment options | [16] | One strain was obtained from the blood of a patient and it was resistant to all antimicrobials tested including the cefiderocol | [16,73] |
| Pseudomonas aeruginosa | In both studies all the collection of 120 and 33 isolates, respectively, were susceptible to cefiderocol. Five consecutive annual SIDERO-WT Studies reports a 99.9% of susceptibility to cefiderocol in a total of 7700 isolates. | [54,60,74] | Whole genome sequencing of P. aeruginosa non-susceptible to cefiderocol identified mutations in major iron transport pathways. The second study reports in vivo development of cefiderocol resistance among four sequential P. aeruginosa clinical isolates ST244 recovered from a single patient, without exposure to cefiderocol. | [75,76] |
| Serratia spp./Serratia marcescens | In SIDERO-WT-2014 study, in 503 isolates of Serratia spp. MIC90 value was 0.25 μg/mL and in another study involving 103 isolates of S. marcescens the MIC90 value was ≤0.0063 μg/mL. | [59,66] | In total, 14 out of 1158 isolates of S. marcescens were classified as cefiderocol-resistant in this study. | [49] |
| Stenotrophomonas maltophilia | Twenty-five meropenem-resistant S. maltophilia were susceptible to cefiderocol. In the second study, all the 7 isolates tested were also susceptible. SIDERO WT studies of 2014 and 2017 reports 100% of susceptibility to cefiderocol in 21 and 187 isolates of S. maltophilia, respectively. | [54,60,74] | S. maltophilia strains evolved cefiderocol resistance through different genetic pathways, but often involved iron transport. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingues, S.; Lima, T.; Saavedra, M.J.; Da Silva, G.J. An Overview of Cefiderocol’s Therapeutic Potential and Underlying Resistance Mechanisms. Life 2023, 13, 1427. https://doi.org/10.3390/life13071427
Domingues S, Lima T, Saavedra MJ, Da Silva GJ. An Overview of Cefiderocol’s Therapeutic Potential and Underlying Resistance Mechanisms. Life. 2023; 13(7):1427. https://doi.org/10.3390/life13071427
Chicago/Turabian StyleDomingues, Sara, Tiago Lima, Maria José Saavedra, and Gabriela Jorge Da Silva. 2023. "An Overview of Cefiderocol’s Therapeutic Potential and Underlying Resistance Mechanisms" Life 13, no. 7: 1427. https://doi.org/10.3390/life13071427
APA StyleDomingues, S., Lima, T., Saavedra, M. J., & Da Silva, G. J. (2023). An Overview of Cefiderocol’s Therapeutic Potential and Underlying Resistance Mechanisms. Life, 13(7), 1427. https://doi.org/10.3390/life13071427









