Therapy in the Course of Kidney Graft Rejection—Implications for the Cardiovascular System—A Systematic Review
Abstract
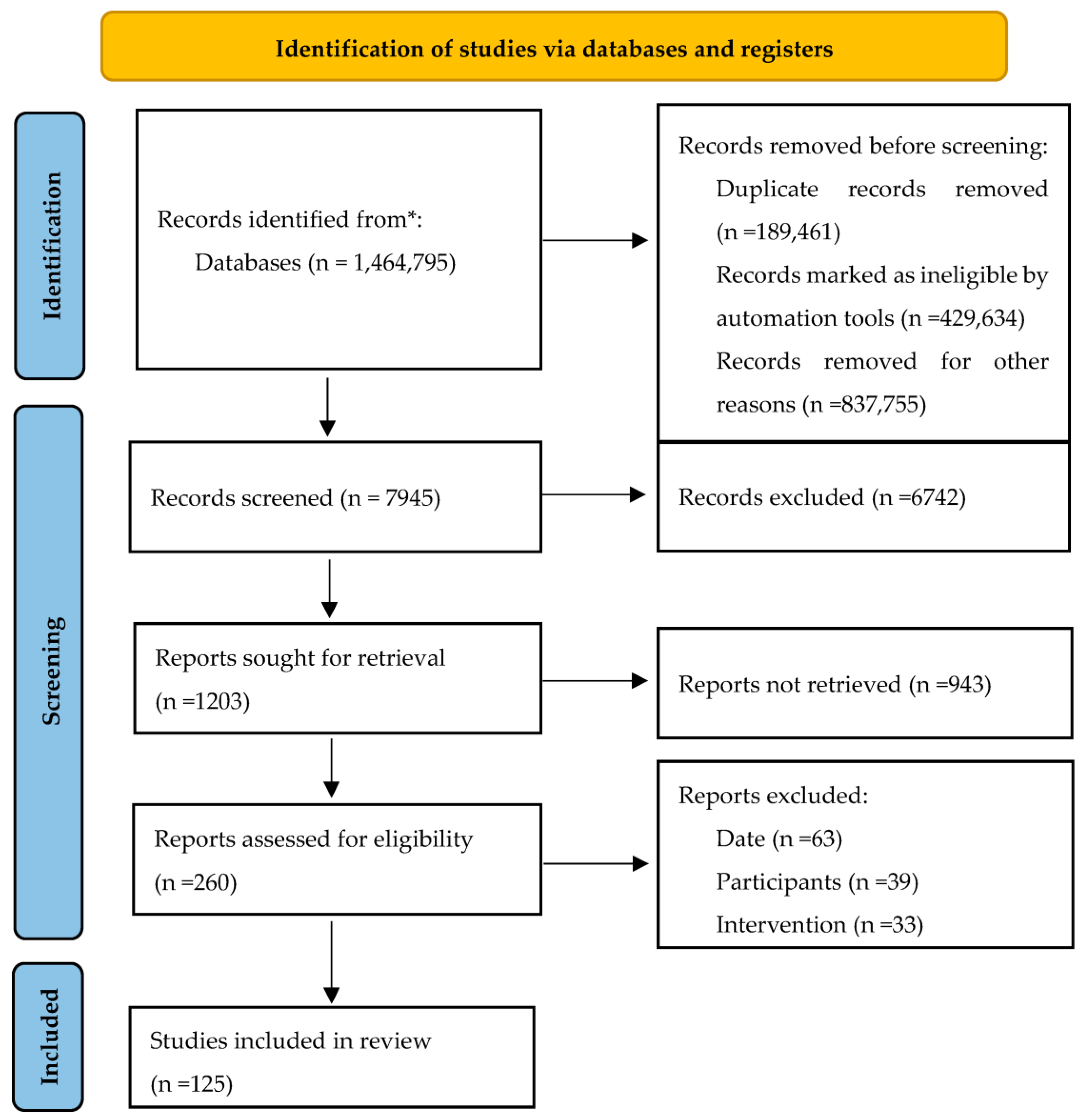
1. Introduction
2. Materials and Methods
3. Antibody-Mediated Rejection
3.1. Active ABMR
3.2. Chronic Active ABMR
3.3. Chronic (Inactive) ABMR
3.4. Evidence-Based Treatment Approaches in Active and Chronic Active ABMR
3.4.1. Plasma Exchange and IVIG
3.4.2. Complement Inhibitors
3.4.3. Rituximab
3.4.4. Imlifidase
3.4.5. Interleukin-6 Inhibitors
3.4.6. Splenectomy
4. T-Cell-Mediated Rejection
4.1. Acute TCMR—Treatment
4.2. Chronic Active TCMR—Treatment
5. Borderline Rejection—Treatment
6. Mixed Rejection—Treatment
- Cyclosporine A (CsA), MMF and steroids;
- Tac, MMF and steroids.
- MMF—the initial dose was 1.5 g per day.
- Calcineurin inhibitors—administered when the serum creatinine level decreased to 50% of pre-transplant levels. The initial dose of Tac was 0.6 mg/kg per day, while CsA was initiated at 4 mg/kg per day. Both were gradually raised in accordance with the restoration of graft function. Tac and CsA maintenance doses were adjusted to trough levels: 6–12 ng/mL for Tac during the first 6 months, followed by 4–8 ng/mL for the next 6 months; 150–250 ng/mL for CsA during the first 6 months, followed by 100–200 ng/mL for the following 6 months.
- Standard corticosteroid tapering—methylprednisolone IV (500 mg) on days 0–2, followed by oral prednisone 80 mg/day on day 3, which was then decreased to 10 mg/day increments to 20 mg/day. The dose of corticosteroid was then reduced slowly to 5 mg/day.
7. Long-Term Implications for the Cardiovascular System Caused by Treatment Methods Applied in Kidney Rejection Treatment
7.1. Prevention of Acute Graft Rejection
7.2. ABMR
7.3. TCMR
7.4. Borderline Rejection
- Tachycardia—Basiliximab 28 (8%) vs. placebo 21 (6%);
- Hypertension—Basiliximab 97 (27%) vs. placebo 93 (26%);
- Hypotension—Basiliximab 30 (8%) vs. placebo 38 (11%) [120].
8. Discussion
- -
- Selection bias: One potential limitation of our study is the possibility of selection bias in the included references. Despite our best efforts to conduct an extensive literature search, it is possible that some relevant studies were inadvertently excluded.
- -
- Publication bias: Another potential limitation is the presence of publication bias, whereby studies with positive or significant results are more likely to be published than those with negative or nonsignificant findings. This bias can impact the overall assessment of the therapy’s efficacy and safety.
- -
- Heterogeneity of study designs: The studies included in our review may exhibit heterogeneity in terms of study design, patient populations, therapeutic interventions and outcome measures. This heterogeneity could affect the comparability and synthesis of the results, potentially limiting the strength of our conclusions.
- -
- Time constraints and knowledge cutoff: Our review article was completed within a specific time frame, and the data collection was limited to the available literature up until May 2023. Newer studies published after this cutoff date may exist, potentially impacting the comprehensiveness of our review.
- -
- Potential confounding factors: The impact of therapy on the cardiovascular system in the context of kidney graft rejection can be influenced by various confounding factors, such as comorbidities, concurrent medications and patient characteristics. It is challenging to control for all these factors in observational studies, which could introduce confounding biases that limit the strength of our conclusions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABMR | Antibody-mediated rejection |
| TCMR | t-cell-mediated rejection |
| IVIG | intravenous immunoglobulin |
| HLA | human leukocyte antigen |
| DSA | donor-specific antibodies |
| CA | chronic active |
| PLEX | plasma exchange |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| IdeS | imlifidase |
| ATG | anti-thymocyte globulin |
| BCR | borderline cellular rejection |
| BL-R | Borderline TCMR |
| KT | kidney transplantation |
| B-CLL | B-cell chronic lymphocytic leukemia |
| MMF | mycophenolate mofetil |
| AHR | acute humoral rejection |
| Tac | tacrolimus |
| CsA | cyclosporine A |
| CNI | calcineurin inhibitor |
| VT | ventricular tachycardia |
References
- Moein, M.; Vlassis, I.; Kim, L.; Hanlon, M.; Saidi, R. Early readmissions post kidney transplantation: Lessons learned. Actas Urol. Esp. 2023. [Google Scholar] [CrossRef] [PubMed]
- Banas, B.; Krämer, B.K.; Krüger, B.; Kamar, N.; Undre, N. Long-Term Kidney Transplant Outcomes: Role of Prolonged-Release Tacrolimus. Transplant. Proc. 2020, 52, 102–110. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Imam, F.; Nadeem, A.; Al-Harbi, M.M.; Iqbal, M.; Rahman, S.; Al-Hosaini, K.A.; Bahashwan, S. Protection against tacrolimus-induced cardiotoxicity in rats by olmesartan and aliskiren. Toxicol. Mech. Methods 2014, 24, 697–702. [Google Scholar] [CrossRef]
- Lentine, K.L.; Brennan, D.C.; Schnitzler, M.A. Incidence and Predictors of Myocardial Infarction after Kidney Transplantation. J. Am. Soc. Nephrol. 2005, 16, 496–506. [Google Scholar] [CrossRef]
- Roufosse, C.; Simmonds, N.; Groningen, M.C.-V.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasińska, A.; Rabant, M.; et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, L.K.; Schagen, M.; de Weerd, A.; Reinders, M.; de Winter, B.; Hesselink, D.A. Investigational drugs for the treatment of kidney transplant rejection. Expert Opin. Investig. Drugs 2022, 31, 1087–1100. [Google Scholar] [CrossRef]
- Banasik, M.; Boratyńska, M.; Kościelska-Kasprzak, K.; Kamińska, D.; Bartoszek, D.; Żabińska, M.; Myszka, M.; Zmonarski, S.; Protasiewicz, M.; Nowakowska, B.; et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transpl. Int. 2014, 27, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Nowańska, K.; Wiśnicki, K.; Kuriata-Kordek, M.; Krajewska, M.; Banasik, M. The role of endothelin II type A receptor (ETAR) in transplant injury. Transpl. Immunol. 2022, 70, 101505. [Google Scholar] [CrossRef]
- Mengel, M.; Mannon, R.B. Banff and ABMR: Are we going in the right direction? Am. J. Transplant. 2021, 21, 2321–2322. [Google Scholar] [CrossRef]
- Racusen, L.C.; Colvin, R.B.; Solez, K.; Mihatsch, M.J.; Halloran, P.F.; Campbell, P.M.; Cecka, M.J.; Cosyns, J.; Demetris, A.J.; Fishbein, M.C.; et al. Antibody-Mediated Rejection Criteria—An Addition to the Banff ’97 Classification of Renal Allograft Rejection. Am. J. Transplant. 2003, 3, 708–714. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell– and antibody-mediated rejection. Am. J. Transplant. 2020, 20, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; Akalin, E.; et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell–mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am. J. Transplant. 2018, 18, 293–307. [Google Scholar] [CrossRef]
- Schinstock, C.A.; Mannon, R.B.; Budde, K.; Chong, A.S.; Haas, M.; Knechtle, S.; Lefaucheur, C.; Montgomery, R.A.; Nickerson, P.; Tullius, S.G.; et al. Recommended Treatment for Antibody-mediated Rejection after Kidney Transplantation: The 2019 Expert Consensus from the Transplantion Society Working Group. Transplantation 2020, 104, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Banasik, M.; Boratyńska, M.; Kościelska-Kasprzak, K.; Mazanowska, O.; Krajewska, M.; Zabińska, M.; Bartoszek, D.; Myszka, M.; Nowakowska, B.; Dawiskiba, T.; et al. The Impact of De Novo Donor-specific Anti-Human Leukocyte Antigen Antibodies on 5-Year Renal Transplant Outcome. Transplant. Proc. 2013, 45, 1449–1452. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.A.; Zachary, A.A.; Racusen, L.C.; Leffell, M.S.; King, K.E.; Burdick, J.; Maley, W.R.; Ratner, L.E. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation 2000, 70, 887–895. [Google Scholar] [CrossRef]
- Roberts, D.M.; Jiang, S.; Chadban, S.J. The Treatment of Acute Antibody-Mediated Rejection in Kidney Transplant Recipients—A Systematic Review. Transplantation 2012, 94, 775–783. [Google Scholar] [CrossRef]
- Loupy, A.; Lefaucheur, C. Antibody-Mediated Rejection of Solid-Organ Allografts. N. Engl. J. Med. 2018, 379, 1150–1160. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. Special Issue: KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Transplant. 2009, 9 (Suppl. S3), S1–S155. [Google Scholar] [CrossRef]
- Wan, S.S.; Ying, T.D.; Wyburn, K.; Roberts, D.M.; Wyld, M.; Chadban, S.J. The Treatment of Antibody-Mediated Rejection in Kidney Transplantation: An updated systematic review and meta-analysis. Transplantation 2018, 102, 557–568. [Google Scholar] [CrossRef]
- Velidedeoglu, E.; Cavaillé-Coll, M.W.; Bala, S.; Belen, O.A.; Wang, Y.; Albrecht, R. Summary of 2017 FDA Public Workshop. Transplantation 2018, 102, e257–e264. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Zeier, M.G.; Chapman, J.R.; Craig, J.C.; Ekberg, H.; Garvey, C.A.; Green, M.D.; Jha, V.; Josephson, M.A.; Kiberd, B.A.; et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int. 2010, 77, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, C.; Nochy, D.; Andrade, J.; Verine, J.; Gautreau, C.; Charron, D.; Hill, G.S.; Glotz, D.; Suberbielle-Boissel, C. Comparison of Combination Plasmapheresis/IVIg/ Anti-CD20 Versus High-Dose IVIg in the Treatment of Antibody-Mediated Rejection. Am. J. Transplant. 2009, 9, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Böhmig, G.A.; Wahrmann, M.; Regele, H.; Exner, M.; Robl, B.; Derfler, K.; Soliman, T.; Bauer, P.; Müllner, M.; Druml, W. Immunoadsorption in Severe C4d-Positive Acute Kidney Allograft Rejection: A Randomized Controlled Trial. Am. J. Transplant. 2007, 7, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Marks, W.H.; Mamode, N.; Montgomery, R.A.; Stegall, M.D.; Ratner, L.E.; Cornell, L.D.; Rowshani, A.T.; Colvin, R.B.; Dain, B.; Boice, J.A.; et al. Safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization therapy: A randomized trial. Am. J. Transplant. 2019, 19, 2876–2888. [Google Scholar] [CrossRef]
- Glotz, D.; Russ, G.; Rostaing, L.; Legendre, C.; Tufveson, G.; Chadban, S.; Grinyó, J.; Mamode, N.; Rigotti, P.; Couzi, L.; et al. Safety and efficacy of eculizumab for the prevention of antibody-mediated rejection after deceased-donor kidney transplantation in patients with preformed donor-specific antibodies. Am. J. Transplant. 2019, 19, 2865–2875. [Google Scholar] [CrossRef]
- Schinstock, C.A.; Bentall, A.J.; Smith, B.H.; Cornell, L.D.; Everly, M.; Gandhi, M.J.; Stegall, M.D. Long-term outcomes of eculizumab-treated positive crossmatch recipients: Allograft survival, histologic findings, and natural history of the donor-specific antibodies. Am. J. Transplant. 2019, 19, 1671–1683. [Google Scholar] [CrossRef]
- Cornell, L.D.; Schinstock, C.A.; Gandhi, M.J.; Kremers, W.K.; Stegall, M.D. Positive Crossmatch Kidney Transplant Recipients Treated with Eculizumab: Outcomes beyond 1 Year. Am. J. Transplant. 2015, 15, 1293–1302. [Google Scholar] [CrossRef]
- Viglietti, D.; Gosset, C.; Loupy, A.; Deville, L.; Verine, J.; Zeevi, A.; Glotz, D.; Lefaucheur, C. C1 Inhibitor in Acute Antibody-Mediated Rejection Nonresponsive to Conventional Therapy in Kidney Transplant Recipients: A Pilot Study. Am. J. Transplant. 2016, 16, 1596–1603. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Orandi, B.J.; Racusen, L.; Jackson, A.M.; Garonzik-Wang, J.M.; Shah, T.; Woodle, E.S.; Sommerer, C.; Fitts, D.; Rockich, K.; et al. Plasma-Derived C1 Esterase Inhibitor for Acute Antibody Mediated Rejection Following Kidney Transplantation: Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study. Am. J. Transplant. 2016, 16, 3468–3478. [Google Scholar] [CrossRef]
- Burton, S.A.; Amir, N.; Asbury, A.; Lange, A.; Hardinger, K.L. Treatment of antibody-mediated rejection in renal transplant patients: A clinical practice survey. Clin. Transplant. 2015, 29, 118–123. [Google Scholar] [CrossRef]
- Macklin, P.; Morris, P.J.; Knight, S.R. A systematic review of the use of rituximab for the treatment of antibody-mediated renal transplant rejection. Transplant. Rev. 2017, 31, 87–95. [Google Scholar] [CrossRef]
- Sautenet, B.; Blancho, G.; Büchler, M.; Morelon, E.; Toupance, O.; Barrou, B.; Ducloux, D.; Chatelet, V.; Moulin, B.; Freguin, C.; et al. One-year Results of the Effects of Rituximab on Acute Antibody-Mediated Rejection in Renal Transplantation: RITUX ERAH, a Multicenter Double-blind Randomized Placebo-controlled Trial. Transplantation 2016, 100, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Moreso, F.; Crespo, M.; Ruiz, J.C.; Torres, A.; Gutierrez-Dalmau, A.; Osuna, A.; Perelló, M.; Pascual, J.; Torres, I.B.; Redondo-Pachón, D.; et al. Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: A multicenter, prospective, randomized, double-blind clinical trial. Am. J. Transplant. 2018, 18, 927–935. [Google Scholar] [CrossRef]
- Budde, K.; Dürr, M. Any Progress in the Treatment of Antibody-Mediated Rejection? J. Am. Soc. Nephrol. 2018, 29, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C.; Lorant, T.; Choi, J.; Kjellman, C.; Winstedt, L.; Bengtsson, M.; Zhang, X.; Eich, T.; Toyoda, M.; Eriksson, B.-M.; et al. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N. Engl. J. Med. 2017, 377, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Winstedt, L.; Järnum, S.; Nordahl, E.A.; Olsson, A.; Runström, A.; Bockermann, R.; Karlsson, C.; Malmström, J.; Palmgren, G.S.; Malmqvist, U.; et al. Complete Removal of Extracellular IgG Antibodies in a Randomized Dose-Escalation Phase I Study with the Bacterial Enzyme IdeS—A Novel Therapeutic Opportunity. PLoS ONE 2015, 10, e0132011. [Google Scholar] [CrossRef] [PubMed]
- Lonze, B.E.; Tatapudi, V.S.; Weldon, E.P.; Min, E.S.; Ali, N.M.; Deterville, C.L.; Gelb, B.E.; Benstein, J.A.; Dagher, N.N.; Wu, M.; et al. IdeS (Imlifidase): A Novel Agent That Cleaves Human IgG and Permits Successful Kidney Transplantation Across High-strength Donor-specific Antibody. Ann. Surg. 2018, 268, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Aubert, O.; Vo, A.; Loupy, A.; Haas, M.; Puliyanda, D.; Kim, I.; Louie, S.; Kang, A.; Peng, A.; et al. Assessment of Tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a Potential Treatment for Chronic Antibody-Mediated Rejection and Transplant Glomerulopathy in HLA-Sensitized Renal Allograft Recipients. Am. J. Transplant. 2017, 17, 2381–2389. [Google Scholar] [CrossRef]
- Eskandary, F.; Dürr, M.; Budde, K.; Doberer, K.; Reindl-Schwaighofer, R.; Waiser, J.; Wahrmann, M.; Regele, H.; Spittler, A.; Lachmann, N.; et al. Clazakizumab in late antibody-mediated rejection: Study protocol of a randomized controlled pilot trial. Trials 2019, 20, 37. [Google Scholar] [CrossRef]
- Locke, J.E.; Zachary, A.A.; Haas, M.; Melancon, J.K.; Warren, D.S.; Simpkins, C.E.; Segev, D.L.; Montgomery, R.A. The Utility of Splenectomy as Rescue Treatment for Severe Acute Antibody Mediated Rejection. Am. J. Transplant. 2007, 7, 842–846. [Google Scholar] [CrossRef]
- Kaplan, B.; Gangemi, A.; Thielke, J.; Oberholzer, J.; Sankary, H.; Benedetti, E. Successful Rescue of Refractory, Severe Antibody Mediated Rejection with Splenectomy. Transplantation 2007, 83, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.H.; Shawar, S.H. Renal Transplantation Rejection. StatPearls Publ. 2023, 1, 1. [Google Scholar]
- Aziz, F.; Parajuli, S.; Garg, N.; Mohamed, M.; Zhong, W.; Djamali, A.; Mandelbrot, D. How Should Acute T-cell Mediated Rejection of Kidney Transplants Be Treated: Importance of Follow-up Biopsy. Transplant. Direct 2022, 8, e1305. [Google Scholar] [CrossRef] [PubMed]
- Kidney Transplantation in Adults: Treatment of Acute T Cell-Mediated (Cellular) Rejection. (n.d.). Available online: https://www.medilib.ir/uptodate/show/7358 (accessed on 22 May 2023).
- Bouatou, Y.; Viglietti, D.; Pievani, D.; Louis, K.; Van Huyen, J.-P.D.; Rabant, M.; Aubert, O.; Taupin, J.-L.; Glotz, D.; Legendre, C.; et al. Response to treatment and long-term outcomes in kidney transplant recipients with acute T cell–mediated rejection. Am. J. Transplant. 2019, 19, 1972–1988. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, C.; Côté, J.-M.; Sénécal, L.; Cardinal, H. Efficacy of Acute Cellular Rejection Treatment According to Banff Score in Kidney Transplant Recipients: A Systematic Review. Transplant. Direct 2016, 2, e115. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E. Evaluation and Treatment of Acute Rejection in Kidney Allografts. Clin. J. Am. Soc. Nephrol. 2020, 15, 430–438. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef]
- Mizera, J.; Pilch, J.; Kamińska, D.; Krajewska, M.; Donizy, P.; Banasik, M. Chronic Active T-Cell Mediated Kidney Rejection as a Clinically Significant Type of Allograft Loss? Diagnostics 2022, 12, 3220. [Google Scholar] [CrossRef]
- Noguchi, H.M.; Nakagawa, K.; Ueki, K.; Tsuchimoto, A.M.; Kaku, K.M.; Okabe, Y.M.; Nakamura, M.M. Response to Treatment for Chronic-active T Cell–mediated Rejection in Kidney Transplantation: A Report of 3 Cases. Transplant. Direct 2020, 6, e628. [Google Scholar] [CrossRef]
- Kung, V.L.; Sandhu, R.; Haas, M.; Huang, E. Chronic active T cell–mediated rejection is variably responsive to immunosuppressive therapy. Kidney Int. 2021, 100, 391–400. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Agrawal, N.; Sharma, A.; Taverniti, A.; P’ng, C.H.; Shingde, M.; Wong, G.; Chapman, J.R. The clinical and pathological significance of borderline T cell–mediated rejection. Am. J. Transplant. 2019, 19, 1452–1463. [Google Scholar] [CrossRef]
- Becker, J.; Chang, A.; Nickeleit, V.; Randhawa, P.; Roufosse, C. Banff Borderline Changes Suspicious for Acute T Cell–Mediated Rejection: Where Do We Stand? Am. J. Transplant. 2016, 16, 2654–2660. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.; Vázquez-Sánchez, T.; Sola, E.; Lopez, V.; Ruiz-Esteban, P.; Caballero, A.; Salido, E.; Leon, M.; Rodriguez, A.; Serra, N.; et al. Treatment of early borderline lesions in low immunological risk kidney transplant patients: A Spanish multicenter, randomized, controlled parallel-group study protocol: The TRAINING study. BMC Nephrol. 2022, 23, 357. [Google Scholar] [CrossRef] [PubMed]
- Klintmalm, G.B.; Feng, S.; Lake, J.R.; Vargas, H.E.; Wekerle, T.; Agnes, S.; Brown, K.A.; Nashan, B.; Rostaing, L.; Meadows-Shropshire, S.; et al. Belatacept-Based Immunosuppression in De Novo Liver Transplant Recipients: 1-Year Experience from a Phase II Randomized Study. Am. J. Transplant. 2014, 14, 1817–1827. [Google Scholar] [CrossRef]
- Onrust, S.V.; Wiseman, L.R. Basiliximab. Drugs 1999, 57, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Asensio, M.; Margarit, C.; Chavez, R.; Ortega, J.; Charco, R.; Iglesias, J. Induction with basiliximab reduces acute rejection in pediatric liver transplant patients treated with tacrolimus and steroids. Transplant. Proc. 2002, 34, 1970–1971. [Google Scholar] [CrossRef]
- Thistlethwaite, J.R.; Nashan, B.; Hall, M.; Chodoff, L.; Lin, T.H. Reduced acute rejection and superior 1-year renal allograft survival with basiliximab in patients with diabetes mellitus1. Transplantation 2000, 70, 784–790. [Google Scholar] [CrossRef]
- Kapic, E.; Becic, F.; Kusturica, J. Basiliximab, mechanism of action and pharmacological properties. Med. Arch. 2004, 58, 373–376. Available online: https://pubmed.ncbi.nlm.nih.gov/15648237/ (accessed on 22 May 2023).
- Swiatecka-Urban, A. Anti-Interleukin-2 Receptor Antibodies for the Prevention of Rejection in Pediatric Renal Transplant Patients. Pediatr. Drugs 2003, 5, 699–716. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Bradley, J.A.; Friend, P.J.; Firth, J.; Taylor, C.J.; Smith, K.G.C.; Thiru, S.; Jamieson, N.V.; Hale, G.; Waldmann, H.; et al. Alemtuzumab (CAMPATH 1H) Induction Therapy in Cadaveric Kidney Transplantation―Efficacy and Safety at Five Years. Am. J. Transplant. 2005, 5, 1347–1353. [Google Scholar] [CrossRef]
- Chan, K.; Taube, D.; Roufosse, C.; Cook, T.; Brookes, P.; Goodall, D.; Galliford, J.; Cairns, T.; Dorling, A.; Duncan, N.; et al. Kidney Transplantation with Minimized Maintenance: Alemtuzumab Induction with Tacrolimus Monotherapy—An Open Label, Randomized Trial. Transplantation 2011, 92, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.J.; Russell, N.K. Alemtuzumab (Campath-1H): A Systematic Review in Organ Transplantation. Transplantation 2006, 81, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.D.; O’Callaghan, J.; Knight, S.; Morris, P.J. Alemtuzumab Induction Therapy in Kidney Transplantation. Transplantation 2012, 93, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Jebrini, A.; Ruiz, A.C.F.; Hosni, M.; Jarmi, T. The Outcome of Tapered Steroid Regimen When Used to Treat Acute Borderline Cellular Rejection after Kidney Transplant: A Single-Center Experience. J. Clin. Med. Res. 2022, 14, 335–340. [Google Scholar] [CrossRef]
- Dale, L.; Brennan, C.; Batal, I.; Morris, H.; Jain, N.G.; Valeri, A.; Husain, S.A.; King, K.; Tsapepas, D.; Cohen, D.; et al. Treatment of borderline infiltrates with minimal inflammation in kidney transplant recipients has no effect on allograft or patient outcomes. Clin. Transplant. 2020, 34, e14019. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, J.; Subrt, P.; Paré, M.; Hartell, D.; Sénécal, L.; Blydt-Hansen, T.; Cardinal, H. Practice Patterns in the Treatment and Monitoring of Acute T Cell–Mediated Kidney Graft Rejection in Canada. Can. J. Kidney Health Dis. 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Kraus, E.S.; Parekh, R.S.; Oberai, P.; Lepley, D.; Segev, D.L.; Bagnasco, S.; Collins, V.; Leffell, M.; Lucas, D.; Rabb, H.; et al. Subclinical Rejection in Stable Positive Crossmatch Kidney Transplant Patients: Incidence and Correlations. Am. J. Transplant. 2009, 9, 1826–1834. [Google Scholar] [CrossRef]
- Feucht, H.E.; Felber, E.; Gokel, M.J.; Hillebrand, G.; Nattermann, U.; Brockmeyer, C.; Held, E.; Riethmüller, G.; Land, W.; Albert, E. Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin. Exp. Immunol. 1991, 86, 464–470. [Google Scholar] [CrossRef]
- Sellarés, J.; De Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the Causes of Kidney Transplant Failure: The Dominant Role of Antibody-Mediated Rejection and Nonadherence. Am. J. Transplant. 2012, 12, 388–399. [Google Scholar] [CrossRef]
- El-Zoghby, Z.M.; Stegall, M.D.; Lager, D.J.; Kremers, W.K.; Amer, H.; Gloor, J.M.; Cosio, F.G. Identifying Specific Causes of Kidney Allograft Loss. Am. J. Transplant. 2009, 9, 527–535. [Google Scholar] [CrossRef]
- Dörje, C.; Midtvedt, K.; Holdaas, H.; Naper, C.; Strøm, E.H.; Øyen, O.; Leivestad, T.; Aronsen, T.; Jenssen, T.; Flaa-Johnsen, L.; et al. Early Versus Late Acute Antibody-Mediated Rejection in Renal Transplant Recipients. Transplantation 2013, 96, 79–84. [Google Scholar] [CrossRef]
- De Freitas, D.G.; Sellarés, J.; Mengel, M.; Chang, J.; Hidalgo, L.G.; Famulski, K.S.; Sis, B.; Einecke, G.; Halloran, P.F. The Nature of Biopsies with “Borderline Rejection” and Prospects for Eliminating This Category. Am. J. Transplant. 2011, 12, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Ramachandran, S.; Banan, B.; Bharat, A.; Wang, X.; Benshoff, N.; Kreisel, D.; Gelman, A.E.; Mohanakumar, T. Immune Response to Tissue-Restricted Self-Antigens Induces Airway Inflammation and Fibrosis Following Murine Lung Transplantation. Am. J. Transplant. 2014, 14, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Moreso, F.; Carrera, M.; Goma, M.; Hueso, M.; Sellares, J.; Martorell, J.; Grinyó, J.M.; Serón, D. Early Subclinical Rejection as a Risk Factor for Late Chronic Humoral Rejection. Transplantation 2012, 93, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Gloor, J.M.; Sethi, S.; Stegall, M.D.; Park, W.D.; Moore, S.B.; DeGoey, S.; Griffin, M.D.; Larson, T.S.; Cosio, F.G. Transplant Glomerulopathy: Subclinical Incidence and Association with Alloantibody. Am. J. Transplant. 2007, 7, 2124–2132. [Google Scholar] [CrossRef]
- Wiebe, C.; Gibson, I.W.; Blydt-Hansen, T.D.; Karpinski, M.; Ho, J.; Storsley, L.J.; Goldberg, A.; Birk, P.E.; Rush, D.N.; Nickerson, P.W. Evolution and Clinical Pathologic Correlations of De Novo Donor-Specific HLA Antibody Post Kidney Transplant. Am. J. Transplant. 2012, 12, 1157–1167. [Google Scholar] [CrossRef]
- Randhawa, P. T-cell-mediated rejection of the kidney in the era of donor-specific antibodies. Curr. Opin. Organ Transplant. 2015, 20, 325–332. [Google Scholar] [CrossRef]
- Haas, M.; Mirocha, J.; Reinsmoen, N.L.; Vo, A.A.; Choi, J.; Kahwaji, J.M.; Peng, A.; Villicana, R.; Jordan, S.C. Differences in pathologic features and graft outcomes in antibody-mediated rejection of renal allografts due to persistent/recurrent versus de novo donor-specific antibodies. Kidney Int. 2017, 91, 729–737. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Z.-H.; Cheng, Z.; Chen, J.; Ji, S.; Zeng, C.; Li, L.-S. Treatment of early mixed cellular and humoral renal allograft rejection with tacrolimus and mycophenolate mofetil. Kidney Int. 2007, 71, 24–30. [Google Scholar] [CrossRef]
- Nickeleit, V. Kidney transplants, antibodies and rejection: Is C4d a magic marker? Nephrol. Dial. Transplant. 2003, 18, 2232–2239. [Google Scholar] [CrossRef]
- Abramowicz, D.; Vanrenterghem, Y.; Squifflet, J.-P.; Kuypers, D.; Mourad, M.; Meurisse, M.; Wissing, M. Efficacy and cardiovascular safety of daclizumab, mycophenolate mofetil, tacrolimus, and early steroid withdrawal in renal transplant recipients: A multicenter, prospective, pilot trial. Clin. Transplant. 2005, 19, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Artz, M.A.; Boots, J.M.M.; Ligtenberg, G.; Roodnat, J.I.; Christiaans, M.H.L.; Vos, P.F.; Moons, P.; Borm, G.; Hilbrands, L.B. Conversion from Cyclosporine to Tacrolimus Improves Quality-of-Life Indices, Renal Graft Function and Cardiovascular Risk Profile. Am. J. Transplant. 2004, 4, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Artz, M.A.; Boots, J.M.; Ligtenberg, G.; Roodnat, J.I.; Christiaans, M.H.; Vos, P.F.; Blom, H.J.; Sweep, F.C.; Demacker, P.N.; Hilbrands, L.B. Improved Cardiovascular Risk Profile and Renal Function in Renal Transplant Patients after Randomized Conversion from Cyclosporine to Tacrolimus. J. Am. Soc. Nephrol. 2003, 14, 1880–1888. [Google Scholar] [CrossRef]
- Ravanshad, Y.; Azarfar, A.; Mehrad-Majd, H.; Esmaeeli, M.; Aval, S.; Emadzadeh, M.; Salehi, M.; Moradi, A.; Golsorkhi, M.; Khazaei, M. Comparison of tacrolimus and cyclosporine for immunosuppression after renal transplantation: An updated systematic review and meta-analysis. Saudi J. Kidney Dis. Transplant. 2018, 29, 1376–1385. [Google Scholar] [CrossRef]
- Mayer, A. Chronic rejection and graft half-life: Five-year follow-up of the european tacrolimus multicenter renal study. Transplant. Proc. 2002, 34, 1491–1492. [Google Scholar] [CrossRef]
- Mycophenolate: MedlinePlus Drug Information. (n.d.). Available online: https://medlineplus.gov/druginfo/meds/a601081.html (accessed on 22 May 2023).
- Morales, J.M.; Domínguez-Gil, B. Impact of Tacrolimus and Mycophenolate Mofetil Combination on Cardiovascular Risk Profile after Kidney Transplantation. J. Am. Soc. Nephrol. 2006, 17, S296–S303. [Google Scholar] [CrossRef]
- Wiśniewska, B. Terapeutyczna plazmafereza w praktyce klinicznej. Hematologia 2019, 9, 306–317. [Google Scholar] [CrossRef]
- Caress, J.B.; Kennedy, B.L.; Eickman, K.D. Safety of intravenous immunoglobulin treatment. Expert Opin. Drug Saf. 2010, 9, 971–979. [Google Scholar] [CrossRef]
- Stenton, S.B.; Dalen, D.; Wilbur, K. Myocardial Infarction Associated with Intravenous Immune Globulin. Ann. Pharmacother. 2005, 39, 2114–2118. [Google Scholar] [CrossRef]
- Core Summary of Product Characteristics for Human Normal Immunoglobulin for Intravenous Administration (IVIg)-Scientific Guideline. European Medicines Agency; (n.d.). Available online: https://www.ema.europa.eu/en/core-summary-product-characteristics-human-normal-immunoglobulin-intravenous-administration-ivig (accessed on 22 May 2023).
- Poterucha, J.T.; Westberg, M.; Nerheim, P.; Lovell, J.P. Rituximab-induced polymorphic ventricular tachycardia. Tex. Heart Inst. J. 2010, 37, 218–220. [Google Scholar] [PubMed]
- Kanamori, H.; Tsutsumi, Y.; Mori, A.; Kawamura, T.; Obara, S.; Shimoyama, N.; Tanaka, J.; Asaka, M.; Imamura, M.; Masauzi, N. Delayed Reduction in Left Ventricular Function following Treatment of Non-Hodgkin’s Lymphoma with Chemotherapy and Rituximab, Unrelated to Acute Infusion Reaction. Cardiology 2006, 105, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Kars, T.U.; Yorgancı, Z.F.; Yaşkıran, O.; Tekinalp, A.; Demircioğlu, S. Rituximab-induced severe acute thrombocytopenia in a patient with splenic marginal zone lymphoma. J. Oncol. Pharm. Pract. 2022. [Google Scholar] [CrossRef]
- Leung, V.S.; Lin, Y. Rituximab-induced acute lympholysis and pancytopenia after COVID-19 vaccination. Clin. Case Rep. 2021, 9, e04517. [Google Scholar] [CrossRef] [PubMed]
- Altheaby, A.; Alloqmani, D.; AlShammari, R.; Alsuhaibani, A.; Hakeem, A.; Alam, S.; Alharbi, S.; Al Zunitan, M.; Bosaeed, M.; Alharbi, N.K. Safety and Efficacy of the COVID-19 Vaccine in Kidney Transplant Recipients. Cureus 2022, 14, e24753. [Google Scholar] [CrossRef]
- Raquet, E.; Nolte, M.W.; May, F.; Müller-Cohrs, J.; Björkqvist, J.; Dickneite, G.; Schürmann, D.; Herzog, E.; Pragst, I. C1-esterase inhibitor treatment: Preclinical safety aspects on the potential prothrombotic risk. Thromb. Haemost. 2014, 112, 960–971. [Google Scholar] [CrossRef]
- Al-Salama, Z.T. Imlifidase: First Approval. Drugs 2020, 80, 1859–1864. [Google Scholar] [CrossRef]
- Idefirix|European Medicines Agency. (n.d.). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/idefirix (accessed on 22 May 2023).
- RoActemra|European Medicines Agency. (n.d.). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/roactemra (accessed on 15 April 2022).
- Sheppard, M.; Laskou, F.; Stapleton, P.P.; Hadavi, S.; Dasgupta, B. Tocilizumab (Actemra). Hum. Vaccines Immunother. 2017, 13, 1972–1988. [Google Scholar] [CrossRef]
- Weledji, E.P. Benefits and risks of splenectomy. Int. J. Surg. 2014, 12, 113–119. [Google Scholar] [CrossRef]
- Seale, J.P.; Compton, M.R. Side-effects of corticosteroid agents. Med. J. Aust. 1986, 144, 139–142. Available online: https://pubmed.ncbi.nlm.nih.gov/3511355/ (accessed on 22 May 2023). [CrossRef]
- Fan, H.; Morand, E.F. Targeting the side effects of steroid therapy in autoimmune diseases: The role of GILZ. Discov. Med. 2012, 13, 123–133. [Google Scholar]
- Methylprednisolone|C22H30O5|CID 6741. PubChem; (n.d.). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Methylprednisolone#section=Antidote-and-Emergency-Treatment (accessed on 22 May 2023).
- Veenstra, D.L.; Best, J.H.; Hornberger, J.; Sullivan, S.D.; Hricik, D.E. Incidence and long-term cost of steroid-related side effects after renal transplantation. Am. J. Kidney Dis. 1999, 33, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Steiner, R.W.; Awdishu, L. Steroids in kidney transplant patients. Semin. Immunopathol. 2011, 33, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Munker, R.; Siddiqui, S.; Cox, J.; Herzig, R.; Palaniyandi, S.; Hildebrandt, G.C. Anti-thymocyte globulin in haematology: Recent developments. Indian J. Med. Res. 2019, 150, 221–227. [Google Scholar] [CrossRef] [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012; Antithymocyte Globulin. [Updated 25 July 2017]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547976/ (accessed on 20 June 2023).
- Kolonko, A.; Więcek, A. Safety of Antithymocyte Globulin Use in Kidney Graft Recipients during the COVID-19 Pandemic. Ann. Transplant. 2021, 26, e933001-1. [Google Scholar] [CrossRef] [PubMed]
- Büchler, M.; de Ligny, B.H.; Madec, C.; Lebranchu, Y.; The French Thymoglobuline Pharmacovigilance Study Group. Induction therapy by anti-thymocyte globulin (rabbit) in renal transplantation: A 1-yr follow-up of safety and efficacy. Clin. Transplant. 2003, 17, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Everolimus: MedlinePlus Drug Information. (n.d.). Available online: https://medlineplus.gov/druginfo/meds/a609032.html#side-effects (accessed on 22 May 2023).
- Arena, C.; Bizzoca, M.E.; Caponio, V.C.A.; Troiano, G.; Zhurakivska, K.; Leuci, S.; Muzio, L.L. Everolimus therapy and side-effects: A systematic review and meta-analysis. Int. J. Oncol. 2021, 59, 54. [Google Scholar] [CrossRef]
- Uchida, M.; Nakano, K.; Fujiwara, M.; Uesawa, Y.; Shimizu, T. Comprehensive analysis of everolimus-induced adverse events using the Japanese real-world database. J. Clin. Pharm. Ther. 2022, 47, 1173–1180. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, C.-S.; Sun, N.; Lv, W.; Chen, B.-M.; Zhang, J.-L. Basiliximab application on liver recipients: A meta-analysis of randomized controlled trials. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 139–146. [Google Scholar] [CrossRef]
- Ponticelli, C. Basiliximab: Efficacy and safety evaluation in kidney transplantation. Expert Opin. Drug Saf. 2014, 13, 373–381. [Google Scholar] [CrossRef]
- Lladó, L.; Xiol, X.; Figueras, J.; Ramos, E.; Memba, R.; Serrano, T.; Torras, J.; Garcia-Gil, A.; Gonzalez-Pinto, I.; Castellote, J.; et al. Immunosuppression without steroids in liver transplantation is safe and reduces infection and metabolic complications: Results from a prospective multicenter randomized study. J. Hepatol. 2006, 44, 710–716. [Google Scholar] [CrossRef]
- Aalamian, Z. Reducing Adverse Effects of Immunosuppressive Agents in Kidney Transplant Recipients. Prog. Transplant. 2001, 11, 271–284. [Google Scholar] [CrossRef]
- Simulect|European Medicines Agency. (n.d.). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/simulect (accessed on 22 May 2023).
- Basquiera, A.L.; Berretta, A.R.; García, J.J.; Palazzo, E.D. Coronary ischemia related to alemtuzumab therapy. Ann. Oncol. 2004, 15, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Monsuez, J.-J.; Charniot, J.-C.; Vignat, N.; Artigou, J.-Y. Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol. 2010, 144, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Attarian, S.; Wang, C.Y.; Romero, J.; Barta, S.K.; Aparo, S.; Menegus, M.A. Alemtuzumab induced ST-segment elevation and acute myocardial dysfunction. J. Cardiol. Cases 2014, 10, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Lemtrada|European Medicines Agency. (n.d.). Available online: https://www.ema.europa.eu/en/medicines/human/referrals/lemtrada (accessed on 22 May 2023).
- Adams, P.S.; Shapiro, R.; Hilmi, I.A. Postoperative Cardiac Tamponade After Kidney Transplantation. Transplantation 2013, 95, e18–e19. [Google Scholar] [CrossRef]
| In order to diagnose Active ABMR, all 3 of the following criteria must be met: | |
| 1. |
Histologic evidence of acute tissue injury, including 1 or more of the following:
|
| 2. |
Evidence of current/recent antibody interaction with vascular endothelium, including 1 or more of the following:
|
| 3. | Serologic evidence of donor-specific antibodies (DSA to HLA or other antigens). C4d staining or expression of validated transcripts/classifiers as noted above in criterion 2 may substitute for DSA; however, thorough DSA testing, including testing for non-HLA antibodies if HLA antibody testing is negative, is strongly advised whenever criteria 1 and 2 are met |
| In order to diagnose Chronic Active ABMR, all 3 of the following criteria must be met: | |
| 1. |
Morphologic evidence of chronic tissue injury, including 1 or more of the following:
|
| 2. | Identical to criterion 2 for active ABMR above. |
| 3. | Identical to criterion 3 for active ABMR above, including strong recommendation for DSA testing whenever criteria 1 and 2 are met. Biopsies meeting criterion 1 but not criterion 2 with current or prior evidence of DSA (post transplant) may be stated as showing chronic ABMR; however, remote DSA should not be considered for diagnosis of chronic active or active ABMR. |
| Chronic (inactive) ABMR | |
| 1. | cg > 0 and/or severe ptcml (ptcml1). |
| 2. | Absence of criterion 2 of current/recent antibody interaction with the endothelium. |
| 3. | Prior documented diagnosis of active or chronic active ABMR and/or documented prior evidence of DSA. |
| i | t | v | |
|---|---|---|---|
| Grade IA | Interstitial inflammation involves more than 25% of non-sclerotic cortical parenchyma | Moderate tubulitis involving at least 1 tubule excluding severely atrophic tubules | - |
| Grade IB | Interstitial inflammation involves more than 25% of non-sclerotic cortical parenchyma | Severe tubulitis involving at least 1 tubule excluding severely atrophic tubules | - |
| Grade IIA | Interstitial inflammation can be present but does not have to | Tubulitis can be present but does not have to | Mild to moderate intimal arteritis |
| Grade II B | Interstitial inflammation can be present but does not have to | Tubulitis can be present but does not have to | Severe intimal arteritis |
| Grade III | Interstitial inflammation can be present but does not have to | Tubulitis can be present but does not have to | Transmural arteritis or/and arterial fibrinoid necrosis, which involve medial smooth muscle together with mononuclear cell intimal arteriris |
| i-IFTA | ti | t | cv | |
|---|---|---|---|---|
| Grade IA | Interstitial inflammation involves not less than 25% of sclerotic cortical parenchyma | Interstitial inflammation involves not less than 25% of total cortical parenchyma | Moderate tubulitis involving at least 1 tubule excluding severely atrophic tubules | - |
| Grade IB | Interstitial inflammation involves not less than 25% of sclerotic cortical parenchyma | Interstitial inflammation involves not less than 25% of total cortical parenchyma | Severe tubulitis involving at least 1 tubule excluding severely atrophic tubules | - |
| Grade II | Chronic allograft arteriopathy including arterial intimal fibrosis together with mononuclear cell inflammation in fibrosis. Moreover, the formation of neointima |
| To diagnose BCR, all 3 of the following criteria must occur: | |
| 1. | Foci of tubulitis (t1, t2 or t3). |
| 2. | Mild interstitial inflammation (i1), or mild (t1) tubulitis with moderate-severe interstitial inflammation (i2 or i3). |
| 3. | No intimal or transmural arteritis (v = 0). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizera, J.; Pilch, J.; Giordano, U.; Krajewska, M.; Banasik, M. Therapy in the Course of Kidney Graft Rejection—Implications for the Cardiovascular System—A Systematic Review. Life 2023, 13, 1458. https://doi.org/10.3390/life13071458
Mizera J, Pilch J, Giordano U, Krajewska M, Banasik M. Therapy in the Course of Kidney Graft Rejection—Implications for the Cardiovascular System—A Systematic Review. Life. 2023; 13(7):1458. https://doi.org/10.3390/life13071458
Chicago/Turabian StyleMizera, Jakub, Justyna Pilch, Ugo Giordano, Magdalena Krajewska, and Mirosław Banasik. 2023. "Therapy in the Course of Kidney Graft Rejection—Implications for the Cardiovascular System—A Systematic Review" Life 13, no. 7: 1458. https://doi.org/10.3390/life13071458
APA StyleMizera, J., Pilch, J., Giordano, U., Krajewska, M., & Banasik, M. (2023). Therapy in the Course of Kidney Graft Rejection—Implications for the Cardiovascular System—A Systematic Review. Life, 13(7), 1458. https://doi.org/10.3390/life13071458






