Comparing Tear Film Viscosity between Sjögren and Non-Sjögren Dry Eye Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment Protocol
2.3. Evaluating the Subjective Severity of Dry Eye via the OSDI Questionnaire
2.4. Determination of Tear Volume on the Ocular Surface
2.5. Assessment of Tear Film Viscosity via Reflective Light Particles Spreading on the Cornea
2.6. Determination of Tear Volume on the Ocular Surface
2.7. Evaluation of Noninvasive Keratograph Break-Up Time (NIKBUT)
2.8. Evaluation of Meibomian Gland Dropout
2.9. Evaluation of Oxford Staining Score
2.10. Sample Size Estimation
2.11. Statistical Analysis
3. Results
3.1. Clinical Profile of Dry Eye Patients with and without Sjögren Syndrome
3.2. Comparison of the Power-Law Fitting Curve between SS- and Non-SS-DED Subjects
3.3. The Momentary Moving Speed of Reflective Light Particles at the Early and Late Tear Film Spreading Phases
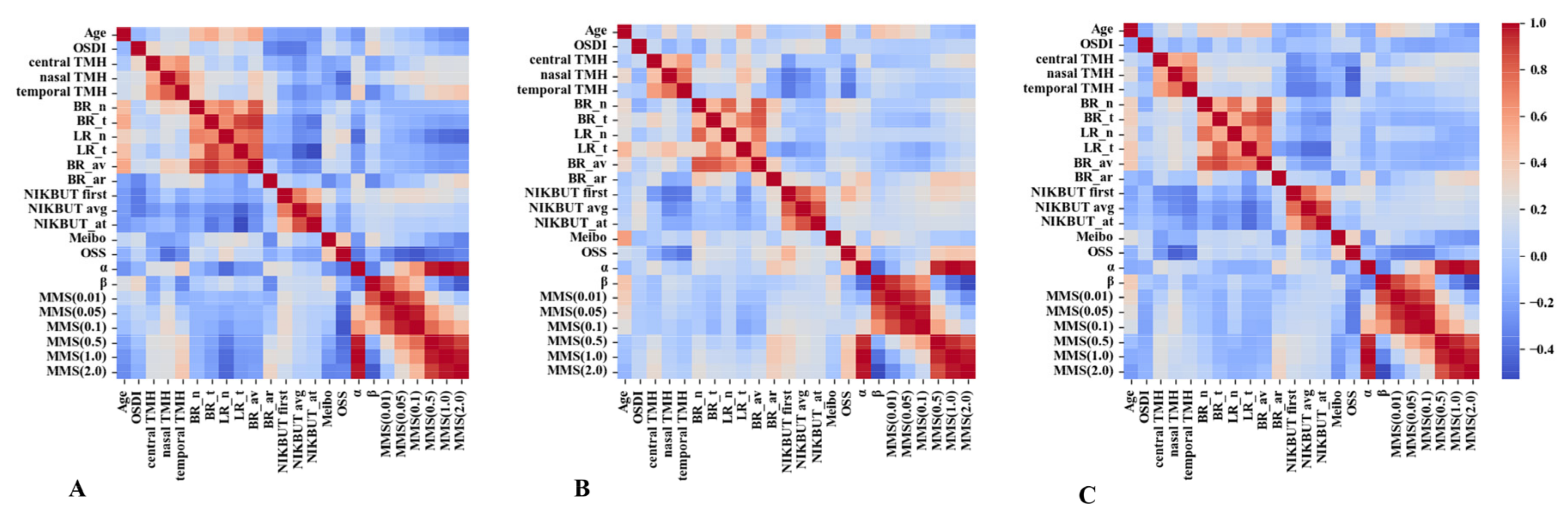
3.4. Correlation between TFV Indices and Classical DED Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pflugfelder, S.C.; Stern, M.E. Biological functions of tear film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Recchioni, A.; Mocciardini, E.; Ponzini, E.; Tavazzi, S. Viscoelastic properties of the human tear film. Exp. Eye Res. 2020, 219, 109083. [Google Scholar] [CrossRef] [PubMed]
- Lievens, C.; Berdy, G.; Douglass, D.; Montaquila, S.; Lin, H.; Simmons, P.; Carlisle-Wilcox, C.; Vehige, J.; Haque, S. Evaluation of an enhanced viscosity artificial tear for moderate to severe dry eye disease: A multicenter, double-masked, randomized 30-day study. Contact Lens Anterior Eye 2019, 42, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Simmons, P.A.; Vehige, J.G. Clinical performance of a mid-viscosity artificial tear for dry eye treatment. Cornea 2007, 26, 294–302. [Google Scholar] [CrossRef]
- Hannemann, E.; Erb, C. The Impact of Artificial Tear Viscosity on the Results of Optical Coherence Tomography. Klin. Monbl. Augenheilkd. 2021, 238, 1004–1009. [Google Scholar] [CrossRef]
- Dalton, K.; Subbaraman, L.N.; Rogers, R.; Jones, L. Physical properties of soft contact lens solutions. Optom. Vis. Sci. 2008, 85, 122–128. [Google Scholar] [CrossRef]
- Pena-Verdeal, H.; Garcia-Queiruga, J.; García-Resúa, C.; Yebra-Pimentel, E.; Giráldez, M.J. Osmolality and pH of commercially available contact lens care solutions and eye drops. Contact Lens Anterior Eye 2021, 44, 101379. [Google Scholar] [CrossRef]
- Gouveia, S.M.; Tiffany, J.M. Human tear viscosity: An interactive role for proteins and lipids. Biochim. Biophys. Acta 2005, 1753, 155–163. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Eftimov, P.; Yokoi, N. Contribution of Mucins towards the Physical Properties of the Tear Film: A Modern Update. Int. J. Mol. Sci. 2019, 20, 6132. [Google Scholar] [CrossRef] [Green Version]
- Bron, A.J.; Mengher, L.S. The ocular surface in keratoconjunctivitis sicca. Eye 1989, 3, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Tiffany, J.M. The viscosity of human tears. Int. Ophthalmol. 1991, 15, 371–376. [Google Scholar] [CrossRef]
- Varikooty, J.; Keir, N.; Simpson, T. Estimating tear film spread and stability through tear hydrodynamics. Optom. Vis. Sci. 2012, 89, E1119–E1124. [Google Scholar] [CrossRef]
- Lai, H.Y.; Fang, P.C.; Chen, A.; Kuo, M.T. Grading reliability of the tear film viscosity examination. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2287–2294. [Google Scholar] [CrossRef]
- Lai, H.Y.; Kuo, M.T.; Fang, P.C.; Lin, C.C.; Chien, C.C.; Cho, W.H.; Chen, A.; Lai, I.C. Tracking the Reflective Light Particles Spreading on the Cornea: An Emerging Assessment for Tear Film Homeostasis. Transl. Vis. Sci. Technol. 2019, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Zang, S.; Cui, Y.; Cui, Y.; Fei, W. Meibomian gland dropout in Sjögren’s syndrome and non-Sjögren’s dry eye patients. Eye 2018, 32, 1681–1687. [Google Scholar] [CrossRef] [Green Version]
- Akpek, E.K.; Bunya, V.Y.; Saldanha, I.J. Sjögren’s Syndrome: More Than Just Dry Eye. Cornea 2019, 38, 658–661. [Google Scholar] [CrossRef]
- Akpek, E.K.; Wu, H.Y.; Karakus, S.; Zhang, Q.; Masli, S. Differential Diagnosis of Sjögren Versus Non-Sjögren Dry Eye through Tear Film Biomarkers. Cornea 2020, 39, 991–997. [Google Scholar] [CrossRef]
- Kang, Y.S.; Lee, H.S.; Li, Y.; Choi, W.; Yoon, K.C. Manifestation of meibomian gland dysfunction in patients with Sjögren’s syndrome, non-Sjögren’s dry eye, and non-dry eye controls. Int. Ophthalmol. 2018, 38, 1161–1167. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Talarico, R.; Tzioufas, A.G.; Bombardieri, S. Classification criteria for Sjogren’s syndrome: A critical review. J. Autoimmun. 2012, 39, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Anaya, J.M.; Rojas-Villarraga, A.; Mantilla, R.D.; Arcos-Burgos, M.; Sarmiento-Monroy, J.C. Polyautoimmunity in Sjögren Syndrome. Rheum. Dis. Clin. N. Am. 2016, 42, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Qu, J.H.; Zhang, X.Y.; Sun, X.G. Repeatability and Reproducibility of Noninvasive Keratograph 5M Measurements in Patients with Dry Eye Disease. J. Ophthalmol. 2016, 2016, 8013621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, W.H.; Fabg, P.C.; Yu, H.J.; Lin, P.W.; Huang, H.M.; Kuo, M.T. Analysis of tear film spatial instability for pediatric myopia under treatment. Sci. Rep. 2020, 10, 14789. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Yang, J.; Wei, R.; Yang, L.; Zhao, S.; Wang, X. Evaluation of Dry Eye and Meibomian Gland Dysfunction in Teenagers with Myopia through Noninvasive Keratograph. J. Ophthalmol. 2016, 2016, 6761206. [Google Scholar] [CrossRef] [Green Version]
- Kuo, M.T.; Fang, P.C.; Kuo, S.F.; Chen, A.C.; Huang, Y.T. Tear Proteomics Study of Dry Eye Disease: Which Eye Do You Adopt as the Representative Eye for the Study? Int. J. Mol. Sci. 2021, 22, 422. [Google Scholar] [CrossRef]
- Hsiao, Y.T.; Huang, Y.T.; Yu, H.J.; Fang, P.C.; Kuo, M.T. Tear Proteomics Approach to Distinguishing Primary from Secondary Sjögren’s Syndrome for Dry Eye Patients with Long-Term Instillation of Eyedrops. Int. J. Mol. Sci. 2022, 23, 15239. [Google Scholar] [CrossRef]
- García-Marqués, J.V.; Talens-Estarelles, C.; García-Lázaro, S.; Cerviño, A. Assessment of condition-induced changes on the ocular surface using novel methods to assess the tear film dynamics and the lipid layer. Contact Lens Anterior Eye 2023, 46, 101799. [Google Scholar] [CrossRef]
- García-Marqués, J.V.; Talens-Estarelles, C.; Martínez-Albert, N.; García-Lázaro, S.; Cerviño, A. An Emerging Method to Assess Tear Film Spread and Dynamics as Possible Tear Film Homeostasis Markers. Curr. Eye Res. 2021, 46, 1291–1298. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, X.; Xu, Y.; Yao, Y.F. Assessment of Tear Film and Bulbar Redness by Keratograph 5M in Pediatric Patients After Orthokeratology. Eye Contact Lens 2018, 44, S382–S386. [Google Scholar] [CrossRef]
- Pult, H.; Riede-Pult, B. Comparison of subjective grading and objective assessment in meibography. Contact Lens Anterior Eye 2013, 36, 22–27. [Google Scholar] [CrossRef]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef]
- Kohn, M.A.; Senyak, J. Sample Size Calculators [Website]. UCSF Clinical & Translational Science Institute. 20 December 2021. Available online: https://www.sample-size.net/ (accessed on 14 April 2023).
- Kuo, M.T.; Fang, P.C.; Chao, T.L.; Chen, A.; Lai, Y.H.; Huang, Y.T.; Tseng, C.Y. Tear Proteomics Approach to Monitoring Sjögren Syndrome or Dry Eye Disease. Int. J. Mol. Sci. 2019, 20, 1932. [Google Scholar] [CrossRef] [Green Version]
- Pandit, J.C.; Nagyová, B.; Bron, A.J.; Tiffany, J.M. Physical properties of stimulated and unstimulated tears. Exp. Eye Res. 1999, 68, 247–253. [Google Scholar] [CrossRef]
- Parkin, S.J.; Knöner, G.; Nieminen, T.A.; Heckenberg, N.R.; Rubinsztein-Dunlop, H. Picoliter viscometry using optically rotated particles. Phys. Rev. E 2007, 76, 041507. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, A.; Lee, J.H.; Makrai, E.; Yeo, L.Y.; Downie, L.E. Tear Film Extensional Viscosity Is a Novel Potential Biomarker of Dry Eye Disease. Ophthalmology 2019, 126, 1196–1198. [Google Scholar] [CrossRef]
- Markoulli, M.; Sobbizadeh, A.; Tan, J.; Briggs, N.; Coroneo, M. The Effect of Optive and Optive Advanced Artificial Tears on the Healthy Tear Film. Curr. Eye Res. 2018, 43, 588–594. [Google Scholar] [CrossRef]
- Tashbayev, B.; Garen, T.; Palm, Ø.; Chen, X.; Herlofson, B.B.; Young, A.; Hove, L.H.; Rykke, M.; Singh, P.B.; Aqrawi, L.A.; et al. Patients with non-Sjögren’s sicca report poorer general and oral health-related quality of life than patients with Sjögren’s syndrome: A cross-sectional study. Sci. Rep. 2020, 10, 2063. [Google Scholar] [CrossRef] [Green Version]
- Cubuk, M.O.; Ucgul, A.Y.; Ozgur, A.; Ozulken, K.; Yuksel, E. Topical cyclosporine a (0.05%) treatment in dry eye patients: A comparison study of Sjogren’s syndrome versus non-Sjogren’s syndrome. Int. Ophthalmol. 2021, 41, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Gong, L.; Lu, Y.; Jin, H.; Robitaille, M. The diagnostic significance of Fourier-domain optical coherence tomography in Sjögren syndrome, aqueous tear deficiency and lipid tear deficiency patients. Acta Ophthalmol. 2012, 90, e359–e366. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Uchino, M.; Goto, E.; Hosaka, E.; Kasuya, Y.; Fukagawa, K.; Dogru, M.; Ogawa, Y.; Tsubota, K. Noninvasive interference tear meniscometry in dry eye patients with Sjögren syndrome. Am. J. Ophthalmol. 2007, 144, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Bandlitz, S.; Purslow, C.; Murphy, P.J.; Pult, H. The relationship between tear meniscus regularity and conjunctival folds. Optom. Vis. Sci. 2014, 91, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Le, Q. Analysis of the first tear film break-up point in Sjögren’s syndrome and non-Sjögren’s syndrome dry eye patients. BMC Ophthalmol. 2022, 22, 1. [Google Scholar] [CrossRef]
- García-Marqués, J.V.; Martínez-Albert, N.; Talens-Estarelles, C.; García-Lázaro, S.; Cerviño, A. Repeatability of Non-invasive Keratograph Break-Up Time measurements obtained using Oculus Keratograph 5M. Int. Ophthalmol. 2021, 41, 2473–2783. [Google Scholar] [CrossRef]
- Koh, S.; Ikeda, C.; Watanabe, S.; Oie, Y.; Soma, T.; Watanabe, H.; Maeda, N.; Nishida, K. Effect of non-invasive tear stability assessment on tear meniscus height. Acta Ophthalmol. 2015, 93, e135–e139. [Google Scholar] [CrossRef]
- Brandt, J.E.; Priori, R.; Valesini, G.; Fairweather, D. Sex differences in Sjögren’s syndrome: A comprehensive review of immune mechanisms. Biol. Sex Differ. 2015, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, F.; Abad, J.C.; Barabino, S.; Burnett, A.; Iyer, G.; Lekhanont, K.; Li, T.; Liu, Y.; Navas, A.; Obinwanne, C.J.; et al. TFOS lifestyle: Impact of societal challenges on the ocular surface. Ocul. Surf. 2023, 28, 165–199. [Google Scholar] [CrossRef]
- Arita, R.; Mizoguchi, T.; Kawashima, M.; Fukuoka, S.; Koh, S.; Shirakawa, R.; Suzuki, T.; Morishige, N. Meibomian Gland Dysfunction and Dry Eye Are Similar but Different Based on a Population-Based Study: The Hirado-Takushima Study in Japan. Am. J. Ophthalmol. 2019, 207, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Vu, C.H.V.; Kawashima, M.; Yamada, M.; Suwaki, K.; Uchino, M.; Shigeyasu, C.; Hiratsuka, Y.; Yokoi, N.; Tsubota, K.; Dry Eye Cross-Sectional Study in Japan Study Group. Influence of Meibomian Gland Dysfunction and Friction-Related Disease on the Severity of Dry Eye. Ophthalmology 2018, 125, 1181–1188. [Google Scholar] [CrossRef]


| Characteristics of Subjects | Dry Eye Disease with Sjögren Syndrome (n = 32) | Dry Eye Disease without Sjögren Syndrome (n = 36) | p-Value |
|---|---|---|---|
| Age (years) | 61.38 ± 9.91 | 65.25 ± 6.80 | 0.089 |
| Male:Female | 0:32 | 9:27 | 0.0025 ** |
| OSDI a | 55.11 ± 24.01 | 38.56 ± 19.30 | 0.0033 ** |
| TMH b | |||
| Central | 0.20 ± 0.22 | 0.21 ± 0.14 | 0.0065 ** |
| Nasal | 0.30 ± 0.18 | 0.42 ± 0.23 | 0.0124 * |
| Temporal | 0.30 ± 0.18 | 0.36 ± 0.23 | 0.4777 |
| Bulbar redness (a.u. c) | |||
| Nasal bulbar score | 1.53 ± 0.80 | 1.53 ± 0.61 | 0.7039 |
| Temporal bulbar score | 1.60 ± 0.69 | 1.51 ± 0.42 | 0.7414 |
| Nasal limbal score | 1.01 ± 0.70 | 1.09 ± 0.51 | 0.1118 |
| Temporal limbal score | 1.14 ± 0.60 | 1.10 ± 0.41 | 0.7718 |
| Mean redness score | 1.56 ± 0.67 | 1.50 ± 0.42 | 0.8259 |
| Mean analyzed area | 7.39 ± 2.80 | 8.26 ± 2.94 | 0.2113 |
| Tear break-up time (s) | |||
| NIKBUT first d | 5.47 ± 3.10 | 4.79 ± 1.95 | 0.4654 |
| NIKBUT avg e | 7.73 ± 4.00 | 7.98 ± 4.52 | 0.7949 |
| Assessable time | 10.84 ± 5.04 | 12.05 ± 5.71 | 0.3789 |
| Meibograde | 2.34 ± 0.97 | 2.03 ± 0.77 | 0.1770 |
| OSS f | 1.22 ± 1.26 | 0.58 ± 0.77 | 0.0455 * |
| Characteristics of Subjects | Dry Eye Disease with Sjögren Syndrome | Dry Eye Disease without Sjögren Syndrome | p-Value |
|---|---|---|---|
| MMS (0.01 s) | 16.81 ± 18.82 | 60.82 ± 70.91 | 0.0002 ** |
| MMS (0.05 s) | 3.64 ± 2.90 | 9.16 ± 6.69 | <10−5 *** |
| MMS (0.1 s) | 2.01 ± 1.39 | 4.34 ± 2.49 | <10−5 *** |
| MMS (0.5 s) | 0.59 ± 0.37 | 0.91 ± 0.40 | 0.0028 ** |
| MMS (1.0 s) | 0.37 ± 0.27 | 0.50 ± 0.27 | 0.0375 * |
| MMS (2.0 s) | 0.25 ± 0.23 | 0.29 ± 0.21 | 0.2983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, H.-Y.; Chen, A.; Fang, P.-C.; Yu, H.-J.; Kuo, M.-T. Comparing Tear Film Viscosity between Sjögren and Non-Sjögren Dry Eye Disease. Life 2023, 13, 1484. https://doi.org/10.3390/life13071484
Lai H-Y, Chen A, Fang P-C, Yu H-J, Kuo M-T. Comparing Tear Film Viscosity between Sjögren and Non-Sjögren Dry Eye Disease. Life. 2023; 13(7):1484. https://doi.org/10.3390/life13071484
Chicago/Turabian StyleLai, Hung-Yin, Alexander Chen, Po-Chiung Fang, Hun-Ju Yu, and Ming-Tse Kuo. 2023. "Comparing Tear Film Viscosity between Sjögren and Non-Sjögren Dry Eye Disease" Life 13, no. 7: 1484. https://doi.org/10.3390/life13071484
APA StyleLai, H.-Y., Chen, A., Fang, P.-C., Yu, H.-J., & Kuo, M.-T. (2023). Comparing Tear Film Viscosity between Sjögren and Non-Sjögren Dry Eye Disease. Life, 13(7), 1484. https://doi.org/10.3390/life13071484







