Will Transcranial Magnetic Stimulation Improve the Treatment of Obsessive–Compulsive Disorder? A Systematic Review and Meta-Analysis of Current Targets and Clinical Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility Criteria
2.2. Study Selection
2.3. Data Extraction and Outcome Measures
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
3.1. Systematic Review and Meta-Analytic Results Overview
3.2. Clinical Moderators of Post-Treatment Y-BOCS Score Reduction
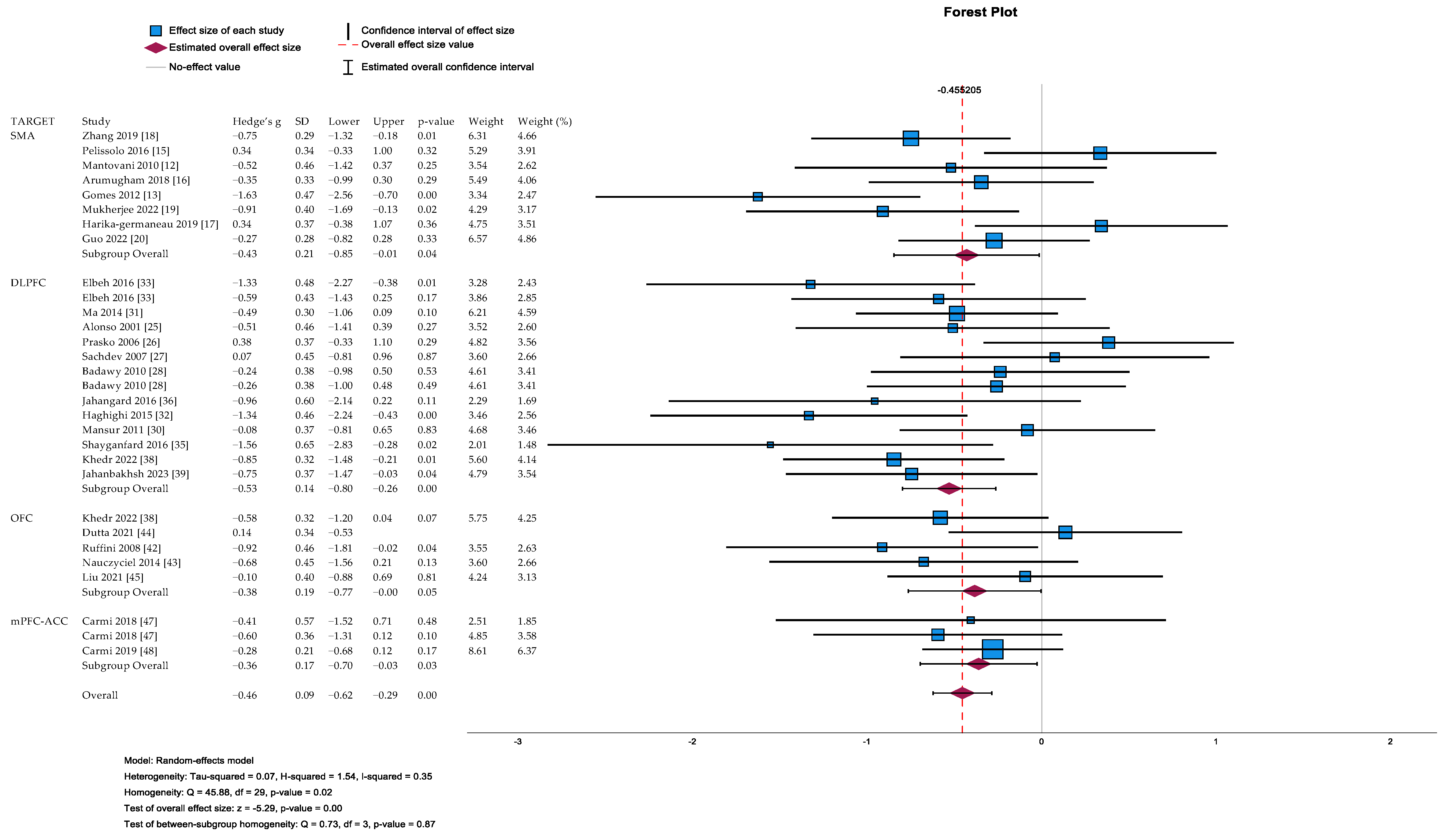
3.3. Systematic Review and Meta-Analytic Results Grouped for Brain Targets
3.3.1. Pre-Supplementary Motor Area (pre-SMA) Studies
Systematic Review Results
Meta-Analytic Results
3.3.2. Dorsolateral Prefrontal Cortex (DLPFC) Studies
Systematic Review Results
Meta-Analytic Results
3.3.3. Orbitofrontal Cortex (OFC) Studies
Systematic Review Results
Meta-Analytic Results
3.3.4. Medial Prefrontal Cortex (mPFC)/Anterior Cingulate Cortex (ACC) Studies
Systematic Review Results
Meta-Analytic Results
3.3.5. Multi-Target Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Roessel, P.J.; Grassi, G.; Aboujaoude, E.N.; Menchón, J.M.; Van Ameringen, M.; Rodríguez, C.I. Treatment-resistant OCD: Pharmacotherapies in adults. Compr. Psychiatry 2023, 120, 152352. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Cecchelli, C.; Vignozzi, L.; Pacini, S. Investigational and Experimental Drugs to Treat Obsessive-Compulsive Disorder. J. Exp. Pharmacol. 2021, 12, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Pallanti, S. Current and up-and-coming pharmacotherapy for obsessive-compulsive disorder in adults. Expert Opin. Pharmacother. 2018, 19, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Figee, M.; Ooms, P.; Righi, L.; Nakamae, T.; Pallanti, S.; Schuurman, R.; Denys, D. Impulsivity and decision-making in obsessive-compulsive disorder after effective deep brain stimulation or treatment as usual. CNS Spectr. 2018, 23, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Goodman, W.K.; Price, L.H.; Rasmussen, S.A.; Mazure, C.; Fleischmann, R.L.; Hill, C.L.; Heninger, G.R.; Charney, D.S. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch. Gen. Psychiatry 1989, 46, 1006–1011. [Google Scholar] [CrossRef]
- Higgins, J.P.; Li, T.; Deeks, J.J. Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; The Cochrane Collaboration: London, UK; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.; Eldridge, S.; Li, T. Including variants on randomized trials. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; The Cochrane Collaboration: London, UK; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Mantovani, A.; Simpson, H.B.; Fallon, B.A.; Rossi, S.; Lisanby, S.H. Randomized sham-controlled trial of repetitive transcranial magnetic stimulation in treatment-resistant obsessive-compulsive disorder. Int. J. Neuropsychopharmacol. 2010, 13, 217–227. [Google Scholar] [CrossRef]
- Gomes, P.V.; Brasil-Neto, J.P.; Allam, N.; Rodrigues de Souza, E. A randomized, double-blind trial of repetitive transcranial magnetic stimulation in obsessive-compulsive disorder with three-month follow-up. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Hawken, E.R.; Dilkov, D.; Kaludiev, E.; Simek, S.; Zhang, F.; Milev, R. Transcranial Magnetic Stimulation of the Supplementary Motor Area in the Treatment of Obsessive-Compulsive Disorder: A Multi-Site Study. Int. J. Mol. Sci. 2016, 17, 420. [Google Scholar] [CrossRef]
- Pelissolo, A.; Harika-Germaneau, G.; Rachid, F.; Gaudeau-Bosma, C.; Tanguy, M.L.; BenAdhira, R.; Bouaziz, N.; Popa, T.; Wassouf, I.; Saba, G.; et al. Repetitive Transcranial Magnetic Stimulation to Supplementary Motor Area in Refractory Obsessive-Compulsive Disorder Treatment: A Sham-Controlled Trial. Int. J. Neuropsychopharmacol. 2016, 19, pyw025. [Google Scholar] [CrossRef] [PubMed]
- Arumugham, S.S.; Vs, S.; Hn, M.B.V.; Ravi, M.; Sharma, E.; Thirthalli, J.; Reddy, Y.C.J. Augmentation Effect of Low-Frequency Repetitive Transcranial Magnetic Stimulation Over Presupplementary Motor Area in Obsessive-Compulsive Disorder: A Randomized Controlled Trial. J. ECT 2018, 34, 253–257. [Google Scholar] [CrossRef]
- Harika-Germaneau, G.; Rachid, F.; Chatard, A.; Lafay-Chebassier, C.; Solinas, M.; Thirioux, B.; Millet, B.; Langbour, N.; Jaafari, N. Continuous theta burst stimulation over the supplementary motor area in refractory obsessive-compulsive disorder treatment: A randomized sham-controlled trial. Brain Stimul. 2019, 12, 1565–1571. [Google Scholar] [CrossRef]
- Zhang, K.; Fan, X.; Yuan, J.; Yin, J.; Su, H.; Hashimoto, K.; Wang, G. Impact of serotonin transporter gene on rTMS augmentation of SSRIs for obsessive compulsive disorder. Neuropsychiatr. Dis. Treat. 2019, 15, 1771–1779. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kumre, P.K.; Goyal, N.; Khanra, S. Adjunctive neuronavigated accelerated continuous theta-burst stimulation in obsessive-compulsive disorder: A randomized sham-controlled study. CNS Spectr. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, K.; Han, H.; Li, P.; Cheng, J.; Zhu, J.; Wang, Z.; Fan, Q. Continuous theta burst stimulation over the bilateral supplementary motor area in obsessive-compulsive disorder treatment: A clinical randomized single-blind sham-controlled trial. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2022, 65, e64. [Google Scholar] [CrossRef]
- Pallanti, S.; Marras, A.; Salerno, L.; Makris, N.; Hollander, E. Better than treated as usual: Transcranial magnetic stimulation augmentation in selective serotonin reuptake inhibitor-refractory obsessive-compulsive disorder, mini-review and pilot open-label trial. J. Psychopharmacol. 2016, 30, 568–578. [Google Scholar] [CrossRef]
- Donse, L.; Sack, A.T.; Fitzgerald, P.B.; Arns, M. Sleep disturbances in obsessive-compulsive disorder: Association with non-response to repetitive transcranial magnetic stimulation (rTMS). J. Anxiety Disord. 2017, 49, 31–39. [Google Scholar] [CrossRef]
- Mantovani, A.; Neri, F.; D’Urso, G.; Mencarelli, L.; Tatti, E.; Momi, D.; Menardi, A.; Sprugnoli, G.; Santarnecchi, E.; Rossi, S. Functional connectivity changes and symptoms improvement after personalized, double-daily dosing, repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: A pilot study. J. Psychiatr. Res. 2021, 136, 560–570. [Google Scholar] [CrossRef]
- Gajadien, P.T.; Postma, T.S.; van Oostrom, I.; Scheepstra KW, F.; van Dijk, H.; Sack, A.T.; van den Heuvel, O.A.; Arns, M. Sleep predicts the response to rTMS and CBT in patients with OCD: An open label effectiveness study. Int. J. Clin. Health Psychol. IJCHP 2023, 23, 100353. [Google Scholar] [CrossRef] [PubMed]
- Alonso, P.; Pujol, J.; Cardoner, N.; Benlloch, L.; Deus, J.; Menchón, J.M.; Capdevila, A.; Vallejo, J. Right prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: A double-blind, placebo-controlled study. Am. J. Psychiatry 2001, 158, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Prasko, J.; Pasková, B.; Záleský, R.; Novák, T.; Kopecek, M.; Bares, M.; Horácek, J. The effect of repetitive transcranial magnetic stimulation (rTMS) on symptoms in obsessive compulsive disorder. A randomized, double blind, sham controlled study. Neuro Endocrinol. Lett. 2006, 27, 327–332. [Google Scholar] [PubMed]
- Sachdev, P.S.; Loo, C.K.; Mitchell, P.B.; McFarquhar, T.F.; Malhi, G.S. Repetitive transcranial magnetic stimulation for the treatment of obsessive compulsive disorder: A double-blind controlled investigation. Psychol. Med. 2007, 37, 1645–1649. [Google Scholar] [CrossRef]
- Badawy, A.A.; El Sawy, H.; Abd El Hay, M. Efficacy of repetitive transcranial magnetic stimulation in the management of obsessive compulsive disorder Egypt. J. Neurol. Psychiatry Neurosurg. 2010, 47, 393–398. [Google Scholar]
- Sarkhel, S.; Sinha, V.K.; Praharaj, S.K. Adjunctive high-frequency right prefrontal repetitive transcranial magnetic stimulation (rTMS) was not effective in obsessive-compulsive disorder but improved secondary depression. J. Anxiety Disord. 2010, 24, 535–539. [Google Scholar] [CrossRef]
- Mansur, C.G.; Myczkowki, M.L.; de Barros Cabral, S.; Sartorelli, M.D.C.B.; Bellini, B.B.; Dias, A.M.; Bernik, M.A.; Marcolin, M.A. Placebo effect after prefrontal magnetic stimulation in the treatment of resistant obsessive-compulsive disorder: A randomized controlled trial. Int. J. Neuropsychopharmacol. 2011, 14, 1389–1397. [Google Scholar] [CrossRef]
- Ma, X.; Huang, Y.; Liao, L.; Jin, Y. A randomized double-blinded sham-controlled trial of α electroencephalogram-guided transcranial magnetic stimulation for obsessive-compulsive disorder. Chin. Med. J. 2014, 127, 601–606. [Google Scholar]
- Haghighi, M.; Shayganfard, M.; Jahangard, L.; Ahmadpanah, M.; Bajoghli, H.; Pirdehghan, A.; Holsboer-Trachsler, E.; Brand, S. Repetitive Transcranial Magnetic Stimulation (rTMS) improves symptoms and reduces clinical illness in patients suffering from OCD—Results from a single-blind, randomized clinical trial with sham cross-over condition. J. Psychiatr. Res. 2015, 68, 238–244. [Google Scholar] [CrossRef]
- Elbeh, K.A.M.; Elserogy, Y.M.B.; Khalifa, H.E.; Ahmed, M.A.; Hafez, M.H.; Khedr, E.M. Repetitive transcranial magnetic stimulation in the treatment of obsessive-compulsive disorders: Double blind randomized clinical trial. Psychiatry Res. 2016, 238, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.J.; Jung, Y.E.; Lim, H.K.; Um, Y.H.; Lee, C.U.; Chae, J.H. Adjunctive Low-frequency Repetitive Transcranial Magnetic Stimulation over the Right Dorsolateral Prefrontal Cortex in Patients with Treatment-resistant Obsessive-compulsive Disorder: A Randomized Controlled Trial. Clin. Psychopharmacol. Neurosci. 2016, 14, 153–160. [Google Scholar] [CrossRef]
- Shayganfard, M.; Jahangard, L.; Nazaribadie, M.; Haghighi, M.; Ahmadpanah, M.; Sadeghi Bahmani, D.; Bajoghli, H.; Holsboer-Trachsler, E.; Brand, S. Repetitive Transcranial Magnetic Stimulation Improved Symptoms of Obsessive-Compulsive Disorders but Not Executive Functions: Results from a Randomized Clinical Trial with Crossover Design and Sham Condition. Neuropsychobiology 2016, 74, 115–124. [Google Scholar] [CrossRef]
- Jahangard, L.; Haghighi, M.; Shyayganfard, M.; Ahmadpanah, M.; Sadeghi Bahmani, D.; Bajoghli, H.; Holsboer-Trachsler, E.; Brand, S. Repetitive Transcranial Magnetic Stimulation Improved Symptoms of Obsessive-Compulsive Disorder, but Also Cognitive Performance: Results from a Randomized Clinical Trial with a Cross-Over Design and Sham Condition. Neuropsychobiology 2016, 73, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Billeri, L.; Cannavò, A.; De Luca, R.; Portaro, S.; Bramanti, P.; Calabrò, R.S. Theta burst stimulation for the treatment of obsessive-compulsive disorder: A pilot study. J. Neural Transm. 2019, 126, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Elbeh, K.; Saber, M.; Abdelrady, Z.; Abdelwarith, A. A double blind randomized clinical trial of the effectiveness of low frequency rTMS over right DLPFC or OFC for treatment of obsessive-compulsive disorder. J. Psychiatr. Res. 2022, 156, 122–131. [Google Scholar] [CrossRef]
- Jahanbakhsh, G.; Alireza Haji Seyed Javadi, S.; Majidi, M.; Khademi, M.; Karimi, R. Effectiveness of adjunctive low-frequency repetitive transcranial magnetic stimulation therapy over the left dorsolateral prefrontal cortex in patients with obsessive-compulsive disorder refractory to medical treatment:A double-blind, randomized clinical trial. Asian J. Psychiatry 2023, 80, 103384. [Google Scholar] [CrossRef]
- Williams, N.R.; Sudheimer, K.D.; Cole, E.J.; Varias, A.D.; Goldstein-Piekarski, A.N.; Stetz, P.; Lombardi, A.; Filippou-Frye, M.; van Roessel, P.; Anderson, K.; et al. Accelerated neuromodulation therapy for Obsessive-Compulsive Disorder. Brain Stimul. 2021, 14, 435–437. [Google Scholar] [CrossRef]
- Topcuoğlu, M.; Cinemre, B.; Erdoğan, A.; Nabiyeva, N. Repetitive Transcranial Magnetic Stimulation in a Group of Treament-Resistant Obsessive-Compulsive Disorder Patients: A Descriptive Study. Acta Med. 2022, 53, 114–122. [Google Scholar] [CrossRef]
- Ruffini, C.; Locatelli, M.; Lucca, A.; Benedetti, F.; Insacco, C.; Smeraldi, E. Augmentation effect of repetitive transcranial magnetic stimulation over the orbitofrontal cortex in drug-resistant obsessive-compulsive disorder patients: A controlled investigation. Prim. Care Companion J. Clin. Psychiatry 2009, 11, 226–230. [Google Scholar] [CrossRef]
- Nauczyciel, C.; Le Jeune, F.; Naudet, F.; Douabin, S.; Esquevin, A.; Vérin, M.; Dondaine, T.; Robert, G.; Drapier, D.; Millet, B. Repetitive transcranial magnetic stimulation over the orbitofrontal cortex for obsessive-compulsive disorder: A double-blind, crossover study. Transl. Psychiatry 2014, 4, e436. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Dhyani, M.; Garg, S.; Tikka, S.K.; Khattri, S.; Mehta, S.; Mishra, J. Efficacy of intensive orbitofrontal continuous Theta Burst Stimulation (iOFcTBS) in Obsessive Compulsive Disorder: A Randomized Placebo Controlled Study. Psychiatry Res. 2021, 298, 113784. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shao, H.; Liao, J.; Yang, D.; Ma, M.; Yang, J. Continuous Theta-Burst Stimulation Over the Right Orbitofrontal Cortex in Treatment-Resistant Obsessive-Compulsive Disorder Treatment: A Randomized Sham-Controlled Trial. Int. J. Gen. Med. 2021, 14, 3109–3118. [Google Scholar] [CrossRef]
- Dunlop, K.; Woodside, B.; Olmsted, M.; Colton, P.; Giacobbe, P.; Downar, J. Reductions in Cortico-Striatal Hyperconnectivity Accompany Successful Treatment of Obsessive-Compulsive Disorder with Dorsomedial Prefrontal rTMS. Neuropsychopharmacology 2016, 41, 1395–1403. [Google Scholar] [CrossRef]
- Carmi, L.; Alyagon, U.; Barnea-Ygael, N.; Zohar, J.; Dar, R.; Zangen, A. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018, 11, 158–165. [Google Scholar] [CrossRef]
- Carmi, L.; Tendler, A.; Bystritsky, A.; Hollander, E.; Blumberger, D.M.; Daskalakis, J.; Ward, H.; Lapidus, K.; Goodman, W.; Casuto, L.; et al. Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry 2019, 176, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Roth, Y.; Tendler, A.; Arikan, M.K.; Vidrine, R.; Kent, D.; Muir, O.; MacMillan, C.; Casuto, L.; Grammer, G.; Sauve, W.; et al. Real-world efficacy of deep TMS for obsessive-compulsive disorder: Post-marketing data collected from twenty-two clinical sites. J. Psychiatr. Res. 2021, 137, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Shreekantiah, U.; Goyal, N.; Roy, C. Brain activation alterations with adjunctive deep transcranial magnetic stimulation in obsessive-compulsive disorder: An fMRI study. CNS Spectr. 2023, 28, 361–366. [Google Scholar] [CrossRef]
- Arıkan, M.K.; İlhan, R.; Esmeray, T.; Laçin Çetin, H.; Aytar, E.K.; Aktas, H.; Günver, M.G.; Tendler, A. Deep Transcranial Magnetic Stimulation Effects on the Electrophysiological Parameters in Obsessive-Compulsive Disorder. Clin. EEG Neurosci. 2022, 53, 484–490. [Google Scholar] [CrossRef]
- Ikawa, H.; Osawa, R.; Sato, A.; Mizuno, H.; Noda, Y. A Case Series of Deep Transcranial Magnetic Stimulation Treatment for Patients with Obsessive-Compulsive Disorder in the Tokyo Metropolitan Area. J. Clin. Med. 2022, 11, 6133. [Google Scholar] [CrossRef]
- Kang, J.I.; Kim, C.H.; Namkoong, K.; Lee, C.I.; Kim, S.J. A randomized controlled study of sequentially applied repetitive transcranial magnetic stimulation in obsessive-compulsive disorder. J. Clin. Psychiatry 2009, 70, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Tadayonnejad, R.; Wilson, A.C.; Chu, S.A.; Corlier, J.; Citrenbaum, C.; Ngo, T.D.P.; Hovhannisyan, E.; Ginder, N.D.; Levitt, J.G.; Wilke, S.A.; et al. Use of right orbitofrontal repetitive transcranial magnetic stimulation (rTMS) augmentation for treatment-refractory obsessive-compulsive disorder with comorbid major depressive disorder. Psychiatry Res. 2022, 317, 114856. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, H.; Murayama, K.; Nemoto, K.; Tomita, M.; Hasuzawa, S.; Mizobe, T.; Kato, K.; Ohno, A.; Tsuruta, S.; Togao, O.; et al. Increased functional connectivity between presupplementary motor area and inferior frontal gyrus associated with the ability of motor response inhibition in obsessive-compulsive disorder. Hum. Brain Mapp. 2022, 43, 974–984. [Google Scholar] [CrossRef] [PubMed]
- De Wit, S.J.; de Vries, F.E.; van der Werf, Y.D.; Cath, D.C.; Heslenfeld, D.J.; Veltman, E.M.; van Balkom, A.J.; Veltman, D.J.; van den Heuvel, O.A. Presupplementary motor area hyperactivity during response inhibition: A candidate endophenotype of obsessive-compulsive disorder. Am. J. Psychiatry 2012, 169, 1100–1108, Erratum in Am. J. Psychiatry 2012, 169, 1218. [Google Scholar] [CrossRef] [PubMed]
- Brakoulias, V.; Nguyen, P.H.D.; Lin, D.; Pham, N.D.K. An international survey of different transcranial magnetic stimulation (TMS) protocols for patients with obsessive-compulsive disorder (OCD). Psychiatry Res. 2021, 298, 113765. [Google Scholar] [CrossRef]
- Grassi, G.; Pallanti, S.; Righi, L.; Figee, M.; Mantione, M.; Denys, D.; Piccagliani, D.; Rossi, A.; Stratta, P. Think twice: Impulsivity and decision making in obsessive-compulsive disorder. J. Behav. Addict. 2015, 4, 263–272. [Google Scholar] [CrossRef]
- Saxena, S.; Rauch, S.L. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr. Clin. N. Am. 2000, 23, 563–586. [Google Scholar] [CrossRef]
- Goodman, W.K.; Storch, E.A.; Sheth, S.A. Harmonizing the Neurobiology and Treatment of Obsessive-Compulsive Disorder. Am. J. Psychiatry 2021, 178, 17–29. [Google Scholar] [CrossRef]
- Ahmari, S.E.; Spellman, T.; Douglass, N.L.; Kheirbek, M.A.; Simpson, H.B.; Deisseroth, K.; Gordon, J.A.; Hen, R. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science 2013, 340, 1234–1239. [Google Scholar] [CrossRef]
- Tzirini, M.; Roth, Y.; Harmelech, T.; Zibman, S.; Pell, G.S.; Kimiskidis, V.K.; Tendler, A.; Zangen, A.; Samaras, T. Detailed measurements and simulations of electric field distribution of two TMS coils cleared for obsessive compulsive disorder in the brain and in specific regions associated with OCD. PLoS ONE 2022, 17, e0263145. [Google Scholar] [CrossRef]
- Brown, J.W.; Braver, T.S. Learned predictions of error likelihood in the anterior cingulate cortex. Science 2005, 307, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Brem, S.; Hauser, T.U.; Iannaccone, R.; Brandeis, D.; Drechsler, R.; Walitza, S. Neuroimaging of cognitive brain function in paediatric obsessive compulsive disorder: A review of literature and preliminary meta-analysis. J. Neural Transm. 2012, 119, 1425–1448. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Morrell, M.J.; Vogt, B.A. Contributions of anterior cin-gulate cortex to behaviour. Brain 1995, 118, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, V.; Carter, C.S. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002, 77, 477–482. [Google Scholar] [CrossRef]
- Mikellides, G.; Michael, P.; Schuhmann, T.; Sack, A.T. TMS-Induced Seizure during FDA-Approved Bilateral DMPFC Protocol for Treating OCD: A Case Report. Case Rep. Neurol. 2021, 13, 584–590. [Google Scholar] [CrossRef]

| Brain Target | Study Design | Stimulation Type/Frequency/Pulse Per Session | Number of Sessions | Subjects (Active Branch) | |
|---|---|---|---|---|---|
| Mantovani 2010 [12] | Bilateral pre-SMA | Sham-controlled | rTMS/1 Hz/1200 | 20 | 9 |
| Gomes 2012 [13] | Bilateral pre-SMA | Sham-controlled | rTMS/1 Hz/1200 | 10 | 12 |
| Hawken 2016 [14] | Bilateral pre-SMA | Sham-controlled | rTMS/1 Hz/1800 | 25 | 10 |
| Pelissolo 2016 [15] | Bilateral pre-SMA | Sham-controlled | rTMS/1 Hz/1500 | 20 | 19 |
| Arumugham 2018 [16] | Bilateral pre-SMA | Sham-controlled | rTMS/1 Hz/1200 | 18 | 19 |
| Harika-germaneau 2019 [17] | Bilateral pre-SMA | Sham-controlled | cTBS/600 | 30 | 14 |
| Zhang 2019 [18] | Bilateral pre-SMA | Sham-controlled | rTMS/1 Hz/1200 | 20 | 25 |
| Mukherjee 2022 [19] | Bilateral pre-SMA | Sham-controlled | cTBS/900 | 30 | 13 |
| Guo 2022 [20] | Bilateral pre-SMA | Sham-controlled | cTBS/1200 | 20 | 26 |
| Pallanti 2016 [21] | Bilateral pre-SMA | Open label | rTMS/1 Hz/1200 | 15 | 25 |
| Donse 2017 [22] | Bilateral pre-SMA | Open label | rTMS/1 Hz/1000 | 10 | 22 |
| Mantovani 2021 [23] | Bilateral pre-SMA | Open label | rTMS/1 Hz/3600 | 10 | 8 |
| Gajadien 2022 [24] | Bilateral pre-SMA | Open label | rTMS/1 Hz/1200 | 10 | 35 |
| Alonso 2001 [25] | rDLPFC | Sham-controlled | rTMS/1/Hz/1200 | 18 | 10 |
| Prasko 2006 [26] | lDLPFC | Sham-controlled | rTMS/1 Hz/1800 | 10 | 18 |
| Sachdev 2007 [27] | lDLPFC | Sham-controlled | rTMS/10 Hz/1500 | 10 | 10 |
| Badawy 2010 [28] | lDLPFC | Sham-controlled | rTMS/20 Hz | 15 | 20 |
| Sarkhel 2010 [29] | rDLPFC | Sham-controlled | rTMS/10 Hz/800 | 10 | 21 |
| Mansur 2011 [30] | rDLPFC | Sham-controlled | rTMS/10 Hz/2000 | 30 | 13 |
| Ma 2014 [31] | Bilateral DLPFC | Sham-controlled | rTMS/8–12 Hz/648–872 | 10 | 25 |
| Haghighi 2015 [32] | Bilateral DLPFC | Sham-controlled | rTMS/20 Hz/750 | 10 | 10 |
| Elbeh 2016 [33] | rDLPFC | Sham-controlled | rTMS/1 H or 10 Hz/2000 | 10 | 15 |
| Seo 2016 [34] | rDLPFC | Sham-controlled | rTMS/1 Hz/1200 | 15 | 14 |
| Shayganfard 2016 [35] | Bilateral DLPFC | Sham-controlled | rTMS/20 Hz/750 | 10 | 5 |
| Jahangard 2016 [36] | Bilateral DLPFC | Sham-controlled | rTMS/20 Hz/750 | 10 | 5 |
| Naro 2019 [37] | lDPFC | Sham-controlled | iTBS/600 | 20 | 5 |
| Khedr 2022 * [38] | rDLPFC | Sham-controlled | rTMS/1 Hz/1500 | 10 | 20 |
| Jahanbakhsh 2023 [39] | lDLPFC | Sham-controlled | rTMS/1 Hz/1200 | 15 | 15 |
| Williams 2021 [40] | rPFC | Open label | cTBS/1800 | 50 | 7 |
| Topcuoglu 2022 [41] | lDLPFC | Open label | rTMS/1 Hz/1200 | 30 | 27 |
| Ruffini 2008 [42] | lOFC | Sham-controlled | rTMS/1 Hz/600 | 15 | 16 |
| Nauczyciel 2014 [43] | rOFC | Sham-controlled | rTMS/1 Hz/1200 | 10 | 10 |
| Dutta 2021 [44] | lOFC | Sham-controlled | cTBS/600 | 10 | 18 |
| Liu 2021 [45] | rOFC | Sham-controlled | cTBS/600 | 20 | 12 |
| Khedr 2022 [38] | rOFC | Sham-controlled | rTMS/1 Hz/1500 | 10 | 20 |
| Dunlop 2016 [46] | Bilateral dmPFC | Open label | rTMS/10 Hz/6000 | 20/30 | 20 |
| Carmi 2018 [47] | mPFC/ACC | Sham-controlled | dTMS/20 Hz/2000 | 25 | 16 |
| Carmi 2018 * [47] | mPFC/ACC | Sham-controlled | dTMS/1 Hz/900 | 25 | 8 |
| Carmi 2019 [48] | mPFC/ACC | Sham-controlled | dTMS/20 Hz/2000 | 29 | 42 |
| Roth 2021 [49] | mPFC/ACC | Open label | dTMS/20 Hz/2000 | 29 | 185 |
| Reddy 2022 [50] | mPFC/ACC | Open label | dTMS/20 Hz/2000 | 10 | 15 |
| Arikan 2022 [51] | mPFC/ACC | Open label | dTMS/20 Hz/2000 | 30 | 29 |
| Ikawa 2022 [52] | mPFC/ACC | Open label | dTMS/20 Hz/2000 | 30 | 26 |
| Kang 2009 [53] | rDLPFC + bilateral pre-SMA | Sham-controlled | rTMS/1 Hz/1200 + 1200 | 10 | 10 |
| Tadayonnejad 2022 [54] | DLPFC + SMA + rOFC | Open label | rTMS/1 Hz/1200 + 1200 + 1200 | 17 ± 6 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, G.; Moradei, C.; Cecchelli, C. Will Transcranial Magnetic Stimulation Improve the Treatment of Obsessive–Compulsive Disorder? A Systematic Review and Meta-Analysis of Current Targets and Clinical Evidence. Life 2023, 13, 1494. https://doi.org/10.3390/life13071494
Grassi G, Moradei C, Cecchelli C. Will Transcranial Magnetic Stimulation Improve the Treatment of Obsessive–Compulsive Disorder? A Systematic Review and Meta-Analysis of Current Targets and Clinical Evidence. Life. 2023; 13(7):1494. https://doi.org/10.3390/life13071494
Chicago/Turabian StyleGrassi, Giacomo, Corinna Moradei, and Chiara Cecchelli. 2023. "Will Transcranial Magnetic Stimulation Improve the Treatment of Obsessive–Compulsive Disorder? A Systematic Review and Meta-Analysis of Current Targets and Clinical Evidence" Life 13, no. 7: 1494. https://doi.org/10.3390/life13071494
APA StyleGrassi, G., Moradei, C., & Cecchelli, C. (2023). Will Transcranial Magnetic Stimulation Improve the Treatment of Obsessive–Compulsive Disorder? A Systematic Review and Meta-Analysis of Current Targets and Clinical Evidence. Life, 13(7), 1494. https://doi.org/10.3390/life13071494






