Maternal Age at Menarche Gene Polymorphisms Are Associated with Offspring Birth Weight
Abstract
:1. Introduction
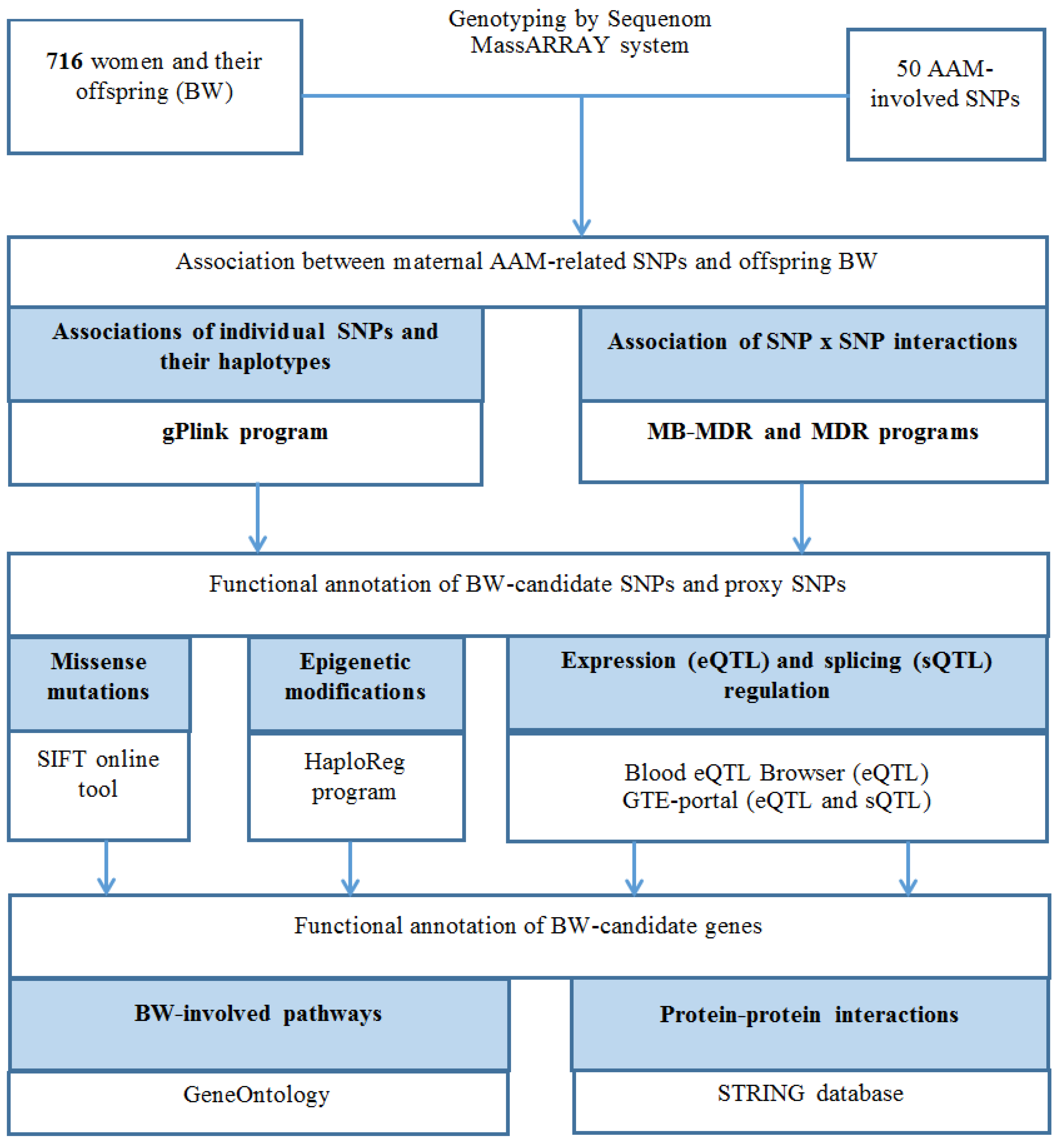
2. Materials and Methods
2.1. Study Subjects
2.2. DNA Extraction, AMM-Involved SNPs Selection, Genotypes Testing
2.3. SNPs Association Analysis
2.4. BW-Involved SNPs/Genes Potential Functions
3. Results
3.1. Study Participants’ Characteristics
3.2. SNPs/Haplotypes Association Analysis
3.3. BW-Involved SNP × SNP Interactions
3.4. Functional of BW-Candidate SNPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horikoshi, M.; Beaumont, R.N.; Day, F.R.; Warrington, N.M.; Kooijman, M.N.; Fernandez-Tajes, J.; Feenstra, B.; van Zuydam, N.R.; Gaulton, K.J.; Grarup, N.; et al. Genome-wide associations for birth weight and correlations with adult disease. Nature 2016, 538, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, D.; Tikkanen, E.; Gustafsson, S.; Priest, J.R.; Burgess, S.; Ingelsson, E. Birthweight, Type 2 Diabetes Mellitus, and Cardiovascular Disease: Addressing the Barker Hypothesis With Mendelian Randomization. Circ. Genom. Precis. Med. 2018, 11, e002054, Erratum in Circ. Genom. Precis. Med. 2018, 11, e000051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrington, N.M.; Beaumont, R.N.; Horikoshi, M.; Day, F.R.; Helgeland, Ø.; Laurin, C.; Bacelis, J.; Peng, S.; Hao, K.; Feenstra, B.; et al. Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat. Genet. 2019, 51, 804–814. [Google Scholar] [CrossRef]
- Risnes, K.R.; Vatten, L.J.; Baker, J.L.; Jameson, K.; Sovio, U.; Kajantie, E.; Osler, M.; Morley, R.; Jokela, M.; Painter, R.C.; et al. Birthweight and mortality in adulthood: A systematic review and meta-analysis. Int. J. Epidemiol. 2011, 40, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Harder, T.; Rodekamp, E.; Schellong, K.; Dudenhausen, J.W.; Plagemann, A. Birth weight and subsequent risk of type 2 diabetes: A meta-analysis. Am. J. Epidemiol. 2007, 165, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Schellong, K.; Schulz, S.; Harder, T.; Plagemann, A. Birth weight and long-term overweight risk: Systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS ONE 2012, 7, e47776. [Google Scholar] [CrossRef] [Green Version]
- Mu, M.; Wang, S.F.; Sheng, J.; Zhao, Y.; Li, H.Z.; Hu, C.L.; Tao, F.B. Birth weight and subsequent blood pressure: A meta-analysis. Arch. Cardiovasc. Dis. 2012, 105, 99–113. [Google Scholar] [CrossRef]
- Horikoshi, M.; Yaghootkar, H.; Mook-Kanamori, D.O.; Sovio, U.; Taal, H.R.; Hennig, B.J.; Bradfield, J.P.; St Pourcain, B.; Evans, D.M.; Charoen, P.; et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat. Genet. 2013, 45, 76–82. [Google Scholar] [CrossRef] [Green Version]
- BIRTH-GENE (BIG) Study Working Group; Huang, T.; Wang, T.; Zheng, Y.; Ellervik, C.; Li, X.; Gao, M.; Fang, Z.; Chai, J.F.; Ahluwalia, T.V.; et al. Association of Birth Weight with Type 2 Diabetes and Glycemic Traits: A Mendelian Randomization Study. JAMA Netw. Open 2019, 2, e1910915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnus, P. Causes of variation in birth weight: A study of offspring of twins. Clin. Genet. 1984, 25, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Magnus, P. Further evidence for a significant effect of fetal genes on variation in birth weight. Clin. Genet. 1984, 26, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Lunde, A.; Melve, K.K.; Gjessing, H.K.; Skjaerven, R.; Irgens, L.M. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am. J. Epidemiol. 2007, 165, 734–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaumont, R.N.; Warrington, N.M.; Cavadino, A.; Tyrrell, J.; Nodzenski, M.; Horikoshi, M.; Geller, F.; Myhre, R.; Richmond, R.C.; Paternoster, L.; et al. Genome-wide association study of offspring birth weight in 86,577 women identifies five novel loci and highlights maternal genetic effects that are independent of fetal genetics. Hum. Mol. Genet. 2018, 27, 742–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Bacelis, J.; Sole-Navais, P.; Srivastava, A.; Juodakis, J.; Rouse, A.; Hallman, M.; Teramo, K.; Melbye, M.; Feenstra, B.; et al. Dissecting maternal and fetal genetic effects underlying the associations between maternal phenotypes, birth outcomes, and adult phenotypes: A mendelian-randomization and haplotype-based genetic score analysis in 10,734 mother-infant pairs. PLoS Med. 2020, 17, e1003305. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.; Richmond, R.C.; Palmer, T.M.; Feenstra, B.; Rangarajan, J.; Metrustry, S.; Cavadino, A.; Paternoster, L.; Armstrong, L.L.; De Silva, N.M.; et al. Genetic Evidence for Causal Relationships Between Maternal Obesity-Related Traits and Birth Weight. JAMA 2016, 315, 1129–1140, Erratum in JAMA 2016, 315, 1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yermachenko, A.; Dvornyk, V. Nongenetic determinants of age at menarche: A systematic review. Biomed. Res. Int. 2014, 2014, 371583. [Google Scholar] [CrossRef] [Green Version]
- Plant, T.M. The hypothalamo–pituitary–gonadal axis. J. Endocrinol. 2015, 226, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Kaprio, J.; Rimpelä, A.; Winter, T.; Viken, R.J.; Rimpelä, M.; Rose, R.J. Common genetic influences on BMI and age at menarche. Hum. Biol. 1995, 67, 739–753. [Google Scholar]
- Anderson, C.A.; Duffy, D.L.; Martin, N.G.; Visscher, P.M. Estimation of variance components for age at menarche in twin families. Behav. Genet. 2007, 37, 668–677. [Google Scholar] [CrossRef]
- Morris, D.H.; Jones, M.E.; Schoemaker, M.J.; Ashworth, A.; Swerdlow, A.J. Familial concordance for age at menarche: Analyses from the Breakthrough Generations Study. Paediatr. Perinat. Epidemiol. 2011, 25, 306–311. [Google Scholar] [CrossRef]
- D’Aloisio, A.A.; DeRoo, L.A.; Baird, D.D.; Weinberg, C.R.; Sandler, D.P. Prenatal and infant exposures and age at menarche. Epidemiology 2013, 24, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Elks, C.E.; Perry, J.R.B.; Sulem, P.; Chasman, D.I.; Franceschini, N.; He, C.; Lunetta, K.L.; Visser, J.A.; Byrne, E.M.; Cousminer, D.L.; et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet. 2010, 42, 1077–1085. [Google Scholar] [CrossRef] [Green Version]
- Perry, J.R.; Day, F.; Elks, C.E.; Sulem, P.; Thompson, D.J.; Ferreira, T.; He, C.; Chasman, D.I.; Esko, T.; Thorleifsson, G.; et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 2014, 514, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Ponomarenko, I.; Reshetnikov, E.; Altuchova, O.; Polonikov, A.; Sorokina, I.; Yermachenko, A.; Dvornyk, V.; Golovchenko, O.; Churnosov, M. Association of genetic polymorphisms with age at menarche in Russian women. Gene 2019, 686, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, D.; Brewer, C.F.; Del Greco, M.F.; Sivakumaran, P.; Bowden, J.; Sheehan, N.A.; Minelli, C. Age at menarche and adult body mass index: A Mendelian randomization study. Int. J. Obes. 2018, 42, 1574–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, J.A.; Carslake, D.; Wade, K.H.; Richmond, R.C.; Langdon, R.J.; Vincent, E.E.; Holmes, M.V.; Timpson, N.J.; Davey Smith, G. Influence of puberty timing on adiposity and cardiometabolic traits: A Mendelian randomisation study. PLoS Med. 2018, 15, e1002641. [Google Scholar] [CrossRef] [Green Version]
- Jo, E.J.; Han, S.; Wang, K. Estimation of Causal Effect of Age at Menarche on Pubertal Height Growth Using Mendelian Randomization. Genes 2022, 13, 710. [Google Scholar] [CrossRef]
- Ponomarenko, I.; Reshetnikov, E.; Polonikov, A.; Sorokina, I.; Yermachenko, A.; Dvornyk, V.; Churnosov, M. Candidate genes for age at menarche are associated with endometrial hyperplasia. Gene 2020, 757, 4933. [Google Scholar] [CrossRef]
- Ponomarenko, I.; Reshetnikov, E.; Polonikov, A.; Verzilina, I.; Sorokina, I.; Elgaeva, E.E.; Tsepilov, Y.A.; Yermachenko, A.; Dvornyk, V.; Churnosov, M. Candidate genes for age at menarche are associated with endometriosis. Reprod. Biomed. Online 2020, 41, 943–956. [Google Scholar] [CrossRef]
- Ponomarenko, I.; Reshetnikov, E.; Polonikov, A.; Verzilina, I.; Sorokina, I.; Yermachenko, A.; Dvornyk, V.; Churnosov, M. Candidate genes for age at menarche are associated with uterine leiomyoma. Front. Genet. 2021, 11, 512940. [Google Scholar] [CrossRef]
- Day, F.R.; Thompson, D.J.; Helgason, H.; Chasman, D.I.; Finucane, H.; Sulem, P.; Ruth, K.S.; Whalen, S.; Sarkar, A.K.; Albrecht, E.; et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 2017, 49, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wan, B.; Sun, M. Mendelian randomization identifies age at menarche as an independent causal effect factor for gestational diabetes mellitus. Diabetes Obes. Metab. 2023, 25, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Tian, Y.; Li, F.; Yan, C.; Wang, H.; Luo, Z.; Jiang, F.; Zhang, J. Maternal age at menarche and offspring body mass index in childhood. BMC Pediatr. 2019, 19, 312. [Google Scholar] [CrossRef] [Green Version]
- Basso, O.; Pennell, M.L.; Chen, A.; Longnecker, M.P. Mother’s age at menarche and offspring size. Int. J. Obes. 2010, 34, 1766–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, K.K.; Northstone, K.; Wells, J.C.; Rubin, C.; Ness, A.R.; Golding, J.; Dunger, D.B. Earlier mother’s age at menarche predicts rapid infancy growth and childhood obesity. PLoS Med. 2007, 4, e132. [Google Scholar] [CrossRef] [Green Version]
- Min, J.; Li, Z.; Liu, X.; Wang, Y. The association between early menarche and offspring’s obesity risk in early childhood was modified by gestational weight gain. Obesity 2014, 22, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Song, L.; Shen, L.; Liu, B.; Zheng, X.; Zhang, L.; Li, Y.; Xia, W.; Lu, B.; Zhang, B.; et al. Age at menarche and prevalence of preterm birth: Results from the Healthy Baby Cohort study. Sci. Rep. 2017, 7, 12594. [Google Scholar] [CrossRef] [Green Version]
- Coall, D.A.; Chisholm, J.S. Evolutionary perspectives on pregnancy: Maternal age at menarche and infant birth weight. Soc. Sci. Med. 2003, 57, 1771–1781. [Google Scholar] [CrossRef]
- Ong, K.K.; Elks, C.E.; Li, S.; Zhao, J.H.; Luan, J.A.; Andersen, L.B.; Bingham, S.A.; Brage, S.; Smith, G.D.; Ekelund, U.; et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat. Genet. 2009, 41, 729–733. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, K.; Juul, A.; Christensen, K.; Skytthe, A.; Scheike, T.; Kold Jensen, T. Birth size and age at menarche: A twin perspective. Hum. Reprod. 2013, 28, 2865–2871. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Liu, R.; Wu, H.; Yu, J.; Jiang, Z.; Huang, H. The Causal Evidence of Birth Weight and Female-Related Traits and Diseases: A Two-Sample Mendelian Randomization Analysis. Front. Genet. 2022, 13, 850892. [Google Scholar] [CrossRef]
- Wang, L.; Xu, F.; Zhang, Q.; Chen, J.; Zhou, Q.; Sun, C. Causal relationships between birth weight, childhood obesity and age at menarche: A two-sample Mendelian randomization analysis. Clin. Endocrinol. 2023, 98, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Reshetnikov, E.; Zarudskaya, O.; Polonikov, A.; Bushueva, O.; Orlova, V.; Krikun, E.; Dvornyk, V.; Churnosov, M. Genetic markers for inherited thrombophilia are associated with fetal growth retardation in the population of Central Russia. J. Obstet. Gynaecol. Res. 2017, 43, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Reshetnikov, E.; Ponomarenko, I.; Golovchenko, O.; Sorokina, I.; Batlutskaya, I.; Yakunchenko, T.; Dvornyk, V.; Polonikov, A.; Churnosov, M. The VNTR polymorphism of the endothelial nitric oxide synthase gene and blood pressure in women at the end of pregnancy. Taiwan. J. Obstet. Gynecol. 2019, 58, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Golovchenko, O.V. Molecular genetic determinants of pre-eclampsia. Res. Results Biomed. 2019, 5, 139–149. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Reshetnikov, E.A. Study of associations of candidate genes differentially expressing in the placenta with the development of placental insufficiency with fetal growth restriction. Res. Results Biomed. 2020, 6, 338–349. (In Russian) [Google Scholar] [CrossRef]
- Golovchenko, O.; Abramova, M.; Ponomarenko, I.; Reshetnikov, E.; Aristova, I.; Polonikov, A.; Dvornyk, V.; Churnosov, M. Functionally significant polymorphisms of ESR1and PGR and risk of intrauterine growth restriction in population of Central Russia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 52–57. [Google Scholar] [CrossRef]
- Abramova, M.; Churnosova, M.; Efremova, O.; Aristova, I.; Reshetnikov, E.; Polonikov, A.; Churnosov, M.; Ponomarenko, I. Effects of pre-pregnancy over-weight/obesity on the pattern of association of hypertension susceptibility genes with preeclampsia. Life 2022, 12, 2018. [Google Scholar] [CrossRef]
- Churnosov, M.; Abramova, M.; Reshetnikov, E.; Lyashenko, I.V.; Efremova, O.; Churnosova, M.; Ponomarenko, I. Polymorphisms of hypertension susceptibility genes as a risk factors of preeclampsia in the Caucasian population of central Russia. Placenta 2022, 129, 51–61. [Google Scholar] [CrossRef]
- Abramova, M.Y. Genetic markers of severe preeclampsia. Res. Results Biomed. 2022, 8, 305–316. (In Russian) [Google Scholar] [CrossRef]
- Tikunova, E.; Ovtcharova, V.; Reshetnikov, E.; Dvornyk, V.; Polonikov, A.; Bushueva, O.; Churnosov, M. Genes of tumor necrosis factors and their receptors and the primary open angle glaucoma in the population of Central Russia. Int. J. Ophthalmol. 2017, 10, 1490–1494. [Google Scholar] [CrossRef]
- Starikova, D.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Novel Data about Association of the Functionally Significant Polymorphisms of the MMP9 Gene with Exfoliation Glaucoma in the Caucasian Population of Central Russia. Ophthalmic Res. 2021, 64, 458–464. [Google Scholar] [CrossRef]
- Eliseeva, N.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. LOXL1 gene polymorphism candidates for exfoliation glaucoma are also associated with a risk for primary open-angle glaucoma in a Caucasian population from central Russia. Mol. Vis. 2021, 27, 262–269. [Google Scholar] [PubMed]
- Ward, L.D.; Kellis, M. HaploReg v4: Systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016, 44, D877–D881. [Google Scholar] [CrossRef] [PubMed]
- Minyaylo, O.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Functionally significant polymorphisms of the MMP-9 gene are associated with peptic ulcer disease in the Caucasian population of Central Russia. Sci. Rep. 2021, 11, 13515. [Google Scholar] [CrossRef]
- Bushueva, O.; Solodilova, M.; Churnosov, M.; Ivanov, V.; Polonikov, A. The Flavin-Containing Monooxygenase 3 Gene and Essential Hypertension: The Joint Effect of Polymorphism E158K and Cigarette Smoking on Disease Susceptibility. Int. J. Hypertens. 2014, 2014, 712169. [Google Scholar] [CrossRef] [Green Version]
- Polonikov, A.; Rymarova, L.; Klyosova, E.; Volkova, A.; Azarova, I.; Bushueva, O.; Bykanova, M.; Bocharova, I.; Zhabin, S.; Churnosov, M.; et al. Matrix metalloproteinases as target genes for gene regulatory networks driving molecular and cellular pathways related to a multistep pathogenesis of cerebrovascular disease. J. Cell. Biochem. 2019, 120, 16467–16482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moskalenko, M.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Polymorphisms of the matrix metalloproteinase genes are associated with essential hypertension in a Caucasian population of Central Russia. Sci. Rep. 2021, 11, 5224. [Google Scholar] [CrossRef]
- Moore, J.H.; Gilbert, J.C.; Tsai, C.T.; Chiang, F.T.; Holden, T.; Barney, N.; White, B.C. A flexible computational framework for detecting, characterizing, and interpreting statistical patterns of epistasis in genetic studies of human disease susceptibility. J. Theor. Biol. 2006, 241, 252–261. [Google Scholar] [CrossRef]
- Ponomarenko, I.V. Using the method of Multifactor Dimensionality Reduction (MDR) and its modifications for analysis of gene-gene and gene-environment interactions in genetic-epidemiological studies (review). Res. Results Biomed. 2019, 5, 4–21. [Google Scholar] [CrossRef]
- Calle, M.L.; Urrea, V.; Malats, N.; Van Steen, K. Mbmdr: An R package for exploring gene-gene interactions associated with binary or quantitative traits. Bioinformatics 2010, 26, 2198–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, R.; Jack, J.R.; Motsinger-Reif, A.A.; Brown, C.C. An adaptive permutation approach for genome-wide association study: Evaluation and recommendations for use. BioData Min. 2014, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, T.; Churnosova, M.; Abramova, M.; Ponomarenko, I.; Reshetnikov, E.; Aristova, I.; Sorokina, I.; Churnosov, M. Risk Effects of rs1799945 Polymorphism of the HFE Gene and Intergenic Interactions of GWAS-Significant Loci for Arterial Hypertension in the Caucasian Population of Central Russia. Int. J. Mol. Sci. 2023, 24, 8309. [Google Scholar] [CrossRef] [PubMed]
- Gauderman, W.; Morrison, J. QUANTO 1.1: A Computer Program for Power and Sample Size Calculations Genetic–Epidemiology Studies. 2006. Available online: http://hydra.usc.edu/gxe (accessed on 6 February 2023).
- Novakov, V.; Novakova, O.; Churnosova, M.; Sorokina, I.; Aristova, I.; Polonikov, A.; Reshetnikov, E.; Churnosov, M. Intergenic Interactions of SBNO1, NFAT5 and GLT8D1 Determine the Susceptibility to Knee Osteoarthritis among Europeans of Russia. Life 2023, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.; Demin, S.; Churnosov, M.; Reshetnikov, E.; Aristova, I.; Churnosova, M.; Ponomarenko, I. Matrix Metalloproteinase Gene Polymorphisms Are Associated with Breast Cancer in the Caucasian Women of Russia. Int. J. Mol. Sci. 2022, 23, 12638. [Google Scholar] [CrossRef]
- Ryzhkov, I.I.; Borzilov, E.E.; Churnosov, M.I.; Ataman, A.V.; Dedkov, A.A.; Polonikov, A.V. Transforming growth factor beta 1 is a novel susceptibility gene for adolescent idiopathic scoliosis. Spine 2013, 38, E699–E704. [Google Scholar] [CrossRef]
- Sirotina, S.; Ponomarenko, I.; Kharchenko, A.; Bykanova, M.; Bocharova, A.; Vagaytseva, K.; Stepanov, V.; Churnosov, M.; Solodilova, M.; Polonikov, A. A Novel Polymorphism in the Promoter of the CYP4A11 Gene Is Associated with Susceptibility to Coronary Artery Disease. Dis. Markers 2018, 2018, 5812802. [Google Scholar] [CrossRef] [Green Version]
- Moskalenko, M.I.; Milanova, S.N.; Ponomarenko, I.V.; Polonikov, A.V.; Churnosov, M.I. Study of associations of polymorphism of matrix metalloproteinases genes with the development of arterial hypertension in men. Kardiologiia 2019, 59, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Polonikov, A.; Kharchenko, A.; Bykanova, M.; Sirotina, S.; Ponomarenko, I.; Bocharova, A.; Vagaytseva, K.; Stepanov, V.; Bushueva, O.; Churnosov, M.; et al. Polymorphisms of CYP2C8, CYP2C9 and CYP2C19 and risk of coronary heart disease in Russian population. Gene 2017, 627, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, N.; Demin, S.; Churnosov, M.; Reshetnikov, E.; Aristova, I.; Churnosova, M.; Ponomarenko, I. The Modifying Effect of Obesity on the Association of Matrix Metalloproteinase Gene Polymorphisms with Breast Cancer Risk. Biomedicines 2022, 10, 2617. [Google Scholar] [CrossRef]
- Golovchenko, I.; Aizikovich, B.; Golovchenko, O.; Reshetnikov, E.; Churnosova, M.; Aristova, I.; Ponomarenko, I.; Churnosov, M. Sex Hormone Candidate Gene Polymorphisms Are Associated with Endometriosis. Int. J. Mol. Sci. 2022, 23, 13691. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.; Churnosova, M.; Abramova, M.; Plotnikov, D.; Ponomarenko, I.; Reshetnikov, E.; Aristova, I.; Sorokina, I.; Churnosov, M. Sex-Specific Features of the Correlation between GWAS-Noticeable Polymorphisms and Hypertension in Europeans of Russia. Int. J. Mol. Sci. 2023, 24, 7799. [Google Scholar] [CrossRef] [PubMed]
- Westra, H.J.; Peters, M.J.; Esko, T.; Yaghootkar, H.; Schurmann, C.; Kettunen, J.; Christiansen, M.W.; Fairfax, B.P.; Schramm, K.; Powell, J.E.; et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat. Genet. 2013, 45, 1238–1243. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 7, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology Consortium. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef] [PubMed]
- GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Delahanty, R.J.; Beeghly-Fadiel, A.; Long, J.R.; Gao, Y.T.; Lu, W.; Xiang, Y.B.; Zheng, Y.; Ji, B.T.; Wen, W.Q.; Cai, Q.Y.; et al. Evaluation of GWAS-identified genetic variants for age at menarche among Chinese women. Hum. Reprod. 2013, 28, 1135–1143. [Google Scholar] [CrossRef]
- Li, S.; Ali, S.; Duan, X.; Liu, S.; Du, J.; Liu, C.; Dai, H.; Zhou, M.; Zhou, L.; Yang, L.; et al. JMJD1B Demethylates H4R3me2s and H3K9me2 to Facilitate Gene Expression for Development of Hematopoietic Stem and Progenitor Cells. Cell Rep. 2018, 23, 389–403. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Chen, X.; Zhou, S.; Liao, L.; Jiang, R.; Xu, J. The histone H3K9 demethylase Kdm3b is required for somatic growth and female reproductive function. Int. J. Biol. Sci. 2015, 11, 494–507. [Google Scholar] [CrossRef]
- GeneCards: The Human Gene Database. Available online: https://www.genecards.org/ (accessed on 22 February 2023).
- Sinnott-Armstrong, N.; Tanigawa, Y.; Amar, D.; Mars, N.; Benner, C.; Aguirre, M.; Venkataraman, G.R.; Wainberg, M.; Ollila, H.M.; Kiiskinen, T.; et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat. Genet. 2021, 53, 185–194, Erratum in Nat. Genet. 2021, 53, 1622. [Google Scholar] [CrossRef] [PubMed]
- Yengo, L.; Vedantam, S.; Marouli, E.; Sidorenko, J.; Bartell, E.; Sakaue, S.; Graff, M.; Eliasen, A.U.; Jiang, Y.; Raghavan, S.; et al. A saturated map of common genetic variants associated with human height. Nature 2022, 610, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huffman, J.E.; Huang, Y.; Do Valle, Í.; Assimes, T.L.; Raghavan, S.; Voight, B.F.; Liu, C.; Barabási, A.L.; Huang, R.D.L.; et al. Genomics and phenomics of body mass index reveals a complex disease network. Nat. Commun. 2022, 13, 7973. [Google Scholar] [CrossRef]
- Chen, C.T.; Fernández-Rhodes, L.; Brzyski, R.G.; Carlson, C.S.; Chen, Z.; Heiss, G.; North, K.E.; Woods, N.F.; Rajkovic, A.; Kooperberg, C.; et al. Replication of loci influencing ages at menarche and menopause in Hispanic women: The Women’s Health Initiative SHARe Study. Hum. Mol. Genet. 2012, 21, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chasman, D.I.; Dreyfus, J.; Hwang, S.J.; Ruiter, R.; Sanna, S.; Buring, J.E.; Fernández-Rhodes, L.; Franceschini, N.; Hankinson, S.E.; et al. Reproductive aging-associated common genetic variants and the risk of breast cancer. Breast Cancer Res. 2012, 14, R54. [Google Scholar] [CrossRef]
- Pickrell, J.K.; Berisa, T.; Liu, J.Z.; Ségurel, L.; Tung, J.Y.; Hinds, D.A. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 2016, 48, 709–717, Published Correction Appears in Nat. Genet. 2016, 48, 1296. [Google Scholar] [CrossRef] [Green Version]
- Kichaev, G.; Bhatia, G.; Loh, P.R.; Gazal, S.; Burch, K.; Freund, M.K.; Schoech, A.; Pasaniuc, B.; Price, A.L. Leveraging Polygenic Functional Enrichment to Improve GWAS Power. Am. J. Hum. Genet. 2019, 104, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wang, W.; Lu, W.; Chen, W.; Shang, A. Inhibin β-A (INHBA) induces epithelial-mesenchymal transition and accelerates the motility of breast cancer cells by activating the TGF-β signaling pathway. Bioengineered 2021, 12, 4681–4696. [Google Scholar] [CrossRef]
- Peng, S.; Deyssenroth, M.A.; Di Narzo, A.F.; Lambertini, L.; Marsit, C.J.; Chen, J.; Hao, K. Expression quantitative trait loci (eQTLs) in human placentas suggest developmental origins of complex diseases. Hum. Mol. Genet. 2017, 26, 3432–3441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, B.; Zhao, Y. Inflammation in Preeclampsia: Genetic Biomarkers, Mechanisms, and Therapeutic Strategies. Front. Immunol. 2022, 13, 883404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Chang, H.M.; Zhu, H.; Klausen, C.; Li, Y.; Leung, P.C.K. Bone Morphogenetic Protein 2 Promotes Human Trophoblast Cell Invasion by Inducing Activin A Production. Endocrinology 2018, 159, 2815–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, B.; Zhou, Y.; Ni, X.; Tong, X.; Xu, X.; Dong, Z.; Sun, R.; Tian, Z.; Wei, H. Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. Immunity 2017, 47, 1100–1113.e6. [Google Scholar] [CrossRef] [Green Version]
- Castriconi, R.; Carrega, P.; Dondero, A.; Bellora, F.; Casu, B.; Regis, S.; Ferlazzo, G.; Bottino, C. Molecular Mechanisms Directing Migration and Retention of Natural Killer Cells in Human Tissues. Front. Immunol. 2018, 9, 2324. [Google Scholar] [CrossRef]
- He, C.; Kraft, P.; Chasman, D.I.; Buring, J.E.; Chen, C.; Hankinson, S.E.; Paré, G.; Chanock, S.; Ridker, P.M.; Hunter, D.J. A large-scale candidate gene association study of age at menarche and age at natural menopause. Hum. Genet. 2010, 128, 515–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Guo, Y.; Shi, H.; Liu, C.L.; Panganiban, R.A.; Chung, W.; O’Connor, L.J.; Himes, B.E.; Gazal, S.; Hasegawa, K.; et al. Shared genetic and experimental links between obesity-related traits and asthma subtypes in UK Biobank. J. Allergy Clin. Immunol. 2020, 145, 537–549, Erratum in J. Allergy Clin. Immunol. 2022, 149, 1486–1489. [Google Scholar] [CrossRef] [Green Version]
- Christakoudi, S.; Evangelou, E.; Riboli, E.; Tsilidis, K.K. GWAS of allometric body-shape indices in UK Biobank identifies loci suggesting associations with morphogenesis, organogenesis, adrenal cell renewal and cancer. Sci. Rep. 2021, 11, 10688. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, T.H.; Lim, A.M.W.; Chang, C.C.; Sie, J.J.; Chen, P.L.; Chang, S.W.; Wu, S.J.; Hsu, C.L.; Hsieh, A.R.; et al. Phenome-wide analysis of Taiwan Biobank reveals novel glycemia-related loci and genetic risks for diabetes. Commun. Biol. 2022, 5, 1175. [Google Scholar] [CrossRef]
- Sakaue, S.; Kanai, M.; Tanigawa, Y.; Karjalainen, J.; Kurki, M.; Koshiba, S.; Narita, A.; Konuma, T.; Yamamoto, K.; Akiyama, M.; et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021, 53, 1415–1424. [Google Scholar] [CrossRef]
- Berndt, S.I.; Gustafsson, S.; Mägi, R.; Ganna, A.; Wheeler, E.; Feitosa, M.F.; Justice, A.E.; Monda, K.L.; Croteau-Chonka, D.C.; Day, F.R.; et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat. Genet. 2013, 45, 501–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harno, E.; Gali Ramamoorthy, T.; Coll, A.P.; White, A. POMC: The Physiological Power of Hormone Processing. Physiol. Rev. 2018, 98, 2381–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.J. 60 YEARS OF POMC: The proopiomelanocortin gene: Discovery, deletion and disease. J. Mol. Endocrinol. 2016, 56, T27–T37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayers, K.L.; Glicksberg, B.S.; Garfield, A.S.; Longerich, S.; White, J.A.; Yang, P.; Du, L.; Chittenden, T.W.; Gulcher, J.R.; Roy, S.; et al. Melanocortin 4 Receptor Pathway Dysfunction in Obesity: Patient Stratification Aimed at MC4R Agonist Treatment. J. Clin. Endocrinol. Metab. 2018, 103, 2601–2612. [Google Scholar] [CrossRef]
- Toumba, M.; Fanis, P.; Vlachakis, D.; Neocleous, V.; Phylactou, L.A.; Skordis, N.; Mantzoros, C.S.; Pantelidou, M. Molecular modelling of novel ADCY3 variant predicts a molecular target for tackling obesity. Int. J. Mol. Med. 2022, 49, 10. [Google Scholar] [CrossRef]
- Xu, T.R.; Yang, Y.; Ward, R.; Gao, L.; Liu, Y. Orexin receptors: Multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell. Signal. 2013, 25, 2413–2423. [Google Scholar] [CrossRef]
- Wang, Z.; Li, V.; Chan, G.C.; Phan, T.; Nudelman, A.S.; Xia, Z.; Storm, D.R. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS ONE 2009, 4, e6979. [Google Scholar] [CrossRef] [Green Version]
- Speliotes, E.K.; Willer, C.J.; Berndt, S.I.; Monda, K.L.; Thorleifsson, G.; Jackson, A.U.; Allen, H.L.; Lindgren, C.M.; Luan, J.; Mägi, R.; et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010, 42, 937–948. [Google Scholar] [CrossRef] [Green Version]
- Seed Ahmed, M.; Kovoor, A.; Nordman, S.; Abu Seman, N.; Gu, T.; Efendic, S.; Brismar, K.; Östenson, C.G.; Gu, H.F. Increased expression of adenylyl cyclase 3 in pancreatic islets and central nervous system of diabetic Goto-Kakizaki rats: A possible regulatory role in glucose homeostasis. Islets 2012, 4, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Hurtado del Pozo, C.; Vesperinas-García, G.; Rubio, M.Á.; Corripio-Sánchez, R.; Torres-García, A.J.; Obregon, M.J.; Calvo, R.M. ChREBP expression in the liver, adipose tissue and differentiated preadipocytes in human obesity. Biochim. Biophys. Acta 2011, 1811, 1194–1200. [Google Scholar] [CrossRef] [Green Version]
- Stergiakouli, E.; Gaillard, R.; Tavaré, J.M.; Balthasar, N.; Loos, R.J.; Taal, H.R.; Evans, D.M.; Rivadeneira, F.; St Pourcain, B.; Uitterlinden, A.G.; et al. Genome-wide association study of height-adjusted BMI in childhood identifies functional variant in ADCY3. Obesity 2014, 22, 2252–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrington, N.M.; Howe, L.D.; Paternoster, L.; Kaakinen, M.; Herrala, S.; Huikari, V.; Wu, Y.Y.; Kemp, J.P.; Timpson, N.J.; St Pourcain, B.; et al. A genome-wide association study of body mass index across early life and childhood. Int. J. Epidemiol. 2015, 44, 700–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xu, M.; Wang, F.; Ji, Y.; DavidsoN, W.S.; Li, Z.; Tso, P. Interaction of ApoA-IV with NR4A1 and NR1D1 Represses G6Pase and PEPCK Transcription: Nuclear Receptor-Mediated Downregulation of Hepatic Gluconeogenesis in Mice and a Human Hepatocyte Cell Line. PLoS ONE 2015, 10, e0142098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, L.; Zhao, X.; Tang, L.; Chen, J.; Zhao, J.; Guo, M.; Chen, C.; Zhou, Y.; Xu, L. Thyroid Transcription Factor-1: Structure, Expression, Function and Its Relationship with Disease. Biomed. Res. Int. 2021, 2021, 9957209. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, T.; Kawaguchi, A.; Hoshi, N.; Kawaguchi, R.; Hoshi, S.; Kimura, S. Thyroid-specific enhancer-binding protein/NKX2.1 is required for the maintenance of ordered architecture and function of the differentiated thyroid. Mol. Endocrinol. 2006, 20, 1796–1809. [Google Scholar] [CrossRef] [Green Version]
- Nagasaki, K.; Minamitani, K.; Nakamura, A.; Kobayashi, H.; Numakura, C.; Itoh, M.; Mushimoto, Y.; Fujikura, K.; Fukushi, M.; Tajima, T. Guidelines for Newborn Screening of Congenital Hypothyroidism (2021 Revision). Clin. Pediatr. Endocrinol. 2023, 32, 26–51. [Google Scholar] [CrossRef]
- Abadi, A.; Peralta-Romero, J.; Suarez, F.; Gomez-Zamudio, J.; Burguete-Garcia, A.I.; Cruz, M.; Meyre, D. Assessing the effects of 35 European-derived BMI-associated SNPs in Mexican children. Obesity 2016, 24, 1989–1995. [Google Scholar] [CrossRef]
- Carroll, J.; Saxena, R.; Welt, C.K. Environmental and genetic factors influence age at menarche in women with polycystic ovary syndrome. J. Pediatr. Endocrinol. Metab. 2012, 25, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Carty, C.L.; Spencer, K.L.; Setiawan, V.W.; Fernandez-Rhodes, L.; Malinowski, J.; Buyske, S.; Young, A.; Jorgensen, N.W.; Cheng, I.; Carlson, C.S.; et al. Replication of genetic loci for ages at menarche and menopause in the multi-ethnic Population Architecture using Genomics and Epidemiology (PAGE) study. Hum. Reprod. 2013, 28, 1695–1706. [Google Scholar] [CrossRef] [Green Version]
- Chenthuran, T.; Galhenagey, G.H.; Jayasekara, R.W.; Dissanayake, V.H. Polymorphism in the epidermal growth factor gene is associated with pre-eclampsia and low birthweight. J. Obstet. Gynaecol. Res. 2014, 5, 1235–1242. [Google Scholar] [CrossRef]
- Cobayashi, F.; Lourenço, B.H.; Cardoso, M.A. 25-Hydroxyvitamin D3 levels, BsmI polymorphism and insulin resistance in Brazilian Amazonian children. Int. J. Mol. Sci. 2015, 16, 12531–12546. [Google Scholar] [CrossRef] [Green Version]
- Cotsapas, C.; Speliotes, E.K.; Hatoum, I.J.; Greenawalt, D.M.; Dobrin, R.; Lum, P.Y.; Suver, C.; Chudin, E.; Kemp, D.; Reitman, M.; et al. Common body mass index-associated variants confer risk of extreme obesity. Hum. Mol. Genet. 2009, 18, 3502–3507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousminer, D.L.; Berry, D.J.; Timpson, N.J.; Ang, W.; Thiering, E.; Byrne, E.M.; Taal, H.R.; Huikari, V.; Bradfield, J.P.; Kerkhof, M.; et al. Genome-wide association and longitudinal analyses reveal genetic loci linking pubertal height growth, pubertal timing and childhood adiposity. Hum. Mol. Genet. 2013, 13, 2735–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousminer, D.L.; Stergiakouli, E.; Berry, D.J.; Ang, W.; Groen-Blokhuis, M.M.; Körner, A.; Siitonen, N.; Ntalla, I.; Marinelli, M.; Perry, J.R.; et al. Genome-wide association study of sexual maturation in males and females highlights a role for body mass and menarche loci in male puberty. Hum. Mol. Genet. 2014, 16, 4452–4464. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, I.; Vaxillaire, M.; Nilsson, M.; Lecoeur, C.; Gu, H.F.; Cavalcanti-Proença, C.; Efendic, S.; Ostenson, C.G.; Brismar, K.; Charpentier, G.; et al. Estrogen receptor alpha gene variants associate with type 2 diabetes and fasting plasma glucose. Pharmacogenetics Genom. 2008, 18, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Demerath, E.W.; Liu, C.T.; Franceschini, N.; Chen, G.; Palmer, J.R.; Smith, E.N.; Chen, C.T.; Ambrosone, C.B.; Arnold, A.M.; Bandera, E.V.; et al. Genome-wide association study of age at menarche in African-American women. Hum. Mol. Genet. 2013, 22, 3329–3346. [Google Scholar] [CrossRef] [Green Version]
- Doo, M.; Kim, Y. Association between ESR1 rs1884051 polymorphism and dietary total energy and plant protein intake on obesity in Korean men. Nutr. Res. Pract. 2011, 5, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Wang, Z.; Zhang, J.; Jia, L.; Zhang, F.; Shi, Y.; Chen, Z. Association between single nucleotide polymorphism of rs2252673 of INSR gene and polycystic ovarian syndrome. Zhonghua Fu Chan Ke Za Zhi 2014, 12, 919–924. [Google Scholar]
- Duicu, C.; Mărginean, C.O.; Voidăzan, S.; Tripon, F.; Bănescu, C. FTO rs9939609 SNP is associated with adiponectin and leptin levels and the risk of obesity in a cohort of Romanian children population. Medicine 2016, 20, e3709. [Google Scholar] [CrossRef]
- Engelman, C.D.; Fingerlin, T.E.; Langefeld, C.D.; Hicks, P.J.; Rich, S.S.; Wagenknecht, L.E.; Bowden, D.W.; Norris, J.M. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J. Clin. Endocrinol. Metab. 2008, 93, 3381–3388. [Google Scholar] [CrossRef]
- Fernandez-Rhodes, L.; Demerath, E.W.; Cousminer, D.L.; Tao, R.; Dreyfus, J.G.; Esko, T.; Smith, A.V.; Gudnason, V.; Harris, T.B.; Launer, L.; et al. Association of adiposity genetic variants with menarche timing in 92,105 women of European descent. Am. J. Epidemiol. 2013, 178, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [Green Version]
- Goodarzi, M.O.; Louwers, Y.V.; Taylor, K.D.; Jones, M.R.; Cui, J.; Kwon, S.; Chen, Y.D.; Guo, X.; Stolk, L.; Uitterlinden, A.G.; et al. Replication of association of a novel insulin receptor gene polymorphism with polycystic ovary syndrome. Fertil. Steril. 2011, 95, 1736–1741.e11. [Google Scholar] [CrossRef] [Green Version]
- Graff, M.; Ngwa, J.S.; Workalemahu, T.; Homuth, G.; Schipf, S.; Teumer, A.; Völzke, H.; Wallaschofski, H.; Abecasis, G.R.; Edward, L.; et al. Genome–wide analysis of BMI in adolescents and young adults reveals additional insight into the effects of genetic loci over the life course. Hum. Mol. Genet. 2013, 22, 3597–3607. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Kraft, P.; Chen, C.; Buring, J.E.; Paré, G.; Hankinson, S.E.; Chanock, S.J.; Ridker, P.M.; David, J.; Chasman, D.I. Genome-wide association studies identify novel loci associated with age at menarche and age at natural menopause. Nat. Genet. 2009, 41, 724–728. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.W.; Oh, B. Recapitulation of genome-wide association studies on body mass index in the Korean population. Int. J. Obes. 2012, 36, 1127–1130. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, T.J.; Lin, E. Association of a common rs9939609 variant in the fat mass and obesity-associated (FTO) gene with obesity and metabolic phenotypes in a Taiwanese population: A replication study. J. Genet. 2016, 95, 595–601. [Google Scholar] [CrossRef]
- Jia, F.; Sun, R.F.; Li, Q.H.; Wang, D.X.; Zhao, F.; Li, J.M.; Pu, Q.; Zhang, Z.Z.; Jin, Y.; Liu, B.L.; et al. Vitamin D receptor BsmI polymorphism and osteoporosis risk: A meta-analysis from 26 studies. Genet. Test. Mol. Biomark. 2013, 1, 30–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitanaka, S.; Isojima, T.; Takaki, M.; Numakura, C.; Hayasaka, K.; Igarashi, T. Association of vitamin D-related gene polymorphisms with manifestation of vitamin D deficiency in children. Endocr. J. 2012, 59, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laczmanski, L.; Lwow, F.; Mossakowska, M.; Puzianowska-Kuznicka, M.; Szwed, M.; Kolackov, K.; Krzyzanowska-Swiniarska, B.; Bar-Andziak, E.; Chudek, J.; Sloka, N.; et al. Association between vitamin D concentration and levels of sex hormones in an elderly Polish population with different genotypes of VDR polymorphisms (rs10735810, rs1544410, rs7975232, rs731236). Gene 2015, 559, 73–76. [Google Scholar] [CrossRef]
- Lango Allen, H.; Estrada, K.; Lettre, G.; Berndt, S.I.; Weedon, M.N.; Rivadeneira, F.; Willer, C.J.; Jackson, A.U.; Vedantam, S.; Raychaudhuri, S.; et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 2010, 7317, 832–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leinonen, J.T.; Surakka, I.; Havulinna, A.S.; Kettunen, J.; Luoto, R.; Salomaa, V.; Wide´n, E. Association of LIN28B with Adult Adiposity-Related Traits in Females. PLoS ONE 2012, 11, e48785. [Google Scholar] [CrossRef]
- Lv, D.; Zhang, D.D.; Wang, H.; Zhang, Y.; Liang, L.; Fu, J.F.; Xiong, F.; Liu, G.L.; Gong, C.X.; Luo, F.H.; et al. Genetic variations in SEC16B, MC4R, MAP2K5 and KCTD15 were associated with childhood obesity and interacted with dietary behaviors in Chinese school-age population. Gene 2015, 560, 149–155. [Google Scholar] [CrossRef]
- Mei, H.; Chen, W.; Jiang, F.; He, J.; Srinivasan, S.; Smith, E.N.; Schork, N.; Murray, S.; Berenson, G.S. Longitudinal replication studies of GWAS risk SNPs influencing body mass index over the course of childhood and adulthood. PLoS ONE 2012, 7, e31470. [Google Scholar] [CrossRef] [Green Version]
- Perry, J.R.; Stolk, L.; Franceschini, N.; Lunetta, K.L.; Zhai, G.; McArdle, P.F.; Smith, A.V.; Aspelund, T.; Bandinelli, S.; Boerwinkle, E.; et al. Meta-analysis of genome-wide association data identifies two loci influencing age at menarche. Nat. Genet. 2009, 41, 648–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, J.R.; Voight, B.F.; Yengo, L.; Amin, N.; Dupuis, J.; Ganser, M.; Grallert, H.; Navarro, P.; Li, M.; Qi, L.; et al. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. PLoS Genet. 2012, 8, e1002741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petkeviciene, J.; Smalinskiene, A.; Klumbiene, J.; Petkevicius, V.; Kriaucioniene, V.; Lesauskaite, V. Physical activity, but not dietary intake, attenuates the effect of the FTO rs9939609 polymorphism on obesity and metabolic syndrome in Lithuanian adult population. Public. Health 2016, 135, 23–29. [Google Scholar] [CrossRef]
- Pyun, J.A.; Kim, S.; Cho, N.H.; Koh, I.; Lee, J.Y.; Shin, C.; Kwack, K. Genome-wide association studies and epistasis analyses of candidate genes related to age at menarche and age at natural menopause in a Korean population. Menopause 2014, 21, 522–529. [Google Scholar] [CrossRef]
- Quan, L.L.; Wang, H.; Tian, Y.; Mu, X.; Zhang, Y.; Tao, K. Association of fat-mass and obesity-associated gene FTO rs9939609 polymorphism with the risk of obesity among children and adolescents: A meta-analysis. Eur Rev Med Pharmacol Sci 2015, 19, 614–623. [Google Scholar]
- Rask-Andersen, M.; Jacobsson, J.A.; Moschonis, G.; Ek, A.E.; Chrousos, G.P.; Marcus, C.; Manios, Y.; Fredriksson, R.; Schiöth, H.B. The MAP2K5-linked SNP rs2241423 is associated with BMI and obesity in two cohorts of Swedish and Greek children. BMC Med. Genet. 2012, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Schweighofer, N.; Lerchbaum, E.; Trummer, O.; Schwetz, V.; Pilz, S.; Pieber, T.R.; Obermayer-Pietsch, B. Androgen levels and metabolic parameters are associated with a genetic variant of F13A1 in women with polycystic ovary syndrome. Gene 2012, 504, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.; Zois, C.; Chatzikyriakidou, A.; Georgiou, I.; Tsatsoulis, A. Combined estrogen receptor α and estrogen receptor β genotypes influence the age of menarche. Hum. Reprod. 2006, 21, 554–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, K.T.; Eun, I.S.; Lee, J.S. Polymorphism in vitamin D receptor is associated with bone mineral density in patients with adolescent idiopathic scoliosis. Eur. Spine J. 2010, 19, 1545–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulem, P.; Gudbjartsson, D.F.; Rafnar, T.; Holm, H.; Olafsdottir, E.J.; Olafsdottir, G.H.; Jonsson, T.; Alexandersen, P.; Feenstra, B.; Boyd, H.A.; et al. Genome-wide association study identifies sequence variants on 6q21 associated with age at menarche. Nat. Genet. 2009, 41, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, H.; Chen, H.; Peng, Y.; Cui, L.; Du, Y.; Wang, Z.; Xu, J.; Chen, Z.-J. Variants in FSHB are associated with polycystic ovary syndrome and luteinizing hormone level in han chinese women. J. Clin. Endocrinol. Metab. 2016, 101, 2178–2184. [Google Scholar] [CrossRef] [Green Version]
- Tu, W.; Wagner, E.K.; Eckert, G.J.; Yu, Z.; Hannon, T.; Pratt, J.H.; He, C. Associations between menarche-related genetic variants and pubertal growth in male and female adolescents. J. Adolesc. Health 2015, 56, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.J.; Hinney, A.; Song, J.Y.; Scherag, A.; Meng, X.R.; Grallert, H.; Illig, T.; Hebebrand, J.; Wang, Y.; Ma, J. Association of common variants identified by recent genome-wide association studies with obesity in Chinese children: A case-control study. BMC Med. Genet. 2016, 17, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.T.; Guo, Y.; Yang, T.L.; Xu, X.H.; Dong, S.S.; Li, M.; Li, T.Q.; Chen, Y.; Deng, H.W. Polymorphisms in the estrogen receptor genes are associated with hip fractures in Chinese. Bone 2008, 43, 910–914. [Google Scholar] [CrossRef]
- Widén, E.; Ripatti, S.; Cousminer, D.L.; Surakka, I.; Lappalainen, T.; Järvelin, M.R.; Eriksson, J.G.; Raitakari, O.; Salomaa, V.; Sovio, U.; et al. Distinct variants at LIN28B influence growth in height from birth to adulthood. Am. J. Hum. Genet. 2010, 86, 773–782. [Google Scholar] [CrossRef]
- Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; Buchkovich, M.L.; Mora, S.; et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013, 45, 1274–1283. [Google Scholar]
- Xu, X.H.; Xiong, D.H.; Liu, X.G.; Guo, Y.; Chen, Y.; Zhao, J.; Recker, R.R.; Deng, H.W. Association analyses of vitamin D-binding protein gene with compression strength index variation in Caucasian nuclear families. Osteoporos. Int. 2010, 21, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.L.; Guo, Y.; Li, S.M.; Li, S.K.; Tian, Q.; Liu, Y.J.; Deng, H.W. Ethnic differentiation of copy number variation on chromosome 16p12.3 for association with obesity phenotypes in European and Chinese populations. Int. J. Obes. 2013, 37, 188–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yermachenko, A.; Dvornyk, V. UGT2B4 previously implicated in the risk of breast cancer is associated with menarche timing in Ukrainian females. Gene 2016, 1, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Liu, Y.; Qu, H.; Qu, S.; Wang, W.; Ren, L. The GC, CYP2R1 and DHCR7 genes are associated with vitamin D levels in northeastern Han Chinese children. Swiss Med. Wkly. 2012, 142, w13636. [Google Scholar] [CrossRef]





| Parameters | N (%) | Birth Weight, g ± SD (min–max) | p-Value |
|---|---|---|---|
| Baseline characteristics | |||
| Maternal age, years | 716 | 26.56 ± 4.95 (16–45) | - |
| Maternal pre-pregnancy BMI, kg/m2 | 716 | 23.86 ± 4.32 (15.06–44.98) | - |
| Age at menarche, years | 716 | 12.63 ± 1.06 (10–16) | |
| Birth weight, g | 716 | 3142.96 ± 584.43 (1050–5220) | - |
| Gestational age, weeks | 716 | 38.7 (24.0–41.0) | - |
| Infant gender, Male/Female | 716 | 379 (52.93%)/337 (47.07%) | - |
| Maternal phenotypic characteristics and offspring birth weight | |||
| Age, years | |||
| 16–25 | 75 (10.48) | 3156.53 ± 519.23 (1490–4770) | 0.22 |
| 21–25 | 238 (33.24) | 3214.90 ± 551.87 (1140–5220) | |
| 26–30 | 253 (35.34) | 3102.98 ± 592.85 (1180–4440) | |
| >30 | 150 (20.95) | 3089.47 ± 642.12 (1050–4340) | |
| Age at menarche, years | |||
| early (<12) | 64 (8.94) | 3291.88 ± 510.58 (1490–3970) | 0.02 |
| average (12–14) | 618 (86.31) | 3131.51 ± 577.60 (1050–5220) | |
| late (>14) | 34 (4.75) | 3020.88 ± 783.82 (1420–4820) | |
| pre-pregnancy BMI, kg/m2 | |||
| underweight (<18.50) | 43(6.01) | 2855.12 ± 641.80 (1220–3990) | <0.0001 |
| normal weight (18.50–24.99) | 441 (61.59) | 3109.62 ± 523.68 (1430–4510) | |
| overweight (25.00–29.99) | 163 (22.77) | 3304.36 ± 613.35(1050–4770) | |
| obesity (>30) | 69 (9.22) | 3354.20 ± 737.65 (1110–5220) | |
| The course of this pregnancy | |||
| normal | 283 (39.5) | 3507.56 ± 325.80 (2510–4440) | 0.0001 |
| preeclampsia | 168 (23.5) | 3483.3 ± 399.99 (2630–5220) | |
| fetal growth restriction | 191 (26.7) | 2568.98 ± 311.79 (1050–2850) | |
| preeclampsia + fetal growth restriction | 74 (10.3) | 2457.43 ± 442.78 (1180–2970) | |
| Smoking: | |||
| yes | 436 (60.89) | 3107.74 ± 586.01 (1050–4770) | 0.07 |
| no | 280 (39.11) | 3197.80 ± 578.75 (1110–5220) | |
| Alcohol: | |||
| yes | 550 (76.82) | 3306.15 ± 509.36 (1420–4820 | 0.08 |
| no | 166 (23.18) | 3539.52 ± 489.22 (2510–5220) | |
| History of arterial hypertension: | |||
| yes | 51 (7.12) | 2780.00 ± 758.84 (1140–4820) | 0.0002 |
| no | 665 (92.88) | 3170.80 ± 559.95 (1050–5220) | |
| History of sexually transmitted diseases: | |||
| yes | 182 (25.42 | 3253.82 ± 526.15 (1430–4070) | 0.10 |
| no | 534 (74.58) | 3396.48 ± 509.78 (1420–5220) | |
| History of preeclampsia: | |||
| yes | 96 (13.41 | 3086.67 ± 534.93 (1530–4070) | 0.34 |
| no | 620 (86.59) | 3151.67 ± 591.65 (1050–5220)) | |
| History of fetal growth restriction: | |||
| yes | 53 (7.40) | 2488.21 ± 423.75 (1110–2970) | <0.0001 |
| no | 663 (92.60) | 3195.30 ± 563.68 (1050–5220) | |
| Minor Allele (SNP) | Gene | Chr | N | Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Add | Dom | Rec | ||||||||||
| β | SE | P | β | SE | P | β | SE | P | ||||
| T (rs1514175) | TNNI3K | 1 | 714 | 0.023 | 0.035 | 0.497 | 0.044 | 0.050 | 0.377 | 0.009 | 0.066 | 0.898 |
| T (rs466639) | RXRG | 1 | 714 | −0.002 | 0.050 | 0.968 | 0.007 | 0.057 | 0.909 | −0.081 | 0.170 | 0.633 |
| G (rs7538038) | KISS1 | 1 | 714 | 0.048 | 0.044 | 0.271 | 0.055 | 0.050 | 0.270 | 0.058 | 0.135 | 0.669 |
| C (rs713586) | RBJ | 2 | 712 | −0.024 | 0.034 | 0.475 | −0.023 | 0.051 | 0.652 | −0.046 | 0.061 | 0.451 |
| A (rs2164808) | POMC | 2 | 714 | 0.034 | 0.035 | 0.335 | 0.049 | 0.054 | 0.362 | 0.038 | 0.059 | 0.518 |
| A (rs7589318) | POMC | 2 | 714 | 0.042 | 0.037 | 0.261 | 0.005 | 0.049 | 0.921 | 0.202 | 0.085 | 0.015 |
| C (rs4374421) | LHCGR | 2 | 707 | −0.018 | 0.038 | 0.647 | −0.029 | 0.049 | 0.559 | −9.917 | 0.088 | 0.999 |
| T (rs7579411) | LHCGR | 2 | 710 | −0.028 | 0.036 | 0.435 | −0.022 | 0.053 | 0.681 | −0.056 | 0.063 | 0.372 |
| C (rs4953616) | LHCGR | 2 | 713 | 0.012 | 0.039 | 0.760 | −0.005 | 0.049 | 0.923 | 0.087 | 0.094 | 0.354 |
| G (rs6732220) | FSHR | 2 | 714 | −0.018 | 0.040 | 0.662 | −0.023 | 0.049 | 0.637 | −0.014 | 0.104 | 0.895 |
| G (rs4953655) | FSHR | 2 | 714 | 0.002 | 0.040 | 0.954 | −0.005 | 0.050 | 0.922 | 0.036 | 0.103 | 0.727 |
| A (rs12617311) | PLCL1 | 2 | 711 | 0.036 | 0.036 | 0.329 | 0.035 | 0.050 | 0.480 | 0.071 | 0.075 | 0.346 |
| C (rs6438424) | IGSF11 | 3 | 710 | −0.043 | 0.035 | 0.215 | −0.037 | 0.054 | 0.494 | −0.083 | 0.060 | 0.168 |
| A (rs2013573) | UGT2B4 | 4 | 714 | 0.016 | 0.046 | 0.732 | 0.020 | 0.051 | 0.694 | −0.006 | 0.156 | 0.972 |
| A (rs13111134) | UGT2B4 | 4 | 712 | 0.002 | 0.042 | 0.968 | −0.020 | 0.049 | 0.691 | 0.134 | 0.122 | 0.272 |
| C (rs222003) | GC | 4 | 715 | 0.137 | 0.065 | 0.036 | 0.157 | 0.068 | 0.021 | −0.238 | 0.377 | 0.529 |
| C (rs222020) | GC | 4 | 713 | −0.008 | 0.056 | 0.881 | −0.022 | 0.059 | 0.707 | 0.259 | 0.266 | 0.331 |
| G (rs3756261) | EGF | 4 | 713 | −0.023 | 0.065 | 0.729 | −0.027 | 0.068 | 0.687 | 0.087 | 0.379 | 0.819 |
| T (rs757647) | KDM3B | 5 | 709 | 0.037 | 0.041 | 0.366 | −0.010 | 0.049 | 0.835 | 0.323 | 0.110 | 0.004 |
| G (rs7766109) | F13A1 | 6 | 715 | −0.019 | 0.035 | 0.580 | 0.006 | 0.055 | 0.919 | −0.060 | 0.058 | 0.299 |
| A (rs4946651) | LIN28B | 6 | 714 | 0.050 | 0.035 | 0.158 | 0.074 | 0.052 | 0.156 | 0.053 | 0.064 | 0.412 |
| C (rs7759938) | LIN28B | 6 | 712 | 0.071 | 0.039 | 0.069 | 0.098 | 0.048 | 0.043 | 0.044 | 0.093 | 0.636 |
| T (rs314280) | LIN28B | 6 | 707 | 0.060 | 0.036 | 0.092 | 0.084 | 0.052 | 0.106 | 0.070 | 0.066 | 0.293 |
| A (rs314276) | LIN28B | 6 | 698 | 0.069 | 0.039 | 0.072 | 0.108 | 0.050 | 0.029 | 0.020 | 0.086 | 0.812 |
| G (rs3020394) | ESR1 | 6 | 715 | −0.071 | 0.037 | 0.053 | −0.058 | 0.049 | 0.233 | −0.182 | 0.080 | 0.023 |
| G (rs1884051) | ESR1 | 6 | 715 | −0.068 | 0.037 | 0.068 | −0.065 | 0.049 | 0.182 | −0.148 | 0.083 | 0.073 |
| C (rs7753051) | IGF2R | 6 | 714 | −0.042 | 0.039 | 0.283 | −0.074 | 0.049 | 0.130 | 0.031 | 0.093 | 0.740 |
| C (rs1079866) | INHBA | 7 | 714 | 0.110 | 0.046 | 0.014 | 0.119 | 0.052 | 0.022 | 0.189 | 0.155 | 0.223 |
| T (rs2288696) | FGFR1 | 8 | 715 | −0.043 | 0.044 | 0.331 | −0.040 | 0.050 | 0.429 | −0.128 | 0.144 | 0.377 |
| A (rs10980926) | ZNF483 | 9 | 715 | 0.014 | 0.037 | 0.711 | 0.006 | 0.049 | 0.900 | 0.052 | 0.084 | 0.538 |
| C (rs10441737) | ZNF483 | 9 | 703 | 0.023 | 0.038 | 0.534 | 0.016 | 0.049 | 0.747 | 0.071 | 0.084 | 0.398 |
| C (rs10769908) | STK33 | 11 | 704 | 0.006 | 0.035 | 0.860 | −0.038 | 0.055 | 0.493 | 0.061 | 0.059 | 0.301 |
| G (rs555621) | FSHB | 11 | 714 | −0.037 | 0.035 | 0.297 | −0.082 | 0.052 | 0.113 | 0.005 | 0.065 | 0.935 |
| A (rs11031010) | FSHB | 11 | 711 | −0.018 | 0.051 | 0.729 | −0.040 | 0.057 | 0.481 | 0.199 | 0.189 | 0.293 |
| C (rs1782507) | FSHB | 11 | 714 | −0.024 | 0.036 | 0.510 | 0.006 | 0.049 | 0.904 | −0.117 | 0.075 | 0.121 |
| A (rs6589964) | BSX | 11 | 715 | 0.031 | 0.035 | 0.368 | 0.055 | 0.054 | 0.312 | 0.026 | 0.059 | 0.663 |
| A (rs1544410) | VDR | 12 | 712 | −0.013 | 0.036 | 0.725 | −0.033 | 0.050 | 0.504 | 0.018 | 0.070 | 0.804 |
| A (rs999460) | NKX2−1 | 14 | 714 | −0.078 | 0.036 | 0.029 | −0.068 | 0.049 | 0.167 | −0.176 | 0.073 | 0.014 |
| A (rs4986938) | ESR2 | 14 | 713 | 0.044 | 0.037 | 0.235 | 0.052 | 0.049 | 0.292 | 0.066 | 0.078 | 0.402 |
| A (rs2241423) | MAP2K5 | 15 | 711 | −0.014 | 0.045 | 0.760 | −0.013 | 0.052 | 0.803 | −0.036 | 0.133 | 0.785 |
| T (rs12444979) | GPRC5B | 16 | 714 | −0.017 | 0.048 | 0.719 | −0.020 | 0.055 | 0.714 | −0.020 | 0.155 | 0.899 |
| A (rs9939609) | FTO | 16 | 715 | 0.046 | 0.034 | 0.181 | 0.075 | 0.052 | 0.153 | 0.043 | 0.060 | 0.472 |
| A (rs12324955) | FTO | 16 | 714 | −4.004 | 0.038 | 0.999 | −0.009 | 0.049 | 0.851 | 0.030 | 0.089 | 0.734 |
| G (rs1398217) | SKOR2 | 18 | 710 | −0.009 | 0.036 | 0.796 | −0.045 | 0.052 | 0.393 | 0.041 | 0.066 | 0.539 |
| G (rs2252673) | INSR | 19 | 711 | −0.036 | 0.043 | 0.409 | −0.031 | 0.050 | 0.536 | −0.110 | 0.127 | 0.386 |
| A (rs1073768) | GHRH | 20 | 713 | 0.017 | 0.035 | 0.634 | 0.015 | 0.055 | 0.785 | 0.031 | 0.060 | 0.609 |
| C (rs4633) | COMT | 22 | 714 | 0.015 | 0.034 | 0.660 | −0.011 | 0.054 | 0.838 | 0.054 | 0.056 | 0.341 |
| A (rs5930973) | CD40LG | 23 | 704 | −0.098 | 0.071 | 0.166 | ||||||
| T (rs3092921) | CD40LG | 23 | 713 | −0.002 | 0.066 | 0.970 | ||||||
| N | SNP × SNP Interaction Models | NH | betaH | WH | NL | betaL | WL | Pperm |
|---|---|---|---|---|---|---|---|---|
| Two-order interaction models (threshold level p < 4 × 10−5, real level p < 2.5 × 10−5) | ||||||||
| 1 | rs222003 GC × rs2013573 UGT2B4 | 1 | 0.726 | 17.98 | 0 | - | - | <0.001 |
| 2 | rs4946651 LIN28B × rs7538038 KISS1 | 2 | 0.353 | 18.46 | 1 | −0.217 | 5.58 | <0.001 |
| 3 | rs7538038 KISS1 × rs314280 LIN28B | 2 | 0.364 | 19.57 | 1 | −0.225 | 6.04 | 0.001 |
| Three-order interaction models (threshold level p < 3 × 10−6, real level p < 5 × 10−8) | ||||||||
| 1 | rs222003 GC x rs4986938 ESR2 × rs2013573 UGT2B4 | 2 | 1.259 | 32.19 | 3 | −0.269 | 5.47 | <0.001 |
| 2 | rs555621 FSHB x rs7538038 KISS1 × rs314280 LIN28B | 5 | 0.474 | 30.41 | 1 | −0.295 | 5.74 | 0.001 |
| 3 | rs999460 NKX2-1 x rs12444979 GPRC5B × rs4374421 LHCGR | 3 | 0.527 | 31.90 | 2 | −0.504 | 6.88 | <0.001 |
| Four-order interaction models (threshold level p < 2 × 10−7, real level p < 3 × 10−13) | ||||||||
| 1 | rs10441737 ZNF483 × rs1073768 GHRH × rs7589318 POMC × rs4633 COMT | 8 | 0.944 | 55.95 | 6 | −0.495 | 23.01 | <0.001 |
| 2 | rs713586 RBJ × rs3020394 ESR1 × rs6589964 BSX × rs12324955 FTO | 5 | 0.551 | 13.57 | 11 | −1.065 | 55.72 | <0.001 |
| 3 | rs713586 RBJ × rs3020394 ESR1 × rs1782507 FSHB × rs12324955 FTO | 4 | 0.465 | 12.05 | 10 | −0.886 | 56.83 | <0.001 |
| 4 | rs713586 RBJ × rs6589964 BSX × rs12324955 FTO × rs1514175 TNNI3K | 5 | 1.142 | 23.63 | 7 | −1.299 | 55.55 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reshetnikova, Y.; Churnosova, M.; Stepanov, V.; Bocharova, A.; Serebrova, V.; Trifonova, E.; Ponomarenko, I.; Sorokina, I.; Efremova, O.; Orlova, V.; et al. Maternal Age at Menarche Gene Polymorphisms Are Associated with Offspring Birth Weight. Life 2023, 13, 1525. https://doi.org/10.3390/life13071525
Reshetnikova Y, Churnosova M, Stepanov V, Bocharova A, Serebrova V, Trifonova E, Ponomarenko I, Sorokina I, Efremova O, Orlova V, et al. Maternal Age at Menarche Gene Polymorphisms Are Associated with Offspring Birth Weight. Life. 2023; 13(7):1525. https://doi.org/10.3390/life13071525
Chicago/Turabian StyleReshetnikova, Yuliya, Maria Churnosova, Vadim Stepanov, Anna Bocharova, Victoria Serebrova, Ekaterina Trifonova, Irina Ponomarenko, Inna Sorokina, Olga Efremova, Valentina Orlova, and et al. 2023. "Maternal Age at Menarche Gene Polymorphisms Are Associated with Offspring Birth Weight" Life 13, no. 7: 1525. https://doi.org/10.3390/life13071525
APA StyleReshetnikova, Y., Churnosova, M., Stepanov, V., Bocharova, A., Serebrova, V., Trifonova, E., Ponomarenko, I., Sorokina, I., Efremova, O., Orlova, V., Batlutskaya, I., Ponomarenko, M., Churnosov, V., Eliseeva, N., Aristova, I., Polonikov, A., Reshetnikov, E., & Churnosov, M. (2023). Maternal Age at Menarche Gene Polymorphisms Are Associated with Offspring Birth Weight. Life, 13(7), 1525. https://doi.org/10.3390/life13071525







