Crosstalk between the Resident Microbiota and the Immune Cells Regulates Female Genital Tract Health
Abstract
:1. Introduction
2. Anatomy of the Female Genital Tract
3. Main Features of the Cervical Mucus
4. The Immune Arsenal of the FGT
4.1. Innate Immunity
4.2. Adaptive Immunity
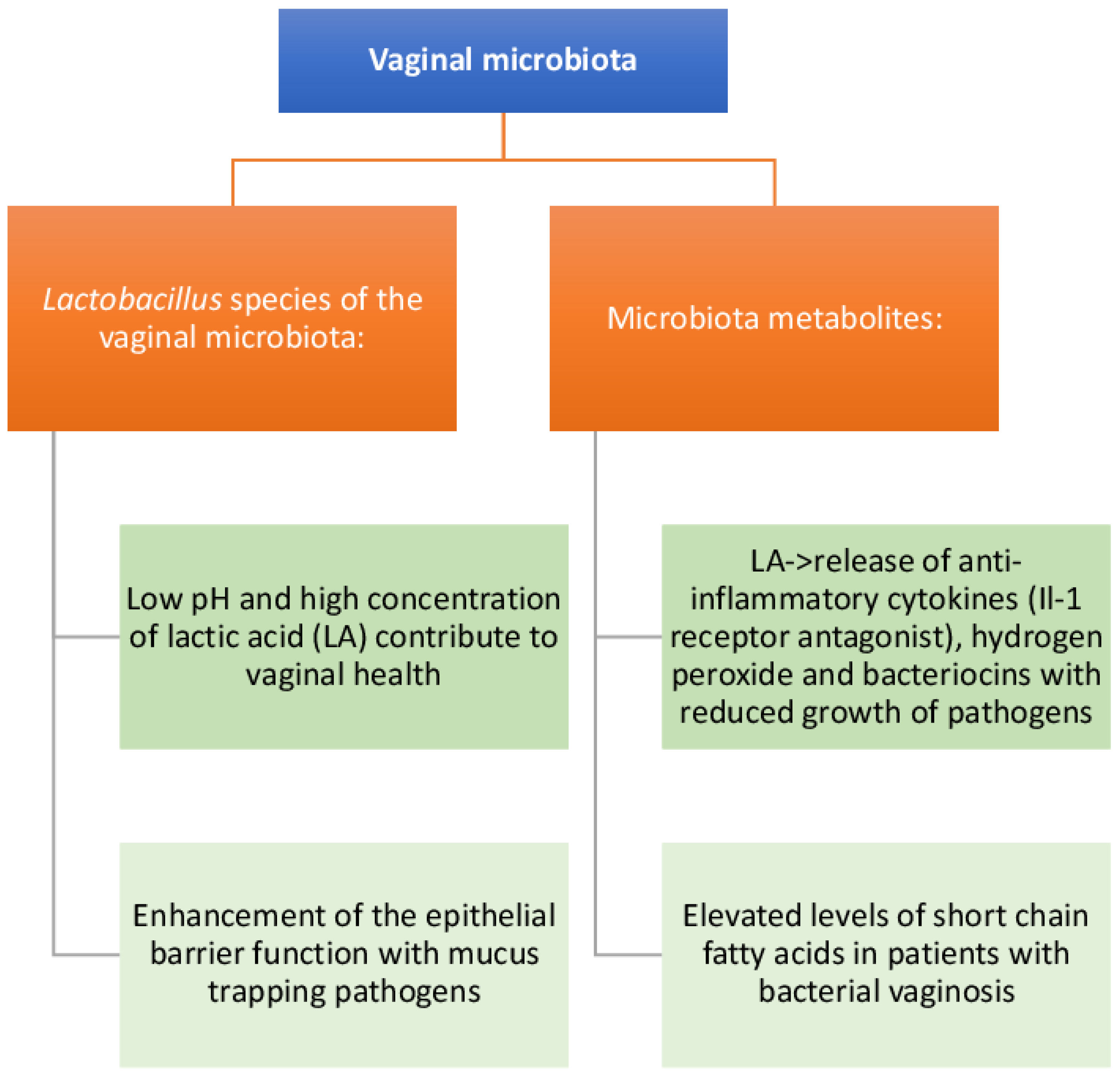
5. Composition and Function of the FGT Microbiota
6. Dysbiosis and FGT Infections
Aerobic Vaginitis
7. Treatment with Natural Products for Maintaining the Health of the FGT
8. Conclusions
Funding
Authors Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPs | Antimicrobial peptides |
| APCs | Antigen-presenting cells |
| AV | Aerobic vaginitis |
| BV | Bacterial vaginosis |
| CSTs | Community state types |
| DCs | Dendritic cells |
| D-LA | D-lactic acid |
| FGT | Female genital tract |
| HBD | Human beta defensin |
| HSV | Herpes simplex virus |
| IL | Interleukin |
| INOS | Inducible nitric oxide |
| LF | Lactoferrin |
| LPS | Lipopolysaccharide |
| L-LA | L-lactic acid |
| NK | Natural killer |
| PID | Pelvic inflammatory disease |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| Th | T helper |
| TGF | Transforming growth factor |
| TJ | Tight junction |
| Treg | T regulatory cell |
| TREM | Tissue resident memory |
| TSST-1 | Toxic shock syndrome toxin-1 |
References
- Rodriguez-Garcia, M.; Patel, M.V.; Shen, Z.; Bodwell, J.; Rossoll, R.M.; Wira, C.R. Tenofovir Inhibits Wound Healing of Epithelial Cells and Fibroblasts from the Upper and Lower Human Female Reproductive Tract. Sci. Rep. 2017, 8, 45725. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.S.; Laven, J.; Louwers, Y.; Budding, A.; Schoenmakers, S. Microbiome as a predictor of implantation. Curr. Opin. Obstet. Gynecol. 2022, 34, 122–132. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhu, M.; Ge, L.; Liu, X.; Su, K.; Chen, Z.; Zhao, W. The Interplay between Cervicovaginal Microbial Dysbiosis and Cervicovaginal Immunity. Front. Immunol. 2022, 13, 857299. [Google Scholar] [CrossRef]
- Mhlekude, B.; Lenman, A.; Sidoyi, P.; Joseph, J.; Kruppa, J.; Businge, C.B.; Mdaka, M.L.; Konietschke, F.; Pich, A.; Gerold, G.; et al. The barrier functions of crude cervical mucus plugs against HIV-1 infection in the context of cell-free and cell-to-cell transmission. AIDS 2021, 35, 2105–2117. [Google Scholar] [CrossRef]
- Le, T.; Reeves, R.K.; McKinnon, L.R. The functional diversity of tissue-resident natural killer cells against infection. Immunology 2022, 167, 28–39. [Google Scholar] [CrossRef]
- Dogra, P.; Farber, D.L. Stealth Killing by Uterine NK Cells for Tolerance and Tissue Homeostasis. Cell 2020, 182, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- Woodward Davis, A.S.; Vick, S.C.; Pattacini, L.; Voillet, V.; Hughes, S.M.; Lentz, G.M.; Kirby, A.C.; Fialkow, M.F.; Gottardo, R.; Hladik, F.; et al. The human memory T cell compartment changes across tissues of the female reproductive tract. Mucosal Immunol. 2021, 14, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.T.; Ruffin, M.T., 4th; Rogers, M.A.; Schloss, P.D. Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions. Genome Med. 2016, 8, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichiyama, T.; Kuroda, K.; Nagai, Y.; Urushiyama, D.; Ohno, M.; Yamaguchi, T.; Nagayoshi, M.; Sakuraba, Y.; Yamasaki, F.; Hata, K.; et al. Analysis of vaginal and endometrial microbiota communities in infertile women with a history of repeated implantation failure. Reprod. Med. Biol. 2021, 20, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Møller, B.R.; Kristiansen, F.V.; Thorsen, P.; Frost, L.; Mogensen, S.C. Sterility of the uterine cavity. Acta Obstet. Gynecol. Scand. 1995, 74, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.D.; Romero, R.; Gervasi, M.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S.S.; Hsu, C.D.; et al. Does the endometrial cavity have a molecular microbial signature? Sci. Rep. 2019, 9, 9905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toson, B.; Simon, C.; Moreno, I. The Endometrial Microbiome and Its Impact on Human Conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Bau, D.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Vilella, F.; Romero, R.; Simón, C. The first glimpse of the endometrial microbiota in early pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 296–305. [Google Scholar] [CrossRef]
- Greenbaum, S.; Greenbaum, G.; Moran-Gilad, J.; Weintraub, A.Y. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obstet. Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.; Zhong, X.; Koenig, S.S.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef] [Green Version]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Torcia, M.G. Interplay among Vaginal Microbiome, Immune Response and Sexually Transmitted Viral Infections. Int. J. Mol. Sci. 2019, 20, 266. [Google Scholar] [CrossRef] [Green Version]
- d’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Sherrard, J.; Wilson, J.; Donders, G.; Mendling, W.; Jensen, J.S. 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int. J. STD AIDS 2018, 29, 1258–1272. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bolan, G.A.; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 2015, 64, 1–137. [Google Scholar] [PubMed]
- Song, S.D.; Acharya, K.D.; Zhu, J.E.; Deveney, C.M.; Walther-Antonio, M.R.S.; Tetel, M.J.; Chia, N. Daily Vaginal Microbiota Fluctuations Associated with Natural Hormonal Cycle, Contraceptives, Diet, and Exercise. mSphere 2020, 5, e00593-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, A.; Li, H.; Yan, Y.; Qi, W.; Wang, Y.; Han, C.; Xue, F. Vaginal bacterial profiles of aerobic vaginitis: A case-control study. Diagn. Microbiol. Infect. Dis. 2020, 96, 114981. [Google Scholar] [CrossRef]
- Bhatia, M.; Mitsi, V.; Court, L.; Thampi, P.; El-Nasharty, M.; Hesham, S.; Randall, W.; Davies, R.; Impey, L. The outcomes of pregnancies with reduced fetal movements: A retrospective cohort study. Acta Obstet. Gynecol. Scand. 2019, 98, 1450–1454. [Google Scholar] [CrossRef]
- Khelaifia, S.; Lagier, J.C.; Nkamga, V.D.; Guilhot, E.; Drancourt, M.; Raoult, D. Aerobic culture of methanogenic archaea without an external source of hydrogen. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Sabo, M.C.; Balkus, J.E.; Richardson, B.A.; Srinivasan, S.; Kimani, J.; Anzala, O.; Schwebke, J.; Feidler, T.L.; Fredricks, D.N.; McClelland, R.S. Association between vaginal washing and vaginal bacterial concentrations. PLoS ONE 2019, 14, e0210825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Houdt, R.; Ma, B.; Bruisten, S.M.; Speksnijder, A.G.C.L.; Ravel, J.; de Vries, H.J.C. Lactobacillus iners-dominated vaginal microbiota is associated with increased susceptibility to Chlamydia trachomatis infection in Dutch women: A case-control study. Sex. Transm. Infect. 2018, 94, 117–123. [Google Scholar] [CrossRef]
- Gargiulo Isacco, C.; Balzanelli, M.G.; Garzone, S.; Lorusso, M.; Inchingolo, F.; Nguyen, K.C.D.; Santacroce, L.; Mosca, A.; Del Prete, R. Alterations of Vaginal Microbiota and Chlamydia trachomatis as Crucial Co-Causative Factors in Cervical Cancer Genesis Procured by HPV. Microorganisms 2023, 11, 662. [Google Scholar] [CrossRef]
- Idahl, A.; Le Cornet, C.; González Maldonado, S.; Waterboer, T.; Bender, N.; Tjønneland, A.; Hansen, L.; Boutron-Ruault, M.C.; Fournier, A.; Kvaskoff, M.; et al. Serologic markers of Chlamydia trachomatis and other sexually transmitted infections and subsequent ovarian cancer risk: Results from the EPIC cohort. Int. J. Cancer 2020, 147, 2042–2052. [Google Scholar] [CrossRef] [Green Version]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef] [Green Version]
- Reid, G. Probiotics: Definition, scope and mechanisms of action. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Nader-Macías, M.E.F.; De Gregorio, P.R.; Silva, J.A. Probiotic lactobacilli in formulas and hygiene products for the health of the urogenital tract. Pharmacol. Res. Perspect. 2021, 9, e00787. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Zimecki, M.; Kruzel, M.L. Lactoferrin for Prevention and Treatment of Anemia and Inflammation in Pregnant Women: A Comprehensive Review. Biomedicines 2021, 9, 898. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacroix, G.; Gouyer, V.; Gottrand, F.; Desseyn, J.L. The Cervicovaginal Mucus Barrier. Int. J. Mol. Sci. 2020, 21, 8266. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, C.J.; Kim, D.J.; Kang, J.H. Immune cells in the female reproductive tract. Immune Netw. 2015, 15, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Charitos, I.A.; Topi, S.; Gagliano-Candela, R.; De Nitto, E.; Polimeno, L.; Montagnani, M.; Santacroce, L. The Toxic Effects of Endocrine Disrupting Chemicals (EDCs) on Gut Microbiota: Bisphenol A (BPA) A Review. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 716–727. [Google Scholar] [CrossRef]
- Wan, F.C.; Zhang, C.; Jin, Q.; Wei, C.; Zhao, H.B.; Zhang, X.L.; You, W.; Liu, X.M.; Liu, G.F.; Liu, Y.F.; et al. Protective effects of astaxanthin on lipopolysaccharide-induced inflammation in bovine endometrial epithelial cells†. Biol. Reprod. 2020, 102, 339–347. [Google Scholar] [CrossRef]
- Benjelloun, F.; Quillay, H.; Cannou, C.; Marlin, R.; Madec, Y.; Fernandez, H.; Chrétien, F.; Le Grand, R.; Barré-Sinoussi, F.; Nugeyre, M.T.; et al. Activation of Toll-Like Receptors Differentially Modulates Inflammation in the Human Reproductive Tract: Preliminary Findings. Front. Immunol. 2020, 11, 1655. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Dietary Approaches to Attain Fish Health with Special Reference to their Immune System. Curr. Pharm. Des. 2018, 24, 4921–4931. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Russo, M.A.; Jirillo, E. Antimicrobial Peptides in Human Disease: Therapeutic Approaches. Second of Two Parts. Curr. Pharm. Des. 2018, 24, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial peptides in the female reproductive tract: A critical component of the mucosal immune barrier with physiological and clinical implications. Hum. Reprod. Update 2015, 21, 353–377. [Google Scholar] [CrossRef] [Green Version]
- Anahtar, M.N.; Gootenberg, D.B.; Mitchell, C.M.; Kwon, D.S. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host Microbe 2018, 23, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Zhang, X.; Wu, H.H.; Wu, S.J.; Tang, X.Y.; Liu, T.Z.; Li, S. Novel T-cell signature based on cell pair algorithm predicts survival and immunotherapy response for patients with bladder urothelial carcinoma. Front. Immunol. 2022, 13, 994594. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Dong, Y.; Bai, J.; Li, H.; Ma, X.; Li, B.; Wang, C.; Li, H.; Qi, W.; Wang, Y.; et al. Interactions between microbiota and cervical epithelial, immune, and mucus barrier. Front. Cell. Infect. Microbiol. 2023, 13, 1124591. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Sardaro, N.; Topi, S.; Pettini, F.; Bottalico, L.; Cantore, S.; Cascella, G.; Del Prete, R.; Dipalma, G.; Inchingolo, F. The pivotal role of oral microbiota in health and disease. J. Biol. Regul. Homeost. Agents 2020, 34, 733–737. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Williams, P.J.; Lash, G.E. Immune cells in the placental bed. Int. J. Dev. Biol. 2010, 54, 281–294. [Google Scholar] [CrossRef]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Murtaza, G.; Kalhoro, D.H.; Kalhoro, M.S.; Yin, Y.; Chughtai, M.I.; Tan, B.; Yaseen, A.; Rehman, Z.U. Understanding the Immune System in Fetal Protection and Maternal Infections during Pregnancy. J. Immunol. Res. 2022, 2022, 7567708. [Google Scholar] [CrossRef]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. (Landmark Ed.) 2021, 26, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Kwon, J.Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Perez-Zsolt, D.; Cantero-Pérez, J.; Erkizia, I.; Benet, S.; Pino, M.; Serra-Peinado, C.; Hernández-Gallego, A.; Castellví, J.; Tapia, G.; Arnau-Saz, V.; et al. Dendritic Cells From the Cervical Mucosa Capture and Transfer HIV-1 via Siglec-1. Front. Immunol. 2019, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Phasouk, K.; Bossard, E.; Klock, A.; Jin, L.; Laing, K.J.; Johnston, C.; Williams, N.A.; Czartoski, J.L.; Varon, D.; et al. Distinct populations of antigen-specific tissue-resident CD8+ T cells in human cervix mucosa. JCI Insight 2021, 6, e149950. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Charles, T.P.; Joag, V.; Bollimpelli, V.S.; Scott, M.K.; Wimmers, F.; Burton, S.L.; Labranche, C.C.; Petitdemange, C.; Gangadhara, S.; et al. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat. Med. 2020, 26, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Bagri, P.; Ghasemi, R.; McGrath, J.J.C.; Thayaparan, D.; Yu, E.; Brooks, A.G.; Stämpfli, M.R.; Kaushic, C. Estradiol Enhances Antiviral CD4+ Tissue-Resident Memory T Cell Responses following Mucosal Herpes Simplex Virus 2 Vaccination through an IL-17-Mediated Pathway. J. Virol. 2020, 95, e01206-20. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Nakashima, A.; Morita, K.; Shima, T.; Yoneda, S.; Kishi, H.; Saito, S. The role of decidual regulatory T cells in the induction and maintenance of fetal antigen-specific tolerance: Imbalance between regulatory and cytotoxic T cells in pregnancy complications. Hum. Immunol. 2021, 82, 346–352. [Google Scholar] [CrossRef]
- Oh, J.E.; Iijima, N.; Song, E.; Lu, P.; Klein, J.; Jiang, R.; Kleinstein, S.H.; Iwasaki, A. Migrant memory B cells secrete luminal antibody in the vagina. Nature 2019, 571, 122–126. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine Microbiota: Residents, Tourists, or Invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef] [Green Version]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.S.; Li, L. Quantifying the human vaginal community state types (CSTs) with the species specificity index. PeerJ 2017, 5, e3366. [Google Scholar] [CrossRef] [Green Version]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Seta, F.; Campisciano, G.; Zanotta, N.; Ricci, G.; Comar, M. The Vaginal Community State Types Microbiome-Immune Network as Key Factor for Bacterial Vaginosis and Aerobic Vaginitis. Front. Microbiol. 2019, 10, 2451. [Google Scholar] [CrossRef] [PubMed]
- Bommana, S.; Richards, G.; Kama, M.; Kodimerla, R.; Jijakli, K.; Read, T.D.; Dean, D. Metagenomic Shotgun Sequencing of Endocervical, Vaginal, and Rectal Samples among Fijian Women with and without Chlamydia trachomatis Reveals Disparate Microbial Populations and Function across Anatomic Sites: A Pilot Study. Microbiol. Spectr. 2022, 10, e0010522. [Google Scholar] [CrossRef]
- Schellenberg, J.J.; Links, M.G.; Hill, J.E.; Dumonceaux, T.J.; Kimani, J.; Jaoko, W.; Wachihi, C.; Mungai, J.N.; Peters, G.A.; Tyler, S.; et al. Molecular definition of vaginal microbiota in East African commercial sex workers. Appl. Environ. Microbiol. 2011, 77, 4066–4074. [Google Scholar] [CrossRef] [Green Version]
- Hedges, S.R.; Barrientes, F.; Desmond, R.A.; Schwebke, J.R. Local and systemic cytokine levels in relation to changes in vaginal flora. J. Infect. Dis. 2006, 193, 556–562. [Google Scholar] [CrossRef] [Green Version]
- Santacroce, L.; Bottalico, L.; Topi, S.; Castellaneta, F.; Charitos, I.A. The “Scourge of the Renaissance”. A Short Review about Treponema pallidum infection. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 335–343. [Google Scholar] [CrossRef]
- Nunn, K.L.; Wang, Y.Y.; Harit, D.; Humphrys, M.S.; Ma, B.; Cone, R.; Ravel, J.; Lai, S.K. Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota. mBio 2015, 6, e01084-15. [Google Scholar] [CrossRef] [Green Version]
- Vagios, S.; Mitchell, C.M. Mutual Preservation: A Review of Interactions Between Cervicovaginal Mucus and Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 676114. [Google Scholar] [CrossRef]
- Borgdorff, H.; Gautam, R.; Armstrong, S.D.; Xia, D.; Ndayisaba, G.F.; van Teijlingen, N.H.; Geijtenbeek, T.B.; Wastling, J.M.; van de Wijgert, J.H. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 2016, 9, 621–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, S.R.; Liu, X.P.; Liao, Q.P. Human defensins and cytokines in vaginal lavage fluid of women with bacterial vaginosis. Int. J. Gynaecol. Obstet. 2008, 103, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.S.; Hackett, C.G.; Abernathy, E.F.; Lee, K.S.; Saff, R.R.; Hohlbaum, A.M.; Moody, K.S.; Hobson, M.W.; Jones, A.; Kolovou, P.; et al. Opposing Roles for Membrane Bound and Soluble Fas Ligand in Glaucoma-Associated Retinal Ganglion Cell Death. PLoS ONE 2011, 6, e17659. [Google Scholar] [CrossRef] [Green Version]
- Hearps, A.C.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.A.; Gugasyan, R.; Anderson, D.J.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallor, A.C.; Antonio, M.A.; Hawes, S.E.; Hillier, S.L. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: Role of hydrogen peroxide production. J. Infect. Dis. 2001, 184, 1431–1436. [Google Scholar] [CrossRef] [Green Version]
- Chopra, C.; Bhushan, I.; Mehta, M.; Koushal, T.; Gupta, A.; Sharma, S.; Kumar, M.; Khodor, S.A.; Sharma, S. Vaginal microbiome: Considerations for reproductive health. Future Microbiol. 2022, 17, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Anton, L.; Sierra, L.J.; DeVine, A.; Barila, G.; Heiser, L.; Brown, A.G.; Elovitz, M.A. Common Cervicovaginal Microbial Supernatants Alter Cervical Epithelial Function: Mechanisms by Which Lactobacillus crispatus Contributes to Cervical Health. Front. Microbiol. 2018, 9, 2181. [Google Scholar] [CrossRef] [Green Version]
- Blacher, E.; Levy, M.; Tatirovsky, E.; Elinav, E. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J. Immunol. (Baltim. Md. 1950) 2017, 198, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Mirmonsef, P.; Gilbert, D.; Zariffard, M.R.; Hamaker, B.R.; Kaur, A.; Landay, A.L.; Spear, G.T. The effects of commensal bacteria on innate immune responses in the female genital tract. Am. J. Reprod. Immunol. 2011, 65, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Moosa, Y.; Kwon, D.; de Oliveira, T.; Wong, E.B. Determinants of Vaginal Microbiota Composition. Front. Cell. Infect. Microbiol. 2020, 10, 467. [Google Scholar] [CrossRef]
- Medina-Bastidas, D.; Camacho-Arroyo, I.; García-Gómez, E. Current findings in endometrial microbiome: Impact on uterine diseases. Reproduction 2022, 163, R81–R96. [Google Scholar] [CrossRef] [PubMed]
- Tortelli, B.A.; Lewis, W.G.; Allsworth, J.E.; Member-Meneh, N.; Foster, L.R.; Reno, H.E.; Peipert, J.F.; Fay, J.C.; Lewis, A.L. Associations between the vaginal microbiome and Candida colonization in women of reproductive age. Am. J. Obstet. Gynecol. 2020, 222, 471.e1–471.e9. [Google Scholar] [CrossRef] [PubMed]
- Hocking, J.S.; Geisler, W.M.; Kong, F.Y.S. Update on the Epidemiology, Screening, and Management of Chlamydia trachomatis Infection. Infect. Dis. Clin. N. Am. 2023, 37, 267–288. [Google Scholar] [CrossRef]
- Xiao, B.; Qin, H.; Mi, L.; Zhang, D. Correlation Analysis of Vaginal Microbiome Changes and Bacterial Vaginosis Plus Vulvovaginal Candidiasis Mixed Vaginitis Prognosis. Front. Cell. Infect. Microbiol. 2022, 12, 860589. [Google Scholar] [CrossRef] [PubMed]
- Olive, A.J.; Sassetti, C.M. Metabolic crosstalk between host and pathogen: Sensing, adapting and competing. Nat. Rev. Microbiol. 2016, 14, 221–234. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [Green Version]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef] [Green Version]
- Budilovskaya, O.V.; Shipitsina, E.V.; Spasibova, E.V.; Pereverzeva, N.A.; Vorob’eva, N.E.; Tsypurdeeva, N.D.; Grigoryev, A.N.; Savicheva, A.M. Differential Expression of Local Immune Response Genes in the Vagina: Implication for the Diagnosis of Vaginal Infections. Bull. Exp. Biol. Med. 2020, 168, 646–650. [Google Scholar] [CrossRef]
- Wagner, B.K.; Dey, M. Chemical strategies to understand and manipulate host-pathogen interactions. Cell Chem. Biol. 2022, 29, 711–712. [Google Scholar] [CrossRef]
- Santacroce, L.; Colella, M.; Charitos, I.A.; Di Domenico, M.; Palmirotta, R.; Jirillo, E. Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications. Metabolites 2023, 13, 461. [Google Scholar] [CrossRef]
- Richardson, A.R.; Libby, S.J.; Fang, F.C. A nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunity. Science 2008, 319, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Edlind, T.D.; Schlievert, P.M.; Nyirjesy, P. Vaginal toxic shock reaction triggering desquamative inflammatory vaginitis. J. Low. Genit. Tract Dis. 2013, 17, 88–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, S.; Morgan, M.T.; Fiedler, T.L.; Djukovic, D.; Hoffman, N.G.; Raftery, D.; Marrazzo, J.M.; Fredricks, D.N. Metabolic signatures of bacterial vaginosis. mBio 2015, 6, e00204-15. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’Antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 14095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeoman, C.J.; Thomas, S.M.; Miller, M.E.; Ulanov, A.V.; Torralba, M.; Lucas, S.; Gillis, M.; Cregger, M.; Gomez, A.; Ho, M.; et al. A multi-omic systems-based approach reveals metabolic markers of bacterial vaginosis and insight into the disease. PLoS ONE 2013, 8, e56111. [Google Scholar] [CrossRef]
- McMillan, A.; Rulisa, S.; Sumarah, M.; Macklaim, J.M.; Renaud, J.; Bisanz, J.E.; Gloor, G.B.; Reid, G. A multi-platform metabolomics approach identifies highly specific biomarkers of bacterial diversity in the vagina of pregnant and non-pregnant women. Sci. Rep. 2015, 5, 14174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mushrif, S.; Eley, A.; Jones, B.M. Inhibition of chemotaxis by organic acids from anaerobes may prevent a purulent response in bacterial vaginosis. J. Med. Microbiol. 2000, 49, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Vitali, B.; Cruciani, F.; Picone, G.; Parolin, C.; Donders, G.; Laghi, L. Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2367–2376. [Google Scholar] [CrossRef]
- Edwards, V.L.; Smith, S.B.; McComb, E.J.; Tamarelle, J.; Ma, B.; Humphrys, M.S.; Gajer, P.; Gwilliam, K.; Schaefer, A.M.; Lai, S.K.; et al. The Cervicovaginal Microbiota-Host Interaction Modulates Chlamydia trachomatis Infection. mBio. 2019, 10, e01548-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolin, C.; Foschi, C.; Laghi, L.; Zhu, C.; Banzola, N.; Gaspari, V.; D’Antuono, A.; Giordani, B.; Severgnini, M.; Consolandi, C.; et al. Insights Into Vaginal Bacterial Communities and Metabolic Profiles of Chlamydia trachomatis Infection: Positioning Between Eubiosis and Dysbiosis. Front. Microbiol. 2018, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, H.; Armstrong, S.D.; Tytgat, H.L.; Xia, D.; Ndayisaba, G.F.; Wastling, J.M.; van de Wijgert, J.H. Unique Insights in the Cervicovaginal Lactobacillus iners and L. crispatus Proteomes and Their Associations with Microbiota Dysbiosis. PLoS ONE 2016, 11, e0150767. [Google Scholar] [CrossRef]
- Sasaki-Imamura, T.; Yoshida, Y.; Suwabe, K.; Yoshimura, F.; Kato, H. Molecular basis of indole production catalyzed by tryptophanase in the genus Prevotella. FEMS Microbiol. Lett. 2011, 322, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.E.; Belland, R.J.; AbdelRahman, Y.M.; Beatty, W.L.; Aiyar, A.A.; Zea, A.H.; Greene, S.J.; Marrero, L.; Buckner, L.R.; Tate, D.J.; et al. Morphologic and molecular evaluation of Chlamydia trachomatis growth in human endocervix reveals distinct growth patterns. Front. Cell. Infect. Microbiol. 2014, 4, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hou, Y.; Yuan, H.; Chen, H. The role of tryptophan in Chlamydia trachomatis persistence. Front. Cell Infect. Microbiol. 2022, 12, 931653. [Google Scholar] [CrossRef]
- Borel, N.; Pospischil, A.; Hudson, A.P.; Rupp, J.; Schoborg, R.V. The role of viable but non-infectious developmental forms in chlamydial biology. Front. Cell. Infect. Microbiol. 2014, 4, 97. [Google Scholar] [CrossRef] [Green Version]
- Abu-Lubad, M.; Meyer, T.F.; Al-Zeer, M.A. Chlamydia trachomatis inhibits inducible NO synthase in human mesenchymal stem cells by stimulating polyamine synthesis. J. Immunol. (Baltim. Md. 1950) 2014, 193, 2941–2951. [Google Scholar] [CrossRef]
- Ziklo, N.; Huston, W.M.; Taing, K.; Katouli, M.; Timms, P. In vitro rescue of genital strains of Chlamydia trachomatis from interferon-γ and tryptophan depletion with indole-positive, but not indole-negative Prevotella spp. BMC Microbiol. 2016, 16, 286. [Google Scholar] [CrossRef] [Green Version]
- Molenaar, M.C.; Singer, M.; Ouburg, S. The two-sided role of the vaginal microbiome in Chlamydia trachomatis and Mycoplasma genitalium pathogenesis. J. Reprod. Immunol. 2018, 130, 11–17. [Google Scholar] [CrossRef]
- Singh, S.; Verma, N.; Taneja, N. The human gut resistome: Current concepts & future prospects. Indian J. Med. Res. 2019, 150, 345–358. [Google Scholar] [CrossRef]
- Lanza, V.F.; Baquero, F.; Martínez, J.L.; Ramos-Ruíz, R.; González-Zorn, B.; Andremont, A.; Sánchez-Valenzuela, A.; Ehrlich, S.D.; Kennedy, S.; Ruppé, E.; et al. In-depth resistome analysis by targeted metagenomics. Microbiome 2018, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Vito, D.; Saini, R.; Inchingolo, F. Probiotics Improve Urogenital Health in Women. Open Access Maced. J. Med. Sci. 2018, 6, 1845–1850. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Ali, S.A. Probiotics and gut microbiota: Mechanistic insights into gut immune homeostasis through TLR pathway regulation. Food Funct. 2022, 13, 7423–7447. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Yoshikata, R.; Yamaguchi, M.; Mase, Y.; Tatsuyuki, A.; Myint, K.Z.Y.; Ohta, H. Evaluation of the efficacy of Lactobacillus-containing feminine hygiene products on vaginal microbiome and genitourinary symptoms in pre- and postmenopausal women: A pilot randomized controlled trial. PLoS ONE 2022, 17, e0270242. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hugerth, L.W.; Schuppe-Koistinen, I.; Du, J. The right bug in the right place: Opportunities for bacterial vaginosis treatment. NPJ Biofilms Microbiomes 2022, 8, 34. [Google Scholar] [CrossRef]
- De Gregorio, P.R.; Juárez Tomás, M.S.; Nader-Macías, M.E. Immunomodulation of Lactobacillus reuteri CRL1324 on Group B Streptococcus Vaginal Colonization in a Murine Experimental Model. Am. J. Reprod. Immunol. 2016, 75, 23–35. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, P.R.; Maldonado, N.C.; Pingitore, E.V.; Terraf, M.C.L.; Tomás, M.S.J.; de Ruiz, C.S.; Santos, V.; Wiese, B.; Bru, E.; Paiz, M.C.; et al. Intravaginal administration of gelatine capsules containing freeze-dried autochthonous lactobacilli: A double-blind, randomised clinical trial of safety. Benef. Microbes 2020, 11, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Kariyama, R.; Nasu, Y.; Ishii, A. Efficacy of Lactobacillus vaginal suppositories for the prevention of recurrent cystitis: A phase II clinical trial. Int. J. Urol. 2021, 28, 1026–1031. [Google Scholar] [CrossRef]
- Vladareanu, R.; Mihu, D.; Mitran, M.; Mehedintu, C.; Boiangiu, A.; Manolache, M.; Vladareanu, S. New evidence on oral L. plantarum P17630 product in women with history of recurrent vulvovaginal candidiasis (RVVC): A randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Verdenelli, M.C.; Cecchini, C.; Coman, M.M.; Silvi, S.; Orpianesi, C.; Coata, G.; Cresci, A.; Di Renzo, G.C. Impact of Probiotic SYNBIO(®) Administered by Vaginal Suppositories in Promoting Vaginal Health of Apparently Healthy Women. Curr. Microbiol. 2016, 73, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Li, D. The role of probiotics in vaginal health. Front. Cell. Infect. Microbiol. 2022, 12, 963868. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.R.; Wierzbicki, M.R.; French, A.L.; Morris, S.; Newmann, S.; Reno, H.; Green, L.; Miller, S.; Powell, J.; Parks, T.; et al. Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis. N. Engl. J. Med. 2020, 382, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Tomusiak, A.; Strus, M.; Heczko, P.B.; Adamski, P.; Stefański, G.; Mikołajczyk-Cichońska, A.; Suda-Szczurek, M. Efficacy and safety of a vaginal medicinal product containing three strains of probiotic bacteria: A multicenter, randomized, double-blind, and placebo-controlled trial. Drug Des. Devel. Ther. 2015, 9, 5345–5354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heczko, P.B.; Tomusiak, A.; Adamski, P.; Jakimiuk, A.J.; Stefański, G.; Mikołajczyk-Cichońska, A.; Suda-Szczurek, M.; Strus, M. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: A randomised, double-blind, placebo-controlled trial. BMC Women’s Health 2015, 15, 115. [Google Scholar] [CrossRef] [Green Version]
- Pendharkar, S.; Brandsborg, E.; Hammarström, L.; Marcotte, H.; Larsson, P.G. Vaginal colonisation by probiotic lactobacilli and clinical outcome in women conventionally treated for bacterial vaginosis and yeast infection. BMC Infect. Dis. 2015, 15, 255. [Google Scholar] [CrossRef] [Green Version]
- Harasim-Dylak, A.; Roguska, M.; Maździarz, A. Role of Trivagin in restoration and maintenance of normal vaginal ecosystem in women treated for recurrent bacterial vaginosis. Curr. Gynecol. Oncol. 2011, 9, 245–252. [Google Scholar]
- Larsson, P.G.; Stray-Pedersen, B.; Ryttig, K.R.; Larsen, S. Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Women’s Health 2008, 8, 3. [Google Scholar] [CrossRef]
- Barbonetti, A.; Cinque, B.; Vassallo, M.R.; Mineo, S.; Francavilla, S.; Cifone, M.G.; Francavilla, F. Effect of vaginal probiotic lactobacilli on in vitro-induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil. Steril. 2011, 95, 2485–2488. [Google Scholar] [CrossRef]
- Mastromarino, P.; Hemalatha, R.; Barbonetti, A.; Cinque, B.; Cifone, M.G.; Tammaro, F.; Francavilla, F. Biological control of vaginosis to improve reproductive health. Indian J. Med. Res. 2014, 140 (Suppl. 1), S91–S97. [Google Scholar]
- Wang, J.; Si, W.; Du, Z.; Zhang, J.; Xue, M. Antioxidants in Animal Feed. Antioxidants 2022, 11, 1760. [Google Scholar] [CrossRef]
- Homayouni, A.; Bastani, P.; Ziyadi, S.; Mohammad-Alizadeh-Charandabi, S.; Ghalibaf, M.; Mortazavian, A.M.; Mehrabany, E.V. Effects of probiotics on the recurrence of bacterial vaginosis: A review. J. Low. Genit. Tract Dis. 2014, 18, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magrone, T.; Jirillo, E. The New Era of Nutraceuticals: Beneficial Effects of Polyphenols in Various Experimental and Clinical Settings. Curr. Pharm. Des. 2018, 24, 5229–5231. [Google Scholar] [CrossRef]
- Topi, S.; Bottalico, L.; Charitos, I.A.; Colella, M.; Di Domenico, M.; Palmirotta, R.; Santacroce, L. Biomolecular Mechanisms of Autoimmune Diseases and Their Relationship with the Resident Microbiota: Friend or Foe? Pathophysiology 2022, 29, 507–536. [Google Scholar] [CrossRef]
- Li, R.; Maimai, T.; Yao, H.; Liu, X.; He, Z.; Xiao, C.; Wang, Y.; Xie, G. Protective effects of polydatin on LPS-induced endometritis in mice. Microb. Pathog. 2019, 137, 103720. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Škalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caccavo, D.; Afeltra, A.; Pece, S.; Giuliani, G.; Freudenberg, M.; Galanos, C.; Jirillo, E. Lactoferrin-lipid A-lipopolysaccharide interaction: Inhibition by anti-human lactoferrin monoclonal antibody AGM 10.14. Infect. Immun. 1999, 67, 4668–4672. [Google Scholar] [CrossRef] [Green Version]
- Alexander, D.B.; Iigo, M.; Yamauchi, K.; Suzui, M.; Tsuda, H. Lactoferrin: An alternative view of its role in human biological fluids. Biochem. Cell Biol. 2012, 90, 279–306. [Google Scholar] [CrossRef]
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-Ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon-A Miracle Molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.M.; Donoval, B.A.; Graham, P.J.; Boksa, L.A.; Spear, G.; Hershow, R.C.; Chen, H.Y.; Landay, A. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin. Vaccine Immunol. CVI 2007, 14, 1102–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sessa, R.; Di Pietro, M.; Schiavoni, G.; Petrucca, A.; Cipriani, P.; Zagaglia, C.; Nicoletti, M.; Santino, I.; del Piano, M. Measurement of Chlamydia pneumoniae bacterial load in peripheral blood mononuclear cells may be helpful to assess the state of chlamydial infection in patients with carotid atherosclerotic disease. Atherosclerosis 2007, 195, e224–e230. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Karadja, E.; De Seta, F. Evidence-based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: A double blind, placebo controlled, randomised clinical trial. Benef. Microbes 2019, 10, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Saccone, G.; Ammendola, A.; Salzano, E.; Iannicelli, M.; De Rosa, R.; Nazzaro, G.; Locci, M. Vaginal lactoferrin in prevention of preterm birth in women with bacterial vaginosis. J. Matern.-Fetal Neonatal Med. 2021, 34, 3704–3708. [Google Scholar] [CrossRef] [PubMed]
- Trentini, A.; Maritati, M.; Rosta, V.; Cervellati, C.; Manfrinato, M.C.; Hanau, S.; Greco, P.; Bonaccorsi, G.; Bellini, T.; Contini, C. Vaginal Lactoferrin Administration Decreases Oxidative Stress in the Amniotic Fluid of Pregnant Women: An Open-Label Randomized Pilot Study. Front. Med. 2020, 7, 555. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Zimecki, M. Antimicrobial and Prebiotic Activity of Lactoferrin in the Female Reproductive Tract: A Comprehensive Review. Biomedicines 2021, 9, 1940. [Google Scholar] [CrossRef]
- Yamauchi, K.; Wakabayashi, H.; Shin, K.; Takase, M. Bovine lactoferrin: Benefits and mechanism of action against infections. Biochem. Cell Biol. 2006, 84, 291–296. [Google Scholar] [CrossRef]

| Disease | Pathogenetic Mechanisms |
|---|---|
| Aerobic vaginitis |
|
| Bacterial vaginosis |
|
| Chlamydia trachomatis (C. t.) infection |
|
| Probiotics | Lactoferrin | Polyphenols |
|---|---|---|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santacroce, L.; Palmirotta, R.; Bottalico, L.; Charitos, I.A.; Colella, M.; Topi, S.; Jirillo, E. Crosstalk between the Resident Microbiota and the Immune Cells Regulates Female Genital Tract Health. Life 2023, 13, 1531. https://doi.org/10.3390/life13071531
Santacroce L, Palmirotta R, Bottalico L, Charitos IA, Colella M, Topi S, Jirillo E. Crosstalk between the Resident Microbiota and the Immune Cells Regulates Female Genital Tract Health. Life. 2023; 13(7):1531. https://doi.org/10.3390/life13071531
Chicago/Turabian StyleSantacroce, Luigi, Raffaele Palmirotta, Lucrezia Bottalico, Ioannis Alexandros Charitos, Marica Colella, Skender Topi, and Emilio Jirillo. 2023. "Crosstalk between the Resident Microbiota and the Immune Cells Regulates Female Genital Tract Health" Life 13, no. 7: 1531. https://doi.org/10.3390/life13071531
APA StyleSantacroce, L., Palmirotta, R., Bottalico, L., Charitos, I. A., Colella, M., Topi, S., & Jirillo, E. (2023). Crosstalk between the Resident Microbiota and the Immune Cells Regulates Female Genital Tract Health. Life, 13(7), 1531. https://doi.org/10.3390/life13071531













