The Role of Systemic Oxidative Status in Coronary Arterial and Peripheral Venous Blood of Patients with Unstable Angina Pectoris
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Patients
2.3. Blood Sampling and Laboratory Test in Study Groups
2.4. Oxidative Stress Evaluating Using Spectrophotometric Method
2.5. Determination of Plasma Prooxidant Parameters (TBARS, NO2−, O2−, H2O2)
2.6. Determination of Hemolysate Antioxidant Parameters (CAT, SOD, GSH)
2.7. Statistical Analysis
3. Results
3.1. Basic Characteristics of Study Population
3.2. Clinical Parameters of Participants in Study Groups
3.3. Laboratory Parameters of Healthy and Participants with UA
3.4. Concentrations of Plasma Pro-Oxidative Markers in Study Population
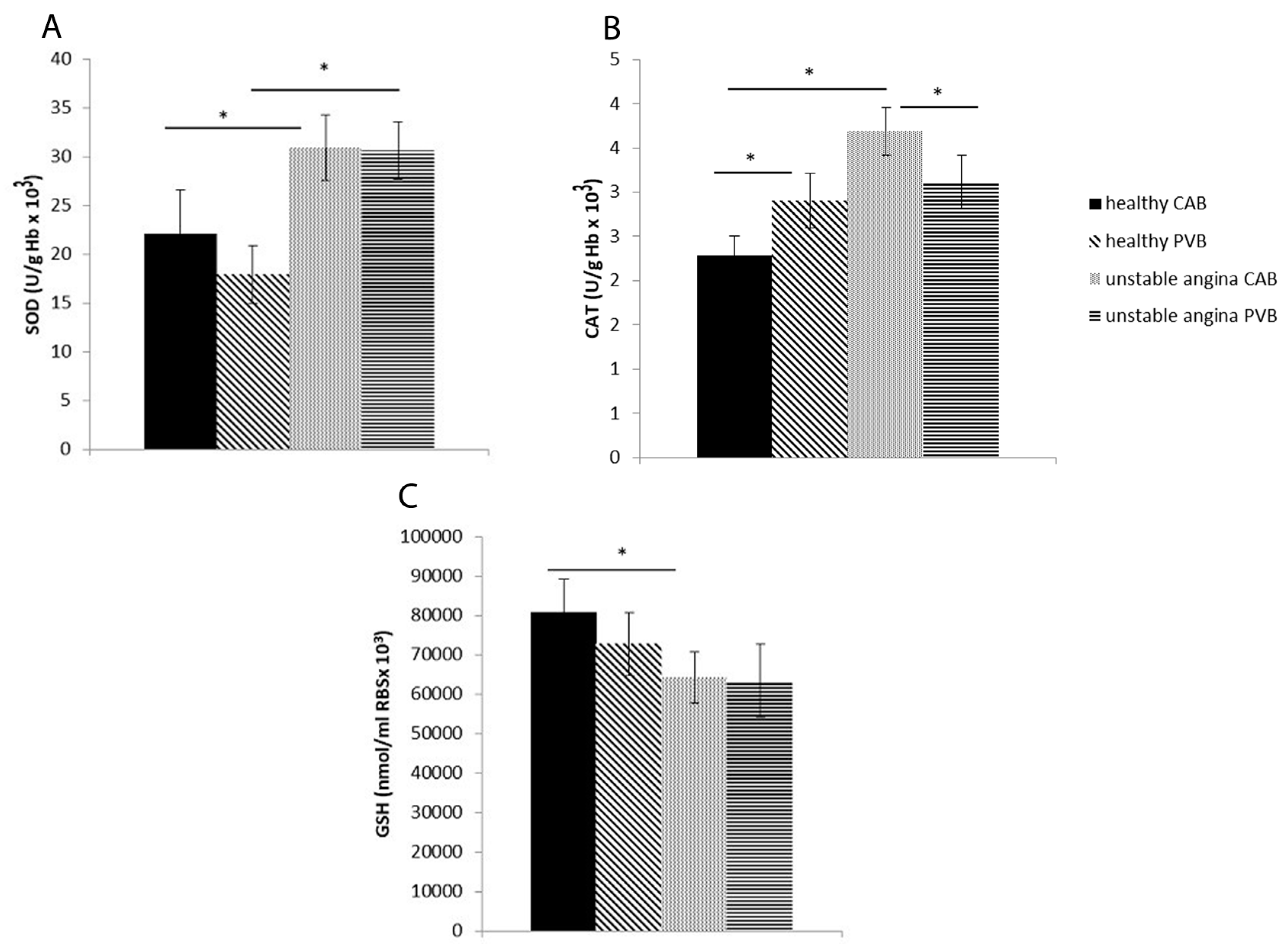
3.5. Antioxidant Enzyme Activity in Study Population
3.6. Regression Analysis of Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vichova, T.; Motovska, Z. Oxidative stress: Predictive marker for coronary artery disease. Exp. Clin. Cardiol. 2013, 18, 88–91. [Google Scholar]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Gracia, K.S.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Review Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Đukić, M. (Ed.) Lipidna peroksidacija indukovana slobodnim radikalima. In Oksidativni Stres: Kliničko-Dijagnostički Značaj; Vulkan izdavaštvo: Beograd, Serbia, 2008; pp. 3–19. [Google Scholar]
- Martin-Ventura, J.L.; Rodrigues-Diez, R.; Martinez-Lopez, D.; Salaices, M.; Blanco-Colio, L.M.; Briones, A.M. Oxidative Stress in Human Atherothrombosis: Sources, Markers and Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 2315. [Google Scholar] [CrossRef] [PubMed]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef]
- Ferrari, R.; Guardigli, G.; Mele, D.; Percoco, G.F.; Ceconi, C.; Curello, S. Oxidative stress during myocardial ischaemia and heart failure. Curr. Pharm. Des. 2004, 10, 1699–1711. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Vinten-Johansen, J. Myocardial apoptosis and ischemic preconditioning. Cardiovasc. Res. 2002, 55, 438–455. [Google Scholar] [CrossRef]
- Naruko, T.; Ueda, M.; Ehara, S.; Itoh, A.; Haze, K.; Shirai, N.; Ikura, Y.; Ohsawa, M.; Itabe, H.; Kobayashi, Y.; et al. Persistent high levels of plasma oxidized low-density lipoprotein after acute myocardial infarction predict stent restenosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 877–883. [Google Scholar] [CrossRef]
- Nagayoshi, Y.; Kawano, H.; Hokamaki, J.; Miyamoto, S.; Kojima, S.; Shimomura, H.; Tsujita, K.; Sakamoto, T.; Yoshimura, M.; Ogawa, H. Urinary 8-hydroxy-2′-deoxyguanosine levels increase after reperfusion in acute myocardial infarction and may predict subsequent cardiac events. Am. J. Cardiol. 2005, 95, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Siwik, D.A.; Colucci, W.S. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail. Rev. 2004, 9, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Keith, M.; Geranmayegan, A.; Sole, M.J.; Kurian, R.; Robinson, A.; Omran, A.S.; Jeejeebhoy, K.N. Increased oxidative stress in patients with congestive heart failure. J. Am. Coll. Cardiol. 1998, 31, 1352–1356. [Google Scholar] [CrossRef]
- Kaneda, H.; Taguchi, J.; Ogasawara, K.; Aizawa, T.; Ohno, M. Increased level of advanced oxidation protein products in patients with coronary artery disease. Atherosclerosis 2002, 162, 221–225. [Google Scholar] [CrossRef]
- Azumi, H.; Inoue, N.; Ohashi, Y.; Terashima, M.; Mori, T.; Fujita, H.; Awano, K.; Kobayashi, K.; Maeda, K.; Hata, K.; et al. Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris: Important role of NAD(P)H oxidase. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Tsutsui, H.; Hayashidani, S.; Kang, D.; Suematsu, N.; Nakamura, K.; Utsumi, H.; Hamasaki, N.; Takeshita, A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ. Res. 2001, 88, 529–535. [Google Scholar] [CrossRef]
- Arroyo, C.M.; Kramer, J.H.; Dickens, B.F.; Weglicki, W.B. Identification of free radicals in myocardial ischemia/reperfusion by spin trapping with nitrone DMPO. FEBS Lett. 1987, 221, 101–104. [Google Scholar] [CrossRef]
- Kurian, G.A.; Rajagopal, R.; Vedantham, S.; Rajesh, M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxid Med Cell Longev. 2016, 2016, 1656450. [Google Scholar] [CrossRef]
- Available online: https://www.thermofisher.com/rs/en/home/references/protocols/cell-and-tissue-analysis/elisa-protocol/elisa-sample-preparation-protocols/plasma-and-serum-preparation.html (accessed on 18 April 2023).
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Auclair, C.; Voisin, E. Nitroblue tetrazolium reduction. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 123–132. [Google Scholar]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Meth. 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Beutler, E. (Ed.) Catalase. In Red Cell Metabolism, a Manual of Biochemical Methods; Grune and Stratton: New York, NY, USA, 1982; pp. 105–106. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide-anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Beutler, E. (Ed.) Reduced glutathione (GSH). In Red Cell Metabolism, a Manual of Biochemical Methods; Grune and Stratton: New York, NY, USA, 1975; pp. 112–114. [Google Scholar]
- Szasz, T.; Thakali, K.; Fink, G.D.; Watts, S.W. A comparison of arteries and veins in oxidative stress: Producers, destroyers, function, and disease. Exp. Biol. Med. 2007, 232, 27–37. [Google Scholar]
- Shi, Y.; Patel, S.; Davenpeck, K.L.; Niculescu, R.; Rodriguez, E.; Magno, M.G.; Ormont, M.L.; Mannion, J.D.; Zalewski, A. Oxidative stress and lipid retention in vascular grafts: Comparison between venous and arterial conduits. Circulation 2001, 103, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Sadowski, J.; Kapelak, B.; Jopek, A.; Rudzinski, P.; Pillai, R.; Korbut, R.; Chanon, K.M. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1614–1620. [Google Scholar] [CrossRef]
- Lantos, J.; Temes, G.; Göbölös, L.; Jaberansari, M.T.; Roth, E. Is peripheral blood a reliable indicator of acute oxidative stress following heart ischemia and reperfusion? Med. Sci. Monit. 2001, 7, 1166–1170. [Google Scholar]
- Jaumdally, R.; Varma, C.; Macfadyen, R.J.; Lip, G.Y.H. Coronary sinus blood sampling: An insight into local cardiac pathophysiology and treatment? Eur. Heart J. 2007, 28, 929–940. [Google Scholar] [CrossRef]
- Bhat, M.A.; Mahajan, M.; Gandhi, G. Oxidative stress status in coronary Artery disease patients. Int. J. Life Sci. Biotechnol. Pharma Res. 2012, 1, 236–243. [Google Scholar]
- Thakur, K.S.; Jaggi, K.; Rathore, B.; Chander, R.; Mahdi, F.; Mathur, A. Assessment of oxidative stress, antioxidant enzymes and lipid profile in the subjects of coronary artery disease (CAD). Int. J. Pharm. Sci. 2014, 5, 3042–3046. [Google Scholar]
- McMurray, J.; Chopra, M.; Abdullah, I.; Smith, W.E.; Dargie, H.J. Evidence for oxidative stress in unstable angina. Br. Heart J. 1992, 68, 454–457. [Google Scholar] [CrossRef]
- Dubois-Randé, J.L.; Artigou, J.Y.; Darmon, J.Y.; Habbal, R.; Manuel, C.; Tayarani, I.; Castaigne, A.; Grosgogeat, Y. Oxidative stress in patients with unstable angina. Eur. Heart J. 1994, 15, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Cavalca, V.; Cighetti, G.; Bamonti, F.; Loaldi, A.; Bortone, L.; Novembrino, C.; De Franceschi, M.; Belardinelli, R.; Guazzi, M.D. Oxidative stress and homocysteine in coronary artery disease. Clin. Chem. 2001, 47, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Uppal, N.; Uppal, V.; Uppal, P. Progression of Coronary Artery Disease (CAD) from Stable Angina (SA) towards Myocardial Infarction (MI): Role of Oxidative Stress. J. Clin. Diagn. Res. 2014, 8, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, M.; Altın, C.; Özyıldız, A.; Müderrisoğlu, H. Are oxidative stress markers helpful for diagnosing the disease and determining its complexity or extent in patients with stable coronary artery disease? Turk. Kardiyol. Dern. Ars. 2017, 45, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Turan, T.; Menteşe, Ü.; Ağaç, M.T.; Akyüz, A.R.; Kul, S.; Aykan, A.Ç.; Bektaş, H.; Korkmaz, L.; Öztaş Menteşe, S.; Dursun, İ.; et al. The relation between intensity and complexity of coronary artery lesion and oxidative stress in patients with acute coronary syndrome. Anatol. J. Cardiol. 2015, 15, 795–800. [Google Scholar] [CrossRef]
- Kharb, S. Low blood glutathione levels in acute myocardial infaction. Ind. J. Med. Sci. 2003, 57, 335–337. [Google Scholar]
- Zuzak, E.; Horecka, A.; Kiełczykowska, M.; Dudek, A.; Musik, I.; Kurzepa, J.; Kurzepa, J. Glutathione level and glutathione reductase activity in serum of coronary heart disease patients. J. Pre-Clin. Clin. Res. 2017, 11, 103–105. [Google Scholar] [CrossRef]
- Kaur, G.; Misra, M.K.; Sanwal, G.G.; Shanker, K.; Chandra, M. Levels of glutathione reductase and glutathione peroxidase of human platelets in unstable angina and myocardial infarction. Boll. Chim. Farm. 1999, 138, 437–439. [Google Scholar]
- Sapira, V.; Cojocaru, I.M.; Socoliuc, G.; Lilios, G.; Grigorian, M.; Craiu, E.; Cojocaru, M. Glutathione reductase levels in patients with unstable angina. Rom. J. Intern. Med. 2011, 49, 197–201. [Google Scholar]
- Lubrano, V.; Balzan, S. Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World J. Exp. Med. 2015, 5, 218–224. [Google Scholar] [CrossRef]
- Gupta, S.; Sodhi, S.; Mahajan, V. Correlation of antioxidants with lipid peroxidation and lipid profile in patients suffering from coronary artery disease. Expert Opin. Ther. Targets 2009, 13, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, V.; Di Cecco, P.; Zucchelli, G.C. Role of superoxide dismutase in vascular inflammation and in coronary artery disease. Clin. Exp. Med. 2006, 6, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Kotur-Stevuljevic, J.; Memon, L.; Stefanovic, A.; Spasic, S.; Spasojevic-Kalimanovska, V.; Bogavac-Stanojevic, N.; Kalimanovska-Ostric, D.; Jelić-Ivanovic, Z.; Zunic, G. Correlation of oxidative stress parameters and inflammatory markers in coronary artery disease patients. Clin. Biochem. 2007, 40, 181–187. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P. Clinical Application of NOX Activity and Other Oxidative Biomarkers in Cardiovascular Disease: A Critical Review. Antioxid. Redox Signal. 2015, 23, 514–532. [Google Scholar] [CrossRef] [PubMed]

| Variable | CTRL Group [n = 45] | UA Group [n = 47] | p Value |
|---|---|---|---|
| Gender (F/M) per group [%] Gender per study population [%] | F 20 (44.4) M 25 (55.6) | F 18 (38.3) M 29 (61.7) | p < 0.05 |
| F 38 (35.9) | M 54 (64.1) | p < 0.05 | |
| Age (years) | 63.07 ± 8.69 | 64.79 ± 7.99 | p > 0.05 |
| Hypertension [%] | 24.2 | 22.5 | p > 0.05 |
| Dyslipidemia [%] | 4.45 | 6.23 | |
| Smoking [%] | 10.2 | 9.4 | |
| Diabetes Mellitus [%] | 3.7 | 23.4 | p < 0.05 |
| Previous vascular events [%] | 4.4 | 42.6 | |
| Previous percutaneous coronary intervention [%] | 8.9 | 12.8 |
| Variable | CTRL Group [n = 45] | UA Group [n = 47] | p Value |
|---|---|---|---|
| Systolic BP [mmHg] | 127.88 ± 20.33 | 132.12 ± 15.77 | p > 0.05 |
| Diastolic BP [mmHg] | 74.72 ± 12.06 | 78.63 ± 9.27 | |
| EF [%] | 53.55 ± 11.11 | 52.21 ± 9.21 |
| Variable | CTRL Group [n = 45] | UA Group [n = 47] | p Value |
|---|---|---|---|
| Hematocrit, mean (SD), (%) | 40.31 ± 5.21 | 40.12 ± 6.22 | p > 0.05 |
| White blood cells/mm3, mean (SD) | 7.55 ± 2.25 | 7.42 ± 2.22 | |
| Neutrophil/lymphocyte ratio, mean (SD) | 2.67 ± 1.10 | 7.73 ± 1.13 | |
| Platelets/mm3, mean (SD) | 223.34 ± 64.23 | 226.87 ± 67.09 | |
| Glucose, mean (SD), (mg/dL) | 111.56 ± 37.21 | 107.23 ± 36.41 | |
| Creatinine, mean (SD), (mg/dL) | 1.01 ± 0.33 | 1.11 ± 0.21 | |
| Total cholesterol, mean (SD), (mg/dL) | 180.23 ± 43.21 | 185. 44 ± 32.12 | |
| High-density cholesterol, mean (SD), (mg/dL) | 39.87 ± 12.87 | 40.45 ± 10.09 | |
| Low-density cholesterol, mean (SD), (mg/dL) | 116.77 ± 13.78 | 119.22 ± 32.12 | |
| Triglycerides, mean (SD), (mg/dL) | 124.66 ± 58.21 | 137.34 ± 67.12 | p < 0.05 * |
| C-reactive protein, mean (SD), (mg/dL) | 1.11 ± 2.83 | 0.99 ± 2.11 | p > 0.05 |
| Variable | B | Std. Error | Beta | t Value | p Value |
|---|---|---|---|---|---|
| Superoxide anion radical [nmol/L] | 18.812 | 3.637 | 0.355 | 5.173 | 0.034 |
| Hydrogen peroxide [nmol/L] | 0.263 | 0.042 | 0.211 | 6.318 | 0.051 |
| Nitric oxide [nmol/L] | 0.178 | 0.001 | 0.231 | 4.321 | 0.011 |
| Index of lipid peroxidation [micromol/L] | 17.12 | 2.043 | 0.111 | 2.341 | 0.023 |
| Superoxide dismutase [U/Hg × 109] | 1.789 | 0.143 | 0.546 | −6.440 | 0.001 |
| Catalase [U/Hg × 109] | 3.789 | 0.301 | 0.234 | −5.431 | 0.001 |
| Reduced glutathione [U/Hg × 109] | 4.121 | 1.212 | 0.398 | −2.341 | 0.111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragan, P.D.; Ivan, S.B.; Goran, D.Z.; Maja, N.D.; Nevena, L.D.; Marijana, A.M.; Jelena, V.M.; Nenad, Z.J.; Vladimir, Z.I.; Turnic, T.N.; et al. The Role of Systemic Oxidative Status in Coronary Arterial and Peripheral Venous Blood of Patients with Unstable Angina Pectoris. Life 2023, 13, 1537. https://doi.org/10.3390/life13071537
Dragan PD, Ivan SB, Goran DZ, Maja ND, Nevena LD, Marijana AM, Jelena VM, Nenad ZJ, Vladimir ZI, Turnic TN, et al. The Role of Systemic Oxidative Status in Coronary Arterial and Peripheral Venous Blood of Patients with Unstable Angina Pectoris. Life. 2023; 13(7):1537. https://doi.org/10.3390/life13071537
Chicago/Turabian StyleDragan, Panic D., Simic B. Ivan, Davidovic Z. Goran, Nikolic D. Maja, Lazarevic D. Nevena, Andjic M. Marijana, Vuckovic M. Jelena, Zornic J. Nenad, Zivkovic I. Vladimir, Tamara Nikolic Turnic, and et al. 2023. "The Role of Systemic Oxidative Status in Coronary Arterial and Peripheral Venous Blood of Patients with Unstable Angina Pectoris" Life 13, no. 7: 1537. https://doi.org/10.3390/life13071537
APA StyleDragan, P. D., Ivan, S. B., Goran, D. Z., Maja, N. D., Nevena, L. D., Marijana, A. M., Jelena, V. M., Nenad, Z. J., Vladimir, Z. I., Turnic, T. N., Vladimir, J. L., & Violeta, I. C. M. (2023). The Role of Systemic Oxidative Status in Coronary Arterial and Peripheral Venous Blood of Patients with Unstable Angina Pectoris. Life, 13(7), 1537. https://doi.org/10.3390/life13071537







