Variation of Circulating Brain-Derived Neurotrophic Factor (BDNF) in Depression: Relationships with Inflammatory Indices, Metabolic Status and Patients’ Clinical Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Psychiatric Evaluation
2.3. Determination of Blood Hematological/Biochemical Parameters and Circulating BDNF Levels
2.3.1. Chemicals, Reagents and Instruments
2.3.2. Blood Sampling, Plasma and Platelet Separation and Storage Conditions
- (1)
- About 8 mL/patient was sent to the Laboratory of Clinical Chemical Analysis of the Department of Laboratory Medicine at the University-Hospital of Pisa, Italy, for the determination of hematological/hematochemical parameters.
- (2)
- About 12 mL/patient was sent to the Laboratory of Biochemistry at the Deartment of Pharmacy, University of Pisa, to determine PPP-BDNF and PLT-BDNF.
2.3.3. Preparation of Platelet Soluble Fractions
2.3.4. BDNF Sandwich ELISA Assay
2.3.5. Statistics and Calculations
3. Results
3.1. Subjects
3.2. Psychiatric Rating Scales and Symptom Presentation
3.3. Inflammatory, Hematological–Metabolic Indexes and BDNF Values
3.4. Correlations and Comparison Analyses between Clinical Demographic Results and Biological Parameters
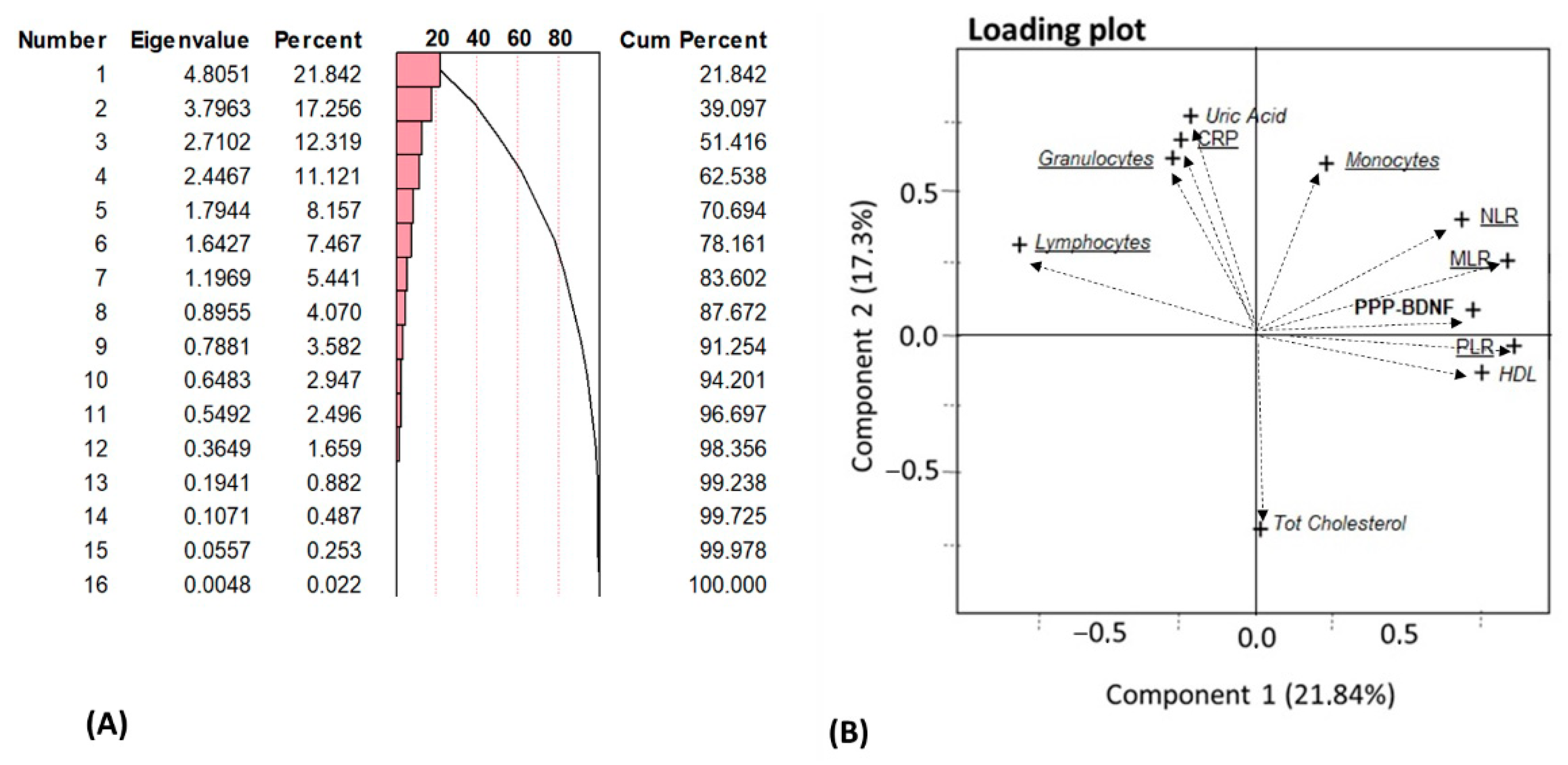
3.5. Principal Component Analysis (PCA) of Biological Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Leonard, B.E. Evidence for a biochemical lesion in depression. J. Clin. Psychiatry 2000, 61 (Suppl. S6), 12–17. [Google Scholar]
- Amodeo, G.; Trusso, M.A.; Fagiolini, A. Depression and inflammation: Disentangling a clear yet complex and multifaceted link. Neuropsychiatry 2017, 7, 448–457. [Google Scholar] [CrossRef]
- Palego, L.; Giannaccini, G.; Betti, L. Neuroendocrine response to psychosocial stressors, inflammation mediators and brain-periphery pathways of adaptation. CNSA Med. Chem. 2021, 21, 2–19. [Google Scholar] [CrossRef]
- Miller, A.H.; Pariante, C.M.; Pearce, B.D. Effects of cytokines on glucocorticoid receptor expression and function. Glucocorticoid resistance and relevance to depression. In Cytokines, Stress, and Depression; Dantzer, R., Wollman, E.E., Yirmiya, R., Eds.; Springer: New York, NY, USA, 1999; Volume 461. [Google Scholar]
- Strawbridge, R.; Young, A.H.; Cleare, A.J. Biomarkers for depression: Recent insights, current challenges and future prospects. Neuropsychiatr. Dis. Treat. 2017, 13, 1245–1262. [Google Scholar] [CrossRef] [Green Version]
- Angst, J.; Sellaro, R.; Merikangas, K.R. Depressive spectrum diagnoses. Compr. Psychiatry 2000, 41, 39–47. [Google Scholar] [CrossRef]
- Alcocer-Gomèz, E.; de Miguel, M.; Casas-Barquero, N.; Nunez-Vasco, J.; Sanchez-Alcazar, J.A.; Fernandez-Rodriguez, A.; Cordero, M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 2014, 36, 111–117. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; Cha, D.S.; Mansur, R.B.; McIntyre, R.S. Inflamed moods: A review of the interactions between inflammation and MDs. Prog. Neur. Psychopharmacol. Biol. Psychiatry 2014, 53, 23–34. [Google Scholar] [CrossRef]
- Fabbrini, E.; Serafini, M.; Colic Baric, I.; Hazen, S.L.; Klein, S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes 2014, 63, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Foley, E.M.; Parkinson, J.T.; Kappelmann, N.; Khandaker, G.M. Clinical phenotypes of depressed patients with evidence of inflammation and somatic symptoms. Compr. Psychoneuroendocrinol. 2021, 8, 100079. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Duivis, H.E.; Beekman, A.T.; Kluft, C.; Neuteboom, J.; Hoogendijk, W.; Smit, J.H.; de Jonge, P.; Penninx, B.W. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl. Psychiatry 2012, 2, e79. [Google Scholar] [CrossRef] [Green Version]
- Mazza, M.G.; Lucchi, S.; Tringali, A.G.M.; Rossetti, A.; Botti, E.R.; Clerici, M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in MDs: A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84 Pt A, 229–236. [Google Scholar] [CrossRef]
- Marazziti, D.; Torrigiani, S.; Carbone, M.G.; Mucci, F.; Flamini, W.; Ivaldi, T.; Dell’Osso, L. Neutrophil/Lymphocyte, Platelet/Lymphocyte, and Monocyte/Lymphocyte Ratios in MDs. Curr. Med. Chem. 2022, 29, 5758–5781. [Google Scholar] [CrossRef]
- Levy, M.J.F.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.L.A.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018, 235, 2195–2220. [Google Scholar] [CrossRef] [Green Version]
- Serra-Millàs, M. Are the changes in the peripheral brain-derived neurotrophic factor levels due to platelet activation? World J. Psychiatry 2016, 6, 84–101. [Google Scholar] [CrossRef]
- Banerjee, R.; Ghosh, A.K.; Ghosh, B.; Bhattacharyya, S.; Mondal, A.C. Decreased mRNA and protein expression of BDNF, NGF, and their receptors in the hippocampus from suicide: An analysis in human postmortem brain. Clin. Med. Ins. Pathol. 2013, 6, 1–11. [Google Scholar] [CrossRef]
- Miyanishi, H.; Nitta, A. A Role of BDNF in the depression pathogenesis and a potential target as antidepressant: The modulator of stress sensitivity “Shati/Nat8l-BDNF system” in the dorsal striatum. Pharmaceuticals 2021, 14, 889. [Google Scholar] [CrossRef]
- Serra-Millàs, M.; López-Vílchez, I.; Navarro, V.; Galán, A.M.; Escolar, G.; Penadés, R.; Catalán, R.; Fañanás, L.; Arias, B.; Gastó, C. Changes in plasma and platelet BDNF levels induced by S-citalopram in major depression. Psychopharmacology 2011, 216, 1–8. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in psychiatry: Concept, definition, types and relevance to the clinical reality. Front. Psychiatry 2020, 11, 432. [Google Scholar] [CrossRef]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. Structured Clinical Interview for DSM-5—Clinician Version (SCID-5 for DSM-5, Clinician Version; SCID-5-RV); American Psychiatric Association: Arlington, VA, USA, 2015. [Google Scholar]
- Cassano, G.B.; Musetti, L.; Perugi, G.; Soriani, A.; Mignani, V.; McNair, D.M.; Akiskal, H.S. A proposed new approach to the clinical sub-classification of depressive illness. Pharmacopsychiatry 1988, 21, 19–23. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef]
- Guy, W. (Ed.) ECDEU Assessment Manual for Psychopharmacology; US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration: Rockville, MD, USA, 1976. [Google Scholar]
- Aas, I.H.M. Global Assessment of Functioning (GAF): Properties and frontier of current knowledge. Ann. Gen. Psychiatry 2010, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Betti, L.; Palego, L.; Unti, E.; Mazzucchi, S.; Kiferle, L.; Palermo, G.; Bonuccelli, U.; Giannaccini, G.; Ceravolo, R. Brain-Derived Neurotrophic Factor (BDNF) and serotonin transporter (SERT) in platelets of patients with mild Huntington’s Disease: Relationships with social cognition symptoms. Arch. Ital. Biol. 2018, 156, 27–39. [Google Scholar] [CrossRef]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci. Rep. 2019, 9, 9655. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gervasoni, N.; Aubry, J.M.; Bondolfi, G.; Osiek, C.; Schwald, M.; Bertschy, G.; Karege, F. Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology 2005, 51, 234–238. [Google Scholar] [CrossRef]
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of low-grade inflammation in depression: A systematic review and meta-analysis of CRP levels. Psychol. Med. 2019, 49, 1958–1970. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Beekman, A.T.F.; de Jonge, P.; Penninx, B.W.J.H. Anxiety disorders and inflammation in a large adult cohort. Transl. Psychiatry 2013, 3, e249. [Google Scholar] [CrossRef] [Green Version]
- Köhler-Forsberg, O.; Buttenschon, H.N.; Tansey, K.E.; Maier, W.; Hauser, J.; Dernovsek, M.Z.; Henigsberg, N.; Souery, D.; Farmer, A.; Rietschel, M.; et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav. Immun. 2017, 62, 344–350. [Google Scholar] [CrossRef]
- Sunbul, E.A.; Sunbul, M.; Yanartas, O.; Cengiz, F.; Bozbay, M.; Sari, I.; Gulec, H. Increased Neutrophil/Lymphocyte Ratio in patients with depression is correlated with the severity of depression and cardiovascular risk factors. Psychiatry Investig. 2016, 13, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Shan, M.; Lu, S.; Cui, R.; Yang, Y.; Sun, Z.; Pan, Y. Association between neutrophil to lymphocyte ratio and depression among US adults: From a large population-based cross-sectional study. J. Psychosom. Res. 2022, 162, 111041. [Google Scholar] [CrossRef] [PubMed]
- Velasco, Á.; Rodríguez-Revuelta, J.; Olié, E.; Abad, I.; Fernández-Peláez, A.; Cazals, A.; Guillaume, S.; de la Fuente-Tomás, L.; Jiménez-Treviño, L.; Gutiérrez, L.; et al. Neutrophil-to-lymphocyte ratio: A potential new peripheral biomarker of suicidal behavior. Eur. Psychiatry 2020, 63, e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Wang, Y.; Wang, X.; Jiang, Z.; Zhu, L.; Fang, S. Neutrophil-to-lymphocyte ratio, platelet-to lymphocyte ratio, and monocyte-to-lymphocyte ratio in depression: An updated systematic review and meta-analysis. Front. Psychiatry 2022, 13, 893097. [Google Scholar] [CrossRef] [PubMed]
- Brinn, A.; Stone, J. Neutrophil–lymphocyte ratio across psychiatric diagnoses: A cross-sectional study using electronic health records. BMJ Open 2020, 10, e036859. [Google Scholar] [CrossRef]
- Tamura, S.; Suzuki, H.; Hirowatari, Y.; Hatase, M.; Nagasawa, A.; Matsuno, K.; Kobayashi, S.; Moriyama, T. Release reaction of brain-derived neurotrophic factor (BDNF) through PAR1 activation and its two distinct pools in human platelets. Thromb. Res. 2011, 128, e55–e61. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Fernández, P.; Säuberli, K.; Colzani, M.; Moreau, T.; Ghevaert, C.; Barde, Y.A. Brain-derived Neurotrophic Factor in megakaryocytes. J. Biol. Chem. 2016, 291, 9872–9881. [Google Scholar] [CrossRef] [Green Version]
- Mucci, F.; Marazziti, D.; Della Vecchia, A.; Baroni, S.; Morana, P.; Carpita, B.; Mangiapane, P.; Morana, F.; Morana, B.; Dell’Osso, L. State-of-the-art: Inflammatory and metabolic markers in mood disorders. Life 2020, 10, 82. [Google Scholar] [CrossRef]
- Colle, R.; Trabado, S.; David, D.J.; Brailly-Tabard, S.; Hardy, P.; Falissard, B.; Fève, B.; Becquemont, L.; Verstuyft, C.; Corruble, E. Plasma BDNF level in major depression: Biomarker of the Val66Met BDNF polymorphism and of the clinical course in Met carrier patients. Neuropsychobiology 2017, 75, 39–45. [Google Scholar] [CrossRef]
- Piccinni, A.; Veltri, A.; Costanzo, D.; Vanelli, F.; Franceschini, C.; Moroni, I.; Domenici, L.; Origlia, N.; Marazziti, D.; Akiskal, H.S.; et al. Decreased plasma levels of brain-derived neurotrophic factor (BDNF) during mixed episodes of bipolar disorder. J. Affect. Dis. 2015, 171, 167–170. [Google Scholar] [CrossRef]
- Uint, L.; Bastos, G.M.; Thurow, H.S.; Bassani Borges, J.B.; Hirata, T.D.C.; França, J.I.D.; Hirata, M.H.; Sousa, A.G.d.M.R. Increased levels of plasma IL-1β and BDNF can predict resistant depression patients. Rev. Assoc. Med. Bras. 2019, 65, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Haas, L.; Portela, L.V.C.; Böhmer, A.E.; Oses, J.P.; Lara, D.R. Increased plasma levels of Brain Derived Neurotrophic Factor (BDNF) in patients with fibromyalgia. Neurochem. Res. 2010, 35, 830–834. [Google Scholar] [CrossRef]
- Buselli, R.; Veltri, A.; Baldanzi, S.; Marino, R.; Bonotti, A.; Chiumiento, M.; Girardi, M.; Pellegrini, L.; Guglielmi, G.; Dell’Osso, L.; et al. Plasma Brain-Derived Neurotrophic Factor (BDNF) and serum cortisol levels in a sample of workers exposed to occupational stress and suffering from Adjustment Disorders. Brain Behav. 2019, 9, e01298. [Google Scholar] [CrossRef] [Green Version]
- Arosio, B.; Guerini, F.R.; Oude Voshaar, R.C.; Aprahamian, I. Blood Brain-Derived Neurotrophic Factor (BDNF) and major depression: Do we have a translational perspective? Front. Behavior. Neurosci. 2021, 15, 626906. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Del Debbio, A.; Veltri, A.; Bianchi, C.; Roncaglia, I.; Carlini, M.; Massimetti, G.; Catena Dell’Osso, M.; Vizzaccaro, C.; Marazziti, D.; et al. Associations between brain-derived neurotrophic factor plasma levels and severity of the illness, recurrence and symptoms in depressed patients. Neuropsychobiology 2010, 62, 207–212. [Google Scholar] [CrossRef]
- Cavaleri, D.; Moretti, F.; Bartoccetti, A.; Mauro, S.; Crocamo, C.; Carrà, G.; Bartoli, F. The role of BDNF in major depressive disorder, related clinical features, and antidepressant treatment: Insight from meta-analyses. Neurosci. Biobehav. Rev. 2023, 149, 105159. [Google Scholar] [CrossRef]
- Ionescu, D.F.; Niciu, M.J.; Mathews, D.C.; Richards, E.M.; Zarate, C.A., Jr. Neurobiology of anxious depression: A review. Depress. Anxiety 2013, 30, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, A.; Cattane, N.; Begni, V.; Pariante, C.M.; Riva, M.A. The human BDNF gene: Peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Transl. Psychiatry 2016, 6, e958. [Google Scholar] [CrossRef]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]




| Patients | Total, Mean ± SD (Median; Range) | Men, Mean ± SD (Median; Range) | Women, Mean ± SD (Median; Range) |
|---|---|---|---|
| Age (years) | 52 ± 14 (55; 18–65) | 53 ± 15 (60; 23–65) | 52 ± 13 (53; 18–65) |
| BMI (kg/m2) | 27 ± 5 (26; 19–37) | 28 ± 6 (28; 20–37) | 27 ± 5 (26; 19–37) |
| Age at onset (years) | 29 ± 12 (29; 6–61) | 29 ± 14 (23; 12–56) | 30 ± 12 (29; 6–61) |
| Illness duration (years) | 24 ± 12 (25; 2–52) | 26 ± 14 (24; 6–52) | 22 ± 11 (25; 2–45) |
| Number of lifetime episodes | 8 ± 5 (6; 1–21) | 11 ± 6 (10; 4–20) | 7 ± 5 (6; 1–21) |
| Patients | Total, n (%) | Men, n | Women, n |
|---|---|---|---|
| Current Episode Severity (DSM-5) * | |||
| Severe (8–9 DSM-5 criteria satisfied) | 23 (59) | 7 | 16 |
| Moderate (6–7 DSM-5 criteria satisfied) | 16 (41) | 5 | 11 |
| SCID-5 Specifier “with anxious distress” | |||
| Yes | 31 (79.5) | 8 | 23 |
| No | 8 (20.5) | 4 | 4 |
| SCID-5 Specifier “with mixed features” | |||
| Yes | 14 (36) | 2 | 12 |
| No | 25 (64) | 10 | 15 |
| SCID-5 Specifier “with melancholic features” | |||
| Yes | 13 (33) | 4 | 9 |
| No | 26 (67) | 8 | 18 |
| SCID-5 Specifier “with psychotic features” | |||
| Yes | 6 (15.4) | 2 | 4 |
| No | 33 (84.6) | 10 | 23 |
| SCID-5 Specifier “with atypical features” | |||
| Yes | 4 (10.3) | 0 | 4 |
| No | 35 (89.7) | 12 | 23 |
| SCID-5 Specifier “with seasonal pattern” | |||
| Yes | 3 (7.7) | 2 | 1 |
| No | 36 (92.3) | 10 | 26 |
| Patients | HAM-D | YMRS | CGI-s | GAF |
|---|---|---|---|---|
| Total | 25 ± 5 (26; 17–36) | 3.0 ± 3.0 (2; 0–12) | 5.0 ± 0.6 (5; 4–6) | 45 ± 8 (45; 30–60) |
| Men | 26 ± 4 (25.5; 20–32) | 3.0 ± 3.0 (3; 0–9) | 4.9 ± 0.5 (5; 4–6) | 48 ± 5 (47; 41–58) |
| Women | 25 ± 5 (26; 17–36) | 3.0 ± 3.0 (2; 0–12) | 5.0 ± 0.6 (5; 4–6) | 44 ± 9 (43; 30–60) |
| Total | Men | Women | Normal Levels | |
|---|---|---|---|---|
| CRP mg/100 mL | 0.9 ± 2.45 (0.15; <0.01–13) | 0.442 ± 0.67 (0.17; <0.01–1.93) | 1.23 ± 3.19 (0.1; <0.01–13) | ≤0.5 |
| ESV mm/h | 15 ± 17 (8.5; 2–76) | 12.9 ± 12.68 (6; 2–37) | 16.1 ± 19.5 (10.5; 2–76) | <25 |
| T-cholesterol mg/100 mL | 178 ± 39 (179; 65–225) | 168.9 ± 44.9 (174.5; 65–225) | 183.8 ± 33.8 (182; 96–224) | <200 |
| LDL mg/100 mL | 116 ± 33 (119; 34–180) | 115.8 ± 45.21 (118; 34–180) | 116.6 ± 22.8 (119; 57–144) | <115 |
| HDL mg/100 mL | 49 ± 17 (49; 24–100) | 40.75 ± 12.6 (43.5; 24–67) | 55.53 ± 17.7 (54; 25–100) | >65 |
| TG mg/100 mL | 129 ± 53 (136; 46–280) | 139 ± 69 (142; 52–280) | 122 ± 38 (129; 46–186) | 10–150 |
| Glucose mg/100 mL | 90 ± 23 (82; 67–163) | 95.2 ± 28.4 (86.5; 70–163) | 86.1 ± 18.72 (82; 67–146) | <125 |
| Uric acid mmol/L | 0.29 ± 0.09 (0.28; 0.16–0.47) | 0.32 ± 0.08 (0.28; 0.2–0.47) | 0.27 ± 0.09 (0.24; 0.16–0.43) | 0.14–0.34 |
| Total bilirubin mg/100 mL | 0.5 ± 0.23 (0.44; 0.11–0.97) | 0.57 ± 0.22 (0.51; 0.28–0.97) | 0.44 ± 0.22 (0.42; 0.11–0.97) | ≤1.2 |
| PLT cells^103/μL | 229 ± 65 (223; 120–389) | 204.3 ± 47.1 (198.5; 120–310) | 246 ± 72.13 (244; 123–389) | 140–450 |
| MPV | 11 ± 0.9 (10.75; 9.4–12.8) | 11.1 ± 0.93 (11.1; 9.4–12.7) | 10.8 ± 0.9 (10.6; 9.5–12.8) | 8.0–11.9 |
| LK cells/μL | 6830 ± 2086 (6670; 3260–11,250) | 7238 ± 2191 (6815; 4190–11,250) | 6542 ± 2025 (6510; 3260–9550) | 4000–11,000 |
| Neutrophils cells/μL | 4022 ± 1719 (3720; 1400–7750) | 4338 ± 1745 (3560; 2650–7750) | 3799 ± 1718 (3840; 1400–7240) | 1800–7000 |
| Eosinophils cells/μL | 190 ± 119 (170; 0–440) | 198.4 ± 126.2 (165; 0–440) | 183.5 ± 117.8 (170; 10–410) | ≤700 |
| Basophils cells/μL | 23 ± 16 (20; 0–80) | 15.8 ± 9 (20; 0–30) | 28.8 ± 18.7 (20; 10–80) | ≤200 |
| Lymphocytes cells/μL | 2060 ± 676 (1990; 1030–4100) | 2121 ± 664.5 (2020; 1030–3061) | 2017 ± 701 (1990; 1040–4100) | 900–4500 |
| Monocytes cells/μL | 516 ± 159 (500; 310–910) | 519.2 ± 146.8 (515; 320–780) | 513.5 ± 172 (500; 310–910) | 100–1200 |
| NLR | 2.09 ± 1.0 (1.71; 0.76–4.45) | 2.17 ± 0.84 (2.24; 1.093–3.52) | 2.03 ± 1.17 (1.5; 0.76–4.45) | 0.78–3.5 |
| MLR | 0.275 ± 0.12 (0.23; 0.12–0.6) | 0.27 ± 0.125 (0.25; 0.12–0.51) | 0.28 ± 0.13 (0.23; 0.17–0.6) | 0.1–0.59 |
| PLR | 119 ± 41 (113.5; 65–209) | 105 ± 40 (83.14; 64.9–174.2) | 128.7 ± 40.7 (118; 70–209) | 40–200 |
| PLT-BDNF ng/mg proteins | 3.05 ± 1.95 (2.44; 0.73–8.73) | 3.2 ± 2.2 (2.54; 0.73–8.67) | 2.97 ± 1.83 (2.4; 0.92–8.73) | - |
| PPP-BDNF ng/mL | 2.075 ± 2.22 (1.54; 0.12–8.95) | 1.41 ± 1.04 (1.5; 0.25–3.97) | 2.37 ± 2.53 (1.6; 0.12–8.95) | - |
| Variables | E 1 | E 2 |
|---|---|---|
| PLT | 0.093 | 0.036 |
| VES | 0.013 | 0.144 |
| CRP | −0.108 | 0.359 |
| Tot Chol | 0.001 | −0.354 |
| HDL | 0.304 | −0.081 |
| LDL | −0.063 | −0.258 |
| Triglycerides | −0.240 | −0.027 |
| Glucose | −0.209 | −0.070 |
| UA | −0.082 | 0.381 |
| Leukocytes | −0.112 | 0.354 |
| Neutrophils | −0.021 | 0.333 |
| Basophils | 0.039 | −0.010 |
| Eosinophils | −0.119 | 0.218 |
| Lymphocytes | −0.358 | 0.169 |
| Monocytes | 0.144 | 0.319 |
| MPV | 0.011 | 0.008 |
| NLR | 0.301 | 0.209 |
| PLR | 0.391 | −0.067 |
| MLR | 0.399 | 0.128 |
| Total Bilirubin | −0.122 | −0.097 |
| PPP-BDNF | 0.344 | 0.053 |
| PLT-BDNF | 0.243 | 0.090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falaschi, V.; Palego, L.; Marazziti, D.; Betti, L.; Musetti, L.; Maglio, A.; Dell’Oste, V.; Sagona, S.; Felicioli, A.; Carpita, B.; et al. Variation of Circulating Brain-Derived Neurotrophic Factor (BDNF) in Depression: Relationships with Inflammatory Indices, Metabolic Status and Patients’ Clinical Features. Life 2023, 13, 1555. https://doi.org/10.3390/life13071555
Falaschi V, Palego L, Marazziti D, Betti L, Musetti L, Maglio A, Dell’Oste V, Sagona S, Felicioli A, Carpita B, et al. Variation of Circulating Brain-Derived Neurotrophic Factor (BDNF) in Depression: Relationships with Inflammatory Indices, Metabolic Status and Patients’ Clinical Features. Life. 2023; 13(7):1555. https://doi.org/10.3390/life13071555
Chicago/Turabian StyleFalaschi, Valentina, Lionella Palego, Donatella Marazziti, Laura Betti, Laura Musetti, Alessandra Maglio, Valerio Dell’Oste, Simona Sagona, Antonio Felicioli, Barbara Carpita, and et al. 2023. "Variation of Circulating Brain-Derived Neurotrophic Factor (BDNF) in Depression: Relationships with Inflammatory Indices, Metabolic Status and Patients’ Clinical Features" Life 13, no. 7: 1555. https://doi.org/10.3390/life13071555
APA StyleFalaschi, V., Palego, L., Marazziti, D., Betti, L., Musetti, L., Maglio, A., Dell’Oste, V., Sagona, S., Felicioli, A., Carpita, B., Brogi, A., Mucci, F., Massimetti, E., Dell’Osso, L., & Giannaccini, G. (2023). Variation of Circulating Brain-Derived Neurotrophic Factor (BDNF) in Depression: Relationships with Inflammatory Indices, Metabolic Status and Patients’ Clinical Features. Life, 13(7), 1555. https://doi.org/10.3390/life13071555









