Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasm of the Pancreas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
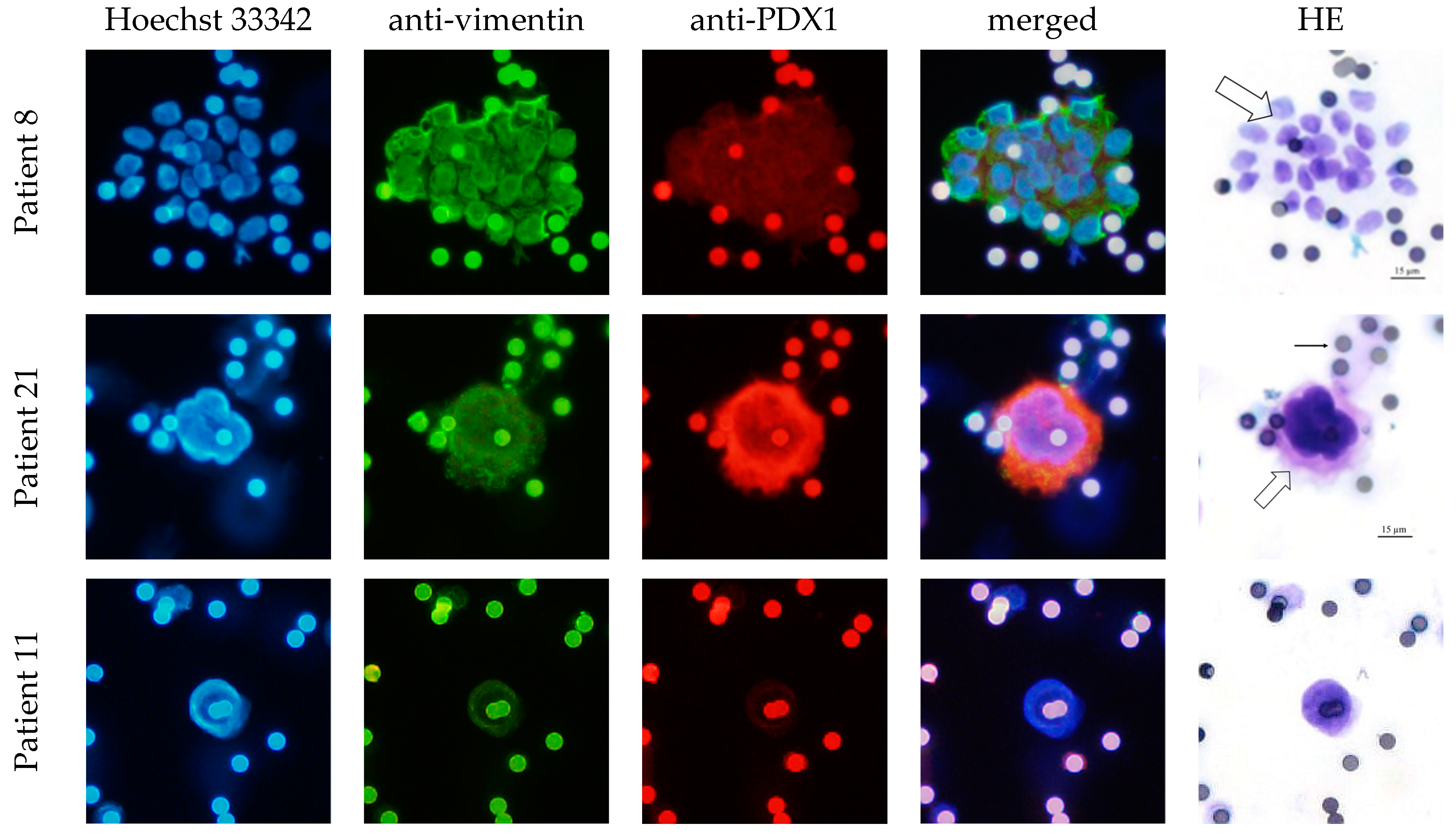
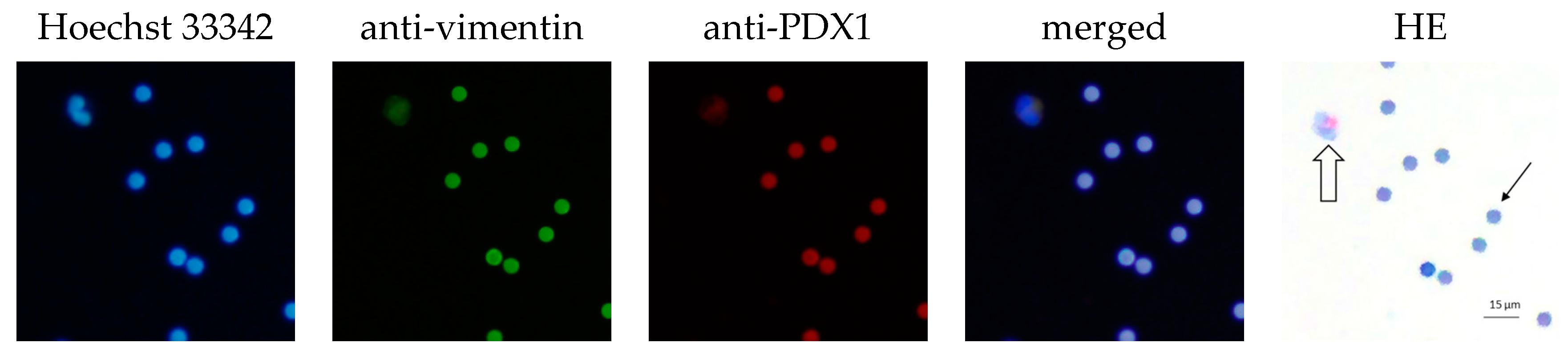
2.2. CEC Isolation Method and Cytological Evaluation
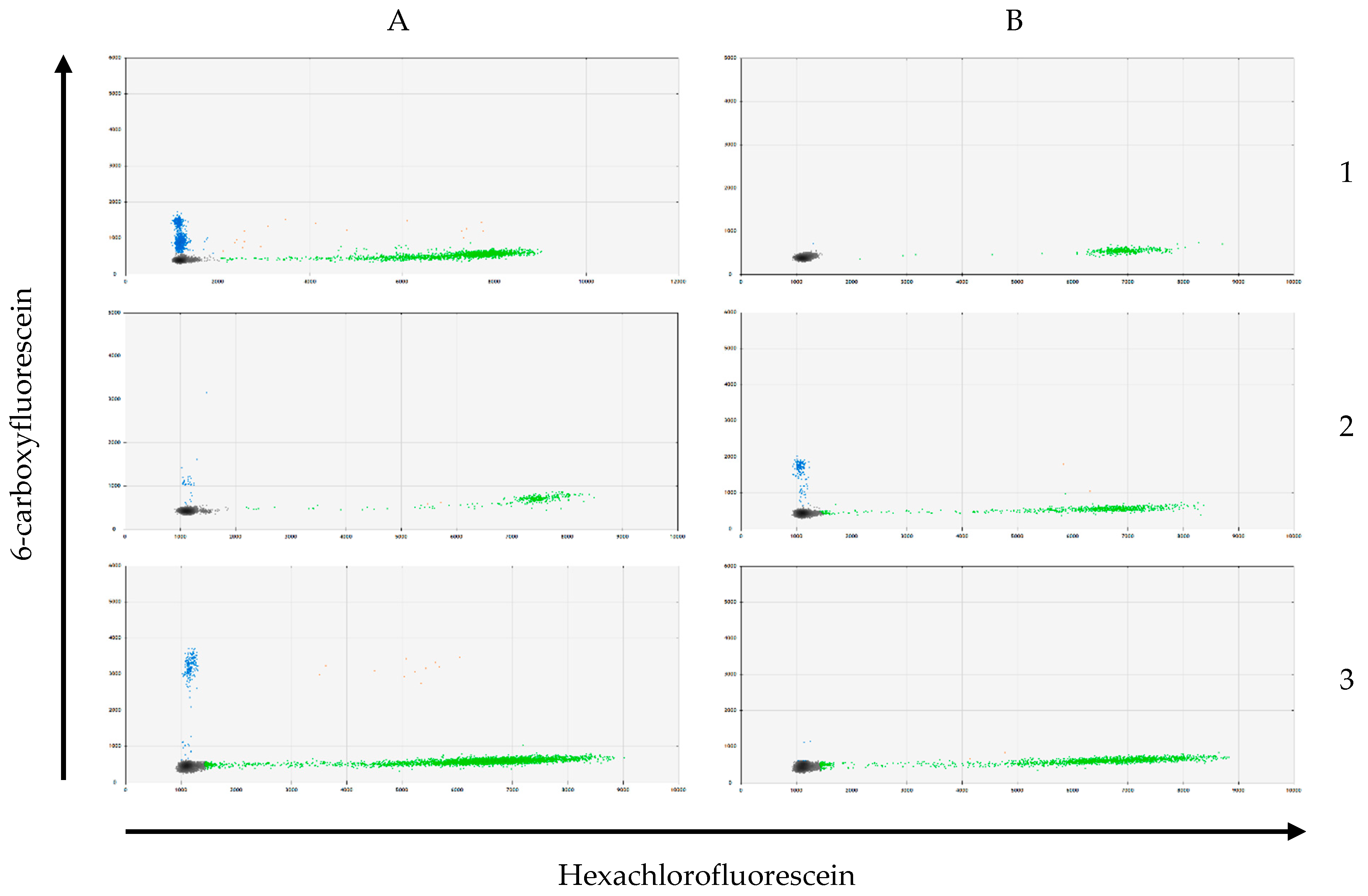
2.3. CEC and Tissue DNA Isolation and KRAS Genotyping
2.4. Histological Evaluation of the IPMN Tissue
2.5. Statistics
3. Results
3.1. Study Population
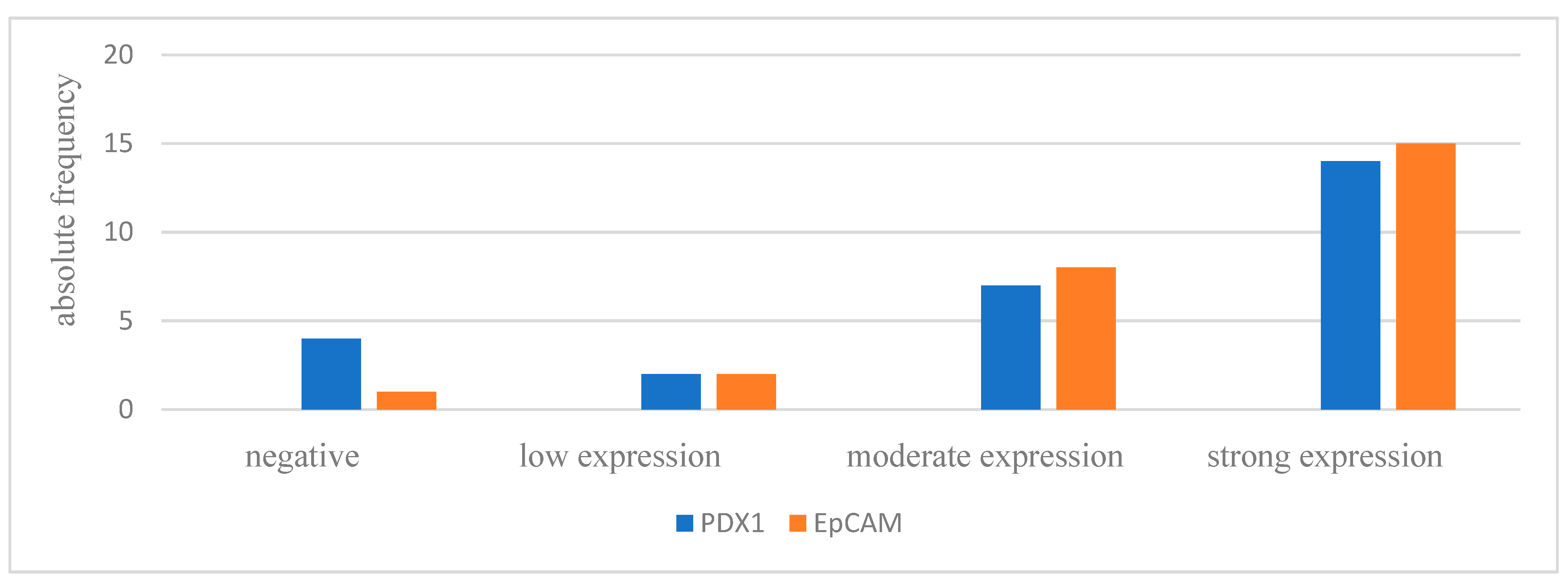
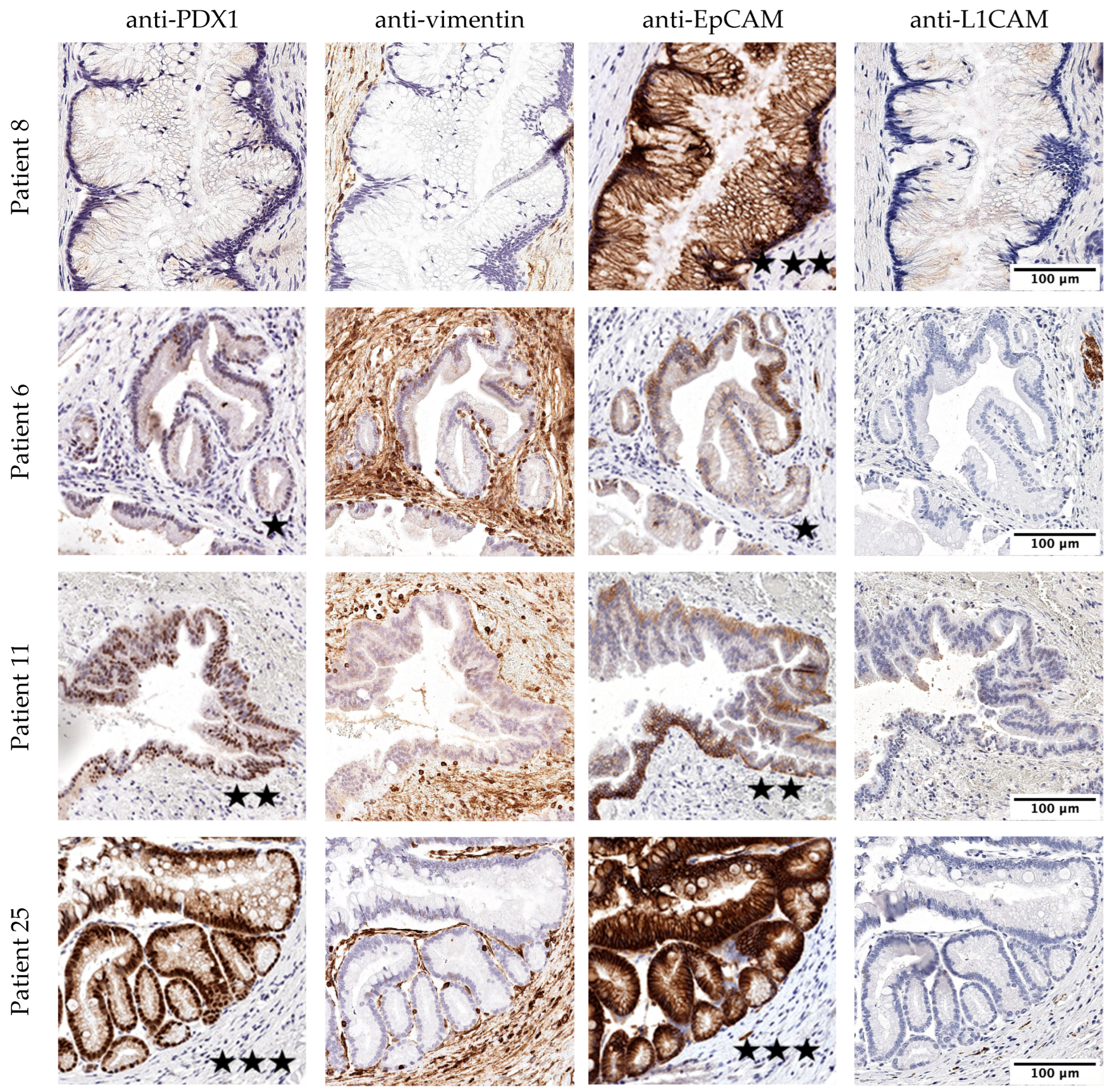
3.2. Histological Evaluation of the IPMN Tissue
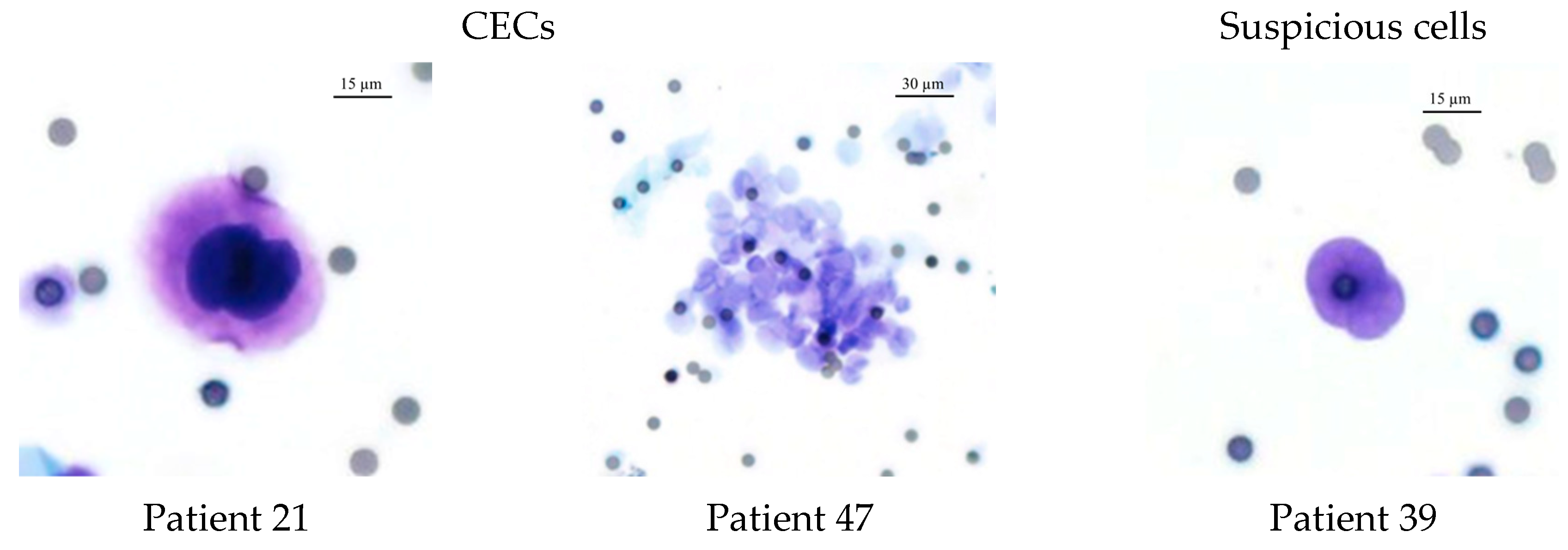
3.3. CEC Isolation Method and Cytological Evaluation
3.4. KRAS Mutation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moris, M.; Bridges, M.D.; Pooley, R.A.; Raimondo, M.; Woodward, T.A.; Stauffer, J.A.; Asbun, H.J.; Wallace, M.B. Association Between Advances in High-Resolution Cross-Section Imaging Technologies and Increase in Prevalence of Pancreatic Cysts From 2005 to 2014. Clin. Gastroenterol. Hepatol. 2016, 14, 585–593.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugge, W.R.; Lauwers, G.Y.; Sahani, D.; Fernandez-del Castillo, C.; Warshaw, A.L. Cystic neoplasms of the pancreas. N. Engl. J. Med. 2004, 351, 1218–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valsangkar, N.P.; Morales-Oyarvide, V.; Thayer, S.P.; Ferrone, C.R.; Wargo, J.A.; Warshaw, A.L.; Fernández-del Castillo, C. 851 resected cystic tumors of the pancreas: A 33-year experience at the Massachusetts General Hospital. Surgery 2012, 152, S4–S12. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.-Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Klöppel, G.; Volkan Adsay, N.; Albores-Saavedra, J.; Fukushima, N.; Horii, A.; Hruban, R.H.; Kato, Y.; Klimstra, D.S.; Longnecker, D.S.; et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: A consensus study. Virchows Arch. Int. J. Pathol. 2005, 447, 794–799. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Stark, A.; Donahue, T.R.; Reber, H.A.; Hines, O.J. Pancreatic Cyst Disease: A Review. JAMA 2016, 315, 1882–1893. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.-L.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Fernández-del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Tanaka, M.; Chari, S.; Adsay, V.; Fernandez-del Castillo, C.; Falconi, M.; Shimizu, M.; Yamaguchi, K.; Yamao, K.; Matsuno, S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006, 6, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Teo, J.-Y.; Chin, Y.-K.; Hennedige, T.; Tan, D.M.; Low, A.S.; Thng, C.H.; Goh, B.K.P. Systematic review of the clinical utility and validity of the Sendai and Fukuoka Consensus Guidelines for the management of intraductal papillary mucinous neoplasms of the pancreas. HPB 2018, 20, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckler, M.; Michalski, C.W.; Schaefle, S.; Kaiser, J.; Büchler, M.W.; Hackert, T. The Sendai and Fukuoka consensus criteria for the management of branch duct IPMN—A meta-analysis on their accuracy. Pancreatology 2017, 17, 255–262. [Google Scholar] [CrossRef]
- Klein, C. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 2009, 9, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Mirek, E.T.; Aiello, N.M.; Maitra, A.; Bailey, J.M.; McAllister, F.; Reichert, M.; Beatty, G.L.; Rustgi, A.K.; Vonderheide, R.H.; et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012, 148, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Rhim, A.D.; Thege, F.I.; Santana, S.M.; Lannin, T.B.; Saha, T.N.; Tsai, S.; Maggs, L.R.; Kochman, M.L.; Ginsberg, G.G.; Lieb, J.G.; et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 2014, 146, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yu, X.; Hartmann, D.; Zhou, J. Circulating tumor cells in peripheral blood of pancreatic cancer patients and their prognostic role: A systematic review and meta-analysis. HPB 2020, 22, 660–669. [Google Scholar] [CrossRef]
- Pang, T.C.Y.; Po, J.W.; Becker, T.M.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Circulating tumour cells in pancreatic cancer: A systematic review and meta-analysis of clinicopathological implications. Pancreatology 2021, 21, 103–114. [Google Scholar] [CrossRef]
- Kulemann, B.; Rösch, S.; Seifert, S.; Timme, S.; Bronsert, P.; Seifert, G.; Martini, V.; Kuvendjiska, J.; Glatz, T.; Hussung, S.; et al. Pancreatic cancer: Circulating Tumor Cells and Primary Tumors show Heterogeneous KRAS Mutations. Sci. Rep. 2017, 7, 4510. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, M.W.; Cauley, C.E.; Kulemann, B.; Liss, A.S.; Castillo, C.F.-D.; Warshaw, A.L.; Lillemoe, K.D.; Thayer, S.P.; Pitman, M.B. Cytologic characteristics of circulating epithelioid cells in pancreatic disease. Cancer Cytopathol. 2017, 125, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wu, G.; Cheng, K.S.; Chen, A.; Neoh, K.H.; Chen, S.; Tang, Z.; Lee, P.F.; Dai, M.; Han, R.P.S. CTC phenotyping for a preoperative assessment of tumor metastasis and overall survival of pancreatic ductal adenocarcinoma patients. EBioMedicine 2019, 46, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Franses, J.W.; Basar, O.; Kadayifci, A.; Yuksel, O.; Choz, M.; Kulkarni, A.S.; Tai, E.; Vo, K.D.; Arora, K.S.; Desai, N.; et al. Improved Detection of Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasms. Oncologist 2018, 23, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poruk, K.E.; Valero, V., 3rd; He, J.; Ahuja, N.; Cameron, J.L.; Weiss, M.J.; Lennon, A.M.; Goggins, M.; Wood, L.D.; Wolfgang, C.L. Circulating Epithelial Cells in Intraductal Papillary Mucinous Neoplasms and Cystic Pancreatic Lesions. Pancreas 2017, 46, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Matthaei, H.; Wu, J.; Hong, S.-M.; Yu, J.; Borges, M.; Hruban, R.H.; Maitra, A.; Kinzler, K.; Vogelstein, B.; et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012, 142, 730–733.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulemann, B.; Pitman, M.B.; Liss, A.S.; Valsangkar, N.; Fernández-del Castillo, C.; Lillemoe, K.D.; Hoeppner, J.; Mino-Kenudson, M.; Warshaw, A.L.; Thayer, S.P. Circulating tumor cells found in patients with localized and advanced pancreatic cancer. Pancreas 2015, 44, 547–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulemann, B.; Liss, A.S.; Warshaw, A.L.; Seifert, S.; Bronsert, P.; Glatz, T.; Pitman, M.B.; Hoeppner, J. KRAS mutations in pancreatic circulating tumor cells: A pilot study. Tumour Biol. 2016, 37, 7547–7554. [Google Scholar] [CrossRef]
- Francescangeli, F.; Magri, V.; de Angelis, M.L.; de Renzi, G.; Gandini, O.; Zeuner, A.; Gazzaniga, P.; Nicolazzo, C. Sequential Isolation and Characterization of Single CTCs and Large CTC Clusters in Metastatic Colorectal Cancer Patients. Cancers 2021, 13, 6362. [Google Scholar] [CrossRef]
- Kuvendjiska, J.; Pitman, M.B.; Martini, V.; Braun, C.; Grebe, K.; Timme, S.; Fichtner-Feigl, S.; Glatz, T.; Schmoor, C.; Guenzle, J.; et al. Cytopathological Heterogeneity of Circulating Tumor Cells in Non-metastatic Esophageal Adenocarcinoma. Anticancer Res. 2020, 40, 5679–5685. [Google Scholar] [CrossRef]
- Kuvendjiska, J.; Bronsert, P.; Martini, V.; Lang, S.; Pitman, M.B.; Hoeppner, J.; Kulemann, B. Non-Metastatic Esophageal Adenocarcinoma: Circulating Tumor Cells in the Course of Multimodal Tumor Treatment. Cancers 2019, 11, 397. [Google Scholar] [CrossRef] [Green Version]
- Hussung, S.; Follo, M.; Klar, R.F.U.; Michalczyk, S.; Fritsch, K.; Nollmann, F.; Hipp, J.; Duyster, J.; Scherer, F.; von Bubnoff, N.; et al. Development and Clinical Validation of Discriminatory Multitarget Digital Droplet PCR Assays for the Detection of Hot Spot KRAS and NRAS Mutations in Cell-Free DNA. J. Mol. Diagn. JMD 2020, 22, 943–956. [Google Scholar] [CrossRef]
- Specht, E.; Kaemmerer, D.; Sänger, J.; Wirtz, R.M.; Schulz, S.; Lupp, A. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology 2015, 67, 368–377. [Google Scholar] [CrossRef]
- Lahat, G.; Lubezky, N.; Loewenstein, S.; Nizri, E.; Gan, S.; Pasmanik-Chor, M.; Hayman, L.; Barazowsky, E.; Ben-Haim, M.; Klausner, J.M. Epithelial-to-mesenchymal transition (EMT) in intraductal papillary mucinous neoplasm (IPMN) is associated with high tumor grade and adverse outcomes. Ann. Surg. Oncol. 2014, 21 (Suppl. S4), S750–S757. [Google Scholar] [CrossRef] [PubMed]
- Karnoub, A.E.; Weinberg, R.A. Ras oncogenes: Split personalities. Nat. Rev. Mol. Cell Biol. 2008, 9, 517–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, J.P., IV; Wang, S.C.; Hebrok, M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat. Rev. Cancer 2010, 10, 683–695. [Google Scholar] [CrossRef] [PubMed]

| Therapy Recommendation | Sendai Consensus | Fukuoka Consensus | |
|---|---|---|---|
| Resection | cyst size > 3 cm presence of mural nodules positive cytology clinical symptoms MPD dilation | High-risk stigmata | obstructive jaundice enhancing mural nodule ≥ 5 mm MPD diameter ≥ 10 mm |
| Surveillance | Worrisome features | MPD diameter 5–9 mm cyst diameter ≥ 30 mm enhancing mural nodules < 5 mm thickened/enhancing cyst walls IPMN-induced acute pancreatitis increased serum level of CA 19-9 cyst growth rate ≥ 5 mm/2 years lymphadenopathy MPD stenosis with distal atrophy | |
| Maximal Dysplasia Grade in IPMN | |||||
|---|---|---|---|---|---|
| Low-Grade | Intermediate-Grade | High-Grade | Total | ||
| n (%) | n (%) | n (%) | n (%) | ||
| IPMN type | branch-duct | 4 (66.7%) | 2 (33.3%) | 0 (0%) | 6 (22.2%) |
| mixed-type | 10 (71.4%) | 2 (14.3%) | 2 (14.3%) | 14 (51.8%) | |
| main-duct | 6 (85.7%) | 1 (14.3%) | 0 (0%) | 7 (25.9%) | |
| Histological subtype | pancreatobiliary | 1 (100%) | 0 (0%) | 0 (0%) | 1 (3.7%) |
| intestinal | 4 (50%) | 3 (37.5%) | 1 (12.5%) | 8 (29.6%) | |
| gastric | 15 (83.3%) | 2 (11.1%) | 1 (5.6%) | 18 (66.7%) | |
| Total | 20 (74.1%) | 5 (18.5%) | 2 (7.4%) | ||
| CEC (n, %) | |||||
|---|---|---|---|---|---|
| Negative (n = 4) | Suspicious (n = 13) | Positive (n = 10) | p | ||
| IPMN type | branch-duct | 0 (0%) | 2 (33.3%) | 4 (66.7%) | 0.38 |
| mixed-type | 2 (14.3%) | 7 (50%) | 5 (35.7%) | ||
| main-duct | 2 (28.6%) | 4 (57.1%) | 1 (14.3%) | ||
| Maximal dysplasia grade | low | 3 (20%) | 10 (50%) | 7 (20%) | 0.52 |
| intermediate | 1 (20%) | 3 (60%) | 1 (20%) | ||
| high | 0 (0%) | 0 (0%) | 2 (100%) | ||
| Enhancing mural nodule | no | 4 (16%) | 12 (48%) | 9 (36%) | 0.78 |
| <5 mm | 0 (0%) | 1 (100%) | 0 (0%) | ||
| ≥5 mm | 0 (0%) | 0 (0%) | 1 (100%) | ||
| Thickened/enhancing cyst walls | no | 4 (16.7%) | 12 (50%) | 8 (33.3%) | 0.73 |
| yes | 0 (0%) | 1 (33.3%) | 2 (66.7%) | ||
| IPMN-induced acute pancreatitis | no | 3 (15.8%) | 10 (52.6%) | 6 (31.6%) | 0.85 |
| yes | 1 (12.5%) | 3 (37.5%) | 4 (50%) | ||
| Pancreatic head cyst with obstructive jaundice | no | 4 (16%) | 11 (44%) | 10 (40%) | 0.63 |
| yes | 0 (0%) | 2 (100%) | 0 (0%) | ||
| Increased serum level of CA 19-9 | no | 3 (15.8%) | 9 (47.4%) | 7 (36.8%) | 0.74 |
| yes | 0 (0%) | 1 (33.3%) | 2 (66.7%) | ||
| Cyst growth-rate ≥5 mm/2 years (n = 13) | no | 2 (25%) | 3 (37.5%) | 3 (37.5%) | 1.0 |
| yes | 1 (20%) | 2 (40%) | 2 (40%) | ||
| Max. cyst size (mm) | 18.8 (SD: 13.7) | 15.8 (SD: 6.4) | 17.7 (SD: 9.3) | 0.79 | |
| KRAS genotype (CEC) | wild-type | 4 (25%) | 8 (50%) | 4 (25%) | 0.15 |
| mutated | 0 (0%) | 1 (20%) | 4 (80%) | ||
| KRAS genotype (tissue) | wild-type | 3 (37.5%) | 4 (50%) | 1 (12.5%) | 0.06 |
| mutated | 1 (5.3%) | 9 (47.4%) | 9 (47.4%) | ||
| Control patients (n = 5) | 5 | 0 | 0 | ||
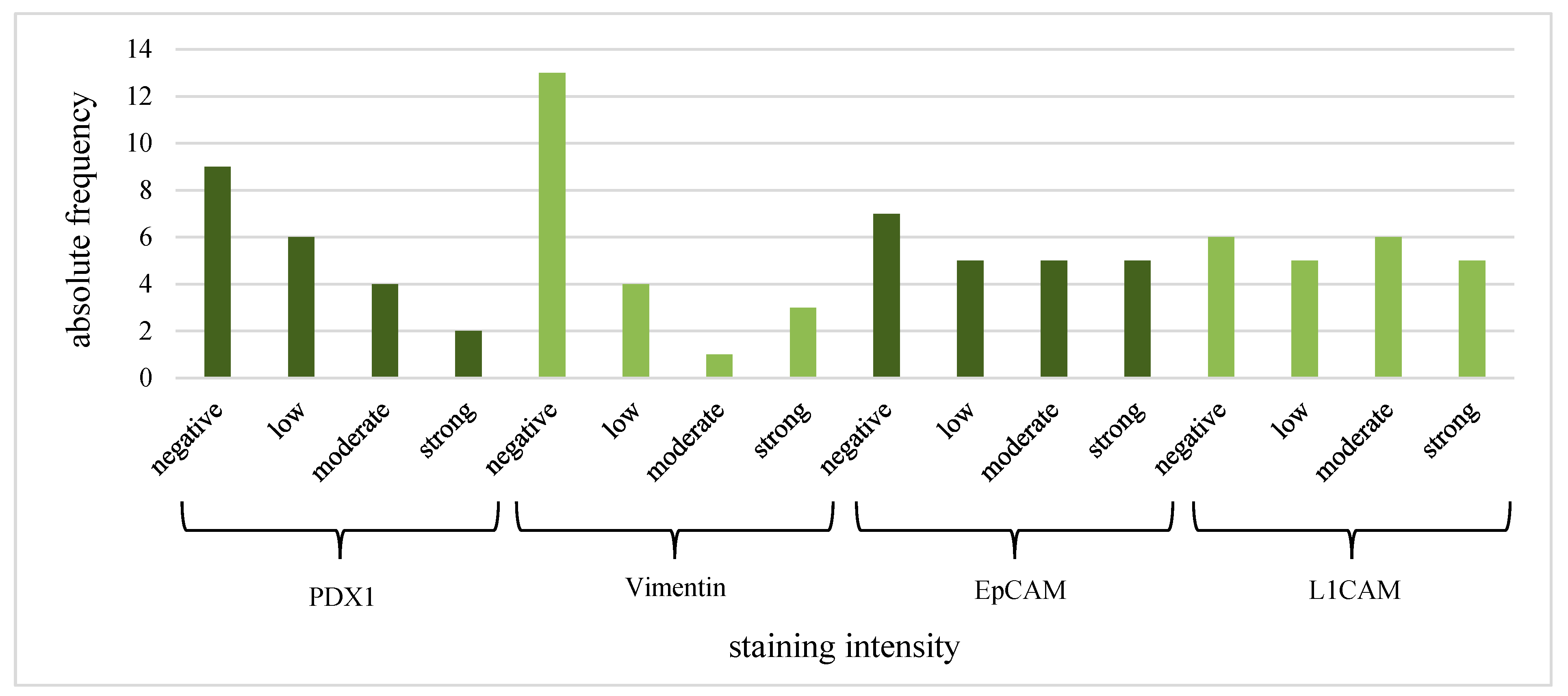
| PDX1 (IF) | Vimentin | EpCAM | L1CAM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | p | Positive | p | Positive | p | Positive | p | ||
| Histological subtype | pancreatobiliary | 0 (0%) | 0.23 | 0 (0%) | 1.00 | 0 (0%) | 0.55 | 0 (0%) | 0.26 |
| gastric | 7 (50.0%) | 6 (42.9%) | 10 (71.4%) | 10 (71.4%) | |||||
| intestinal | 5 (83.3%) | 2 (33.3%) | 5 (71.4%) | 6 (85.7%) | |||||
| CEC | suspicious | 5 (41.7%) | 0.18 | 1 (8.3%) | 0.002 | 7 (58.3%) | 0.38 | 8 (66.7%) | 0.65 |
| positive | 7 (77.8%) | 7 (77.8%) | 8 (80.0%) | 8 (80.0%) | |||||
| Cluster | no | 2 (50.0%) | 0.17 | 2 (50.0%) | 0.17 | 3 (60.0%) | 0.44 | 3 (60.0%) | 0.44 |
| yes | 5 (100%) | 5 (100%) | 5 (100%) | 5 (100%) | |||||
| KRAS (tissue) | wild-type | 3 (75.0%) | 0.60 | 0 (0%) | 0.13 | 2 (50.0%) | 0.57 | 2 (50.0%) | 0.29 |
| mutated | 9 (52.9%) | 8 (47.1%) | 13 (72.2%) | 14 (77.8%) | |||||
| KRAS in CECs | ||||
|---|---|---|---|---|
| Wild-Type | Mutation | p | ||
| IPMN type | branch-duct | 2 (50%) | 2 (50%) | 0.21 |
| mixed-type | 10 (90.9%) | 1 (9.1%) | ||
| main-duct | 4 (66.7%) | 2 (33.3%) | ||
| Max. cyst size (mm) | 15.2 (SD: 8) | 19.2 (SD: 10.5) | 0.37 | |
| Histological subtype | pancreatobiliary | 0 (0%) | 1 (100%) | 0.44 |
| gastric | 11 (78.6%) | 3 (21.4%) | ||
| intestinal | 5 (83.3%) | 1 (16.7%) | ||
| Maximal dysplasia grade | low | 13 (72.2%) | 5 (27.8%) | 0.55 |
| intermediate | 3 (100%) | 0 (0%) | ||
| Enhancing mural nodule | no | 15 (78.9%) | 4 (21.1%) | 0.43 |
| <5 mm | 0 (0%) | 1 (100%) | ||
| ≥5 mm | 1 (100%) | 0 (0%) | ||
| Thickened/enhancing cyst walls | no | 14 (77.8%) | 4 (22.2%) | 1.0 |
| yes | 2 (66.7%) | 1 (33.3%) | ||
| IPMN-induced acute pancreatitis | no | 11 (73.3%) | 4 (26.7%) | 1.0 |
| yes | 5 (83.3%) | 1 (16.7%) | ||
| Pancreatic head cyst with obstructive jaundice | no | 15 (78.9%) | 4 (21.1%) | 0.43 |
| yes | 1 (50%) | 1 (50%) | ||
| Increased serum level of CA 19-9 | no | 12 (85.7%) | 2 (14.3%) | 0.47 |
| yes | 2 (66.7%) | 1 (33.3%) | ||
| Cyst growth rate ≥5 mm/2 years (n = 10) | no | 5 (71.4%) | 2 (28.6%) | 1.0 |
| yes | 2 (66.7%) | 1 (33.3%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuvendjiska, J.; Müller, F.; Bronsert, P.; Timme-Bronsert, S.; Fichtner-Feigl, S.; Kulemann, B. Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasm of the Pancreas. Life 2023, 13, 1570. https://doi.org/10.3390/life13071570
Kuvendjiska J, Müller F, Bronsert P, Timme-Bronsert S, Fichtner-Feigl S, Kulemann B. Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasm of the Pancreas. Life. 2023; 13(7):1570. https://doi.org/10.3390/life13071570
Chicago/Turabian StyleKuvendjiska, Jasmina, Felix Müller, Peter Bronsert, Sylvia Timme-Bronsert, Stefan Fichtner-Feigl, and Birte Kulemann. 2023. "Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasm of the Pancreas" Life 13, no. 7: 1570. https://doi.org/10.3390/life13071570





