The Role of Blood Oxygen Level Dependent Signal Variability in Pediatric Neuroscience: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Operational Definitions
2.2. Article Search Strategy
2.3. Study Inclusion and Exclusion
2.4. Study Selection and Quality Assessment
2.5. Data Synthesis
3. Results
3.1. Study Sample
| Title | Author and Year | Location (Region, Country) | Study Design | Age of Subjects | Sex | Sample Size | Case Definition |
|---|---|---|---|---|---|---|---|
| Age-Associated Patterns in Gray Matter Volume, Cerebral Perfusion and BOLD Oscillations in Children and Adolescents | Bray et al., 2017 [26] | Calgary, Alberta, Canada | Cross-Sectional | Mean = 13.8, SD = 3.12 Range = 7–18 | Typically developing females = 34 Typically developing males = 25 | Typically developing = 59 | All participants healthy (No cases) |
| BOLD SV and complexity in children and adolescents with and without autism spectrum disorder | Easson, et al., 2019 [17] | Toronto, Ontario, Canada | Cross-Sectional | ASD Group Mean = 13.25, SD = 2.87 ASD Group Range = [9.6–17.80] Typically Developing Mean = 13.42 SD = 3.21 Typically Developing Range [8.10–17.60] | ASD Males = 20 Typically Developing Males = 17 | ASD = 20 Typically Developing = 17 Total Sample Size = 37 | Autism spectrum disorder was defined by the Autism Brain Imaging Data Exchange (ABIDE) II database (Where cases ascertained from) |
| Changes in BOLD variability are linked to the development of variable response inhibition: BOLD variability and variable response inhibition | Thompson et al., 2020 [7] | London, UK | Cross-Sectional | Children Range = [10–12] Children Mean = 11.56, SD = 0.83 Adult Range = [18–26] Adult Mean = 21.55, SD = 2.31 | Females = 10 Males = 9 | Children 10–12 = 19 Adults 18–26 = 26 Total = 45 | All participants healthy (No cases) |
| Creative internally directed cognition is associated with reduced BOLD variability | Roberts, et al., 2020 [18] | Auckland, New Zealand | Cross-Sectional | Range = [17–25] Mean = 21 years, SD = 4 years | 8 Males and 16 Females | 24 typically developing | All participants healthy (No cases) |
| Disentangling resting-state BOLD variability and PCC functional connectivity in 22q11.2 deletion syndrome | Zöller et al., 2017 [4] | Geneva, Switzerland | Case Control | 22q11.2 Gene Age Range = [9.0–24.8] Mean 22q11.2 Gene Age = 16.53 ± 4.25 Control Group Age Range = [9.5–24.9] Mean Control Group Age = 16.44 ± 4.20 | Males = 21 Females = 29 | Healthy Controls = 50 (22/28) 2q11.2DS = 50 (21/29) Total = 100 | 50 patients with 22q11.2DS, which is a specific type of microdeletion in chromosome 22 |
| Individual Differences in Reading Skill Are Related to Tiral-by-Trial Neural Activation Variability in the Reading Network | Malins et al., 2017 [21] | United States | Cross-Sectional | Discovery Sample Range = [7.8–11.3] Discovery Sample Mean = 9.3, SD = 0.6 Confirmation Sample Range = [7.5–11.3] Confirmation Sample Mean = 9.4, SD = 1.1 | Sample 1 females: 18 female Sample 1 males: 26 male Sample 2 females: 14 female Sample 2 males: 18 males | Sample 1 = 44 Sample 2 = 32 Total = 76 | All participants healthy (No cases) |
| Moment-to-Moment BOLD Signal Variability Reflects Regional Changes in Neural Flexibility across the Lifespan | Nomi et al., 2017 [16] | Miami, Florida USA | Cross-Sectional | Slow repetition time Range = [6–85] Slow repetition time Mean = 42.26, SD = 23.60 Fast repetition time Range = [6–85] Fast repetition time Mean = 42.46, SD = 23.30 | 191 participants, 132 Female, 59 male | 191 Participants | All participants healthy (No cases) |
| Neural correlates of inhibitory control and functional genetic variation in the dopamine D4 receptor gene | Mulligan et al., 2014 [23] | Alberta, Canada | Cross-Sectional | All Participants are 18 | Female population = 33 Male population = 29 | 7R+ = 23 7R− control = 39 Total = 62 | (R7+) group (dopamine D4 receptor gene (DRD4) with 7 repeats in the Variable Number of Tandem Repeats section (VNTR) of DRD4) |
| Neural, electrophysiological and anatomical basis of brain-network variability and its characteristic changes in mental disorders | Zhang et al., 2016 [22] | Nanjing, PR, China | Case Control | Total Study Age Range = [8–25] UM Sample Controls = 15.1 +/− 3.7 Autism = 3.6 +/− 2.4 Peking University-PKU Sample Controls = (11.4 +/− 1.9) ADHD = (12.1 +/− 2.0) New York University-NYU Controls = (12.2 +/− 3.1) ADHD = (12.2 +/−13.1) | Autism UM dataset controls = (48/16) Autism UM dataset Autism = (31/7) ADHD PKU dataset controls = (84/59) ADHD PKU dataset ADHD = (89/10) ADHD NYU dataset controls = (54/54) ADHD NYU dataset ADHD = (106/34) | Autism MU dataset controls = 64 Autism MU dataset Autism = 38 ADHD PKU dataset controls = 143 ADHD PKU dataset ADHD = 99 ADHD NYU dataset controls = 108 ADHD NYU dataset ADHD = 140 Total = 592 (we only use a subset of 1180 total in this study due to age exclusions) | Schizophrenia case definition as defined in the Taiwan Dataset 1 (Guo et al., 2014); and the COBRE Dataset 2. Autism case definition as defined by the definition as defined in the New York University-NYU Dataset 3 and University of Melbourne-UM Dataset 4 (which are from ABIDE Consortium) ADHD case definition was defined by the Peking University-PKU Dataset 5; and New York University-NYU Dataset 6 which are included from 1000 participants derived from the Functional Connectome Project |
| Psychotic symptoms influence the development of anterior cingulate BOLD variability in 22q11.2 deletion syndrome | Zöller et al., 2018 [29] | Geneva Switzerland | Case-Control | Between 10 and 30 years old | PS+ = 28 (12/16) PS− = 29 (14/15) Healthy controls = 69 (30/39) | 22q11.2 gene = 57 Healthy Controls = 69 Total = 126 | Chromosome 22q11.2 deletion syndrome (22q11DS) is defined as a neurodevelopmental disorder associated with “a broad phenotype of clinical, cognitive, and psychiatric features”. It is a specific type of microdeletion in chromosome 22 |
| Temporal fractal analysis of the rs-BOLD signal identifies brain abnormalities in autism spectrum disorder | Dona et al., 2017 [24] | Austin, Texas, United States | Case-Control | ASD (12.7 ± 2.4 y/o) 55 age-matched (14.1 ± 3.1 y/o) healthy controls | ASD = 46 male and 9 females, Healthy controls = 38 male and 9 females. | ASD = 55 Healthy Control = 55 Total = 110 | ASD and age-matched controls. Definition of ASD defined by NITRC database and the ABIDE project |
| Variability of the hemodynamic response as a function of age and frequency of epileptic discharge in children with epilepsy | Jacobs et al., 2007 [28] | Germany and Montreal Canada | Cross-Sectional | Range = [5 months-18 years] (Mean and SD not calculated) | 12 Female, 25 Male | 37 | Epilepsy, case definition of epilepsy not explicit but EEG-fMRI data were ascertained in children who met the following criteria: (1) An indication for an anatomical scan on the basis of the necessity to investigate a lesion seen on a prior anatomical MRI scan or to diagnose them with epilepsy syndrome and exclude pathological changes. (2) Participants had frequent spikes (N 10 in 20 min) identified on EEG outside the scanner, without occurrence in bursts. |
| Evaluation of spontaneous regional brain activity in weight-recovered anorexia nervosa | Seidel et al., 2020 [19] | Germany | Case Control Study | Total Study Range = 15.5–29.7 recAN Mean = 22.06, SD = 3.38 HC Mean = 22.05, SD = 3.34 | Healthy Control = 65 Female recAN = 65 female | Healthy Control = 65 recAN = 65 Total = 130 | Recovered Anorexia Nervosa (Weight Recovered). Defined as recAN subjects had to (1) maintain a body mass index (BMI) (kg/m2) > 18.5 (if older than 18 years) or above the 10th age percentile (if younger than 18 years); (2) menstruate; and (3) have not binged, purged, or engaged in restrictive eating patterns during at least 6 months before the study. |
| Complexity of low-frequency blood oxygen level-dependent fluctuations covaries with local connectivity | Anderson et al., 2013 [20] | N/A | Cross-Sectional | Range = [7–30] Mean = 8.3, SD = 5.6 | Male = 590 Female = 429 | 1019 | Not Specified |
| Fractal Analysis of Brain Blood Oxygenation Level Dependent (BOLD) Signals from Children with Mild Traumatic Brain Injury (mTBI) | Dona et al., 2017 [25] | N/A | Cross-Sectional | mTBI Subjects = 13.4 ± 2.3 Age-matched Healthy Controls = 13.5 ± 2.34 | N/A | mTBI = 15 Healthy Control = 56 Total = 71 | Case Control |
| The longitudinal relationship between BOLD signal variability changes and white matter maturation during early childhood | Wang et al., 2021 [10] | Canada and Australia | Cross-Sectional | Range = 1.97–8.0 years Mean age at intake = 4.42 ± 1.27 | Females = 43 Males = 40 | 83 | None |
| Frequency-specific alterations of the resting-state BOLD signals in nocturnal enuresis: an fMRI Study | Zheng et al., 2021 [27] | China | Case Control | Range approx. = [7–12] NE Patients 9.27(± 1.760) Control 9.68(± 1.601) | NE males = 57 NE Females = 14 Control Males = 19 Control Females = 16 | Children with nocturnal enuresis (NE) = 129 Healthy controls = 37 | Case Control |
| Metric Type | Authors | Variability Metric | Description | Findings and Associations |
|---|---|---|---|---|
| Deviation from Average BOLD Signal | (Roberts et al., 2020 [18], Zöller et al., 2017 [4], Zöller et al., 2018 [30], Wang et al., 2021 [10], Anderson et al., 2013 [20]) | BOLDSD | Quantified the deviation of average BOLD signal from the mean signal. | BOLD Signal variability globally increased with age in all metrics (some regions decrease) BOLD SV in dACC did not change over age in PS+ patients and increased in PS−. Variability increased with age in the DMN. Positively correlated with GE in structural networks and negatively correlated with performance in ASD behavioral severity (SRS). Negative associations with indexes of creativity |
| (Nomi et al., 2017a [16], Seidel et al., 2020 [19], Amanda K. Easson and McIntosh 2019 [17]) | sMSSD | Calculated by subtracting the amplitude of the signal at time point t from time point t + 1, squaring, and then averaging the resulting values from the entire voxel time course. | ||
| Correlational Measures of BOLD Signal Variance | (Zhang et al., 2016 [22]) | Temporal Variance | The BOLD time series were segmented into non-overlapping windows, a whole brain signal measure is obtained using Pearson correlation, and a region’s variability is compared to others. | Lower variability of DMN in schizophrenia, and increased variability in Autism/ADHD. Changes in variability were closely related to symptom scores and in the 10% most variable regions. Variability increases with age in the inhibition network. More variability in the network was associated with less variability in behavioral performance. Low variability in the DMN was correlated with high FC. Lower variability in 7R+ when compared to 7R− when participants successfully inhibited a prepotent motor response. Primarily seen in the prefrontal cortex, occipital lobe, and cerebellum. |
| (Malins et al., 2018 [21], and Mulligan et al., 2014 [23]) | GLM Derived Variance | GLM produced trial β series estimates of the signal which was used to estimate a variance. | ||
| (Thompson et al., 2021 [7]) | Differences of Residuals | The difference in the variability between the two residual models. | ||
| Signal Complexity | (Amanda K. Easson and McIntosh 2019 [17]) | Sample Entropy | SE was used in identifying repetitive patterns in a time series. The degree of regularity of these patterns of activation was also observed, with fewer complex signals being more random. | Positive correlations were identified between entropy, GE, and age. Negative correlations with SRS severity scores and FD in social and non-social tasks, ADIR and ADOS. Grey matter rs-BOLD FD in mTBI patients had reduced FD. Power law exponents remained unchanged or decreased with age and are linearly related to ReHo, which covaried across subjects and gray matter regions. Grey matter rs-BOLD FD in mTBI patients had reduced FD. The fALFF increased with age, distinguishing posterior, and anterior regions. Higher fALFF values in recAN patient’s cerebellum and the inferior temporal gyrus compared to controls. The fALFF decreased in the right insula in children with NE. |
| (Dona et al., 2017a [24] and Dona et al., 2017b [25]) | Fractal Dimension | Measure of the structural complexity of a signal derived from hurst exponents and quantified structural complexity across different predefined time windows. | ||
| (Anderson et al., 2013 [20]) | Power Law Exponents | Power-based index of sinusoidal amplitudes in the BOLD signal. Signal that follows fractal characteristics that were self-similar within and across frequencies over a time series were measured. | ||
| (Seidal et al., 2020 [19], Zheng et al., 2021 [27], Bray 2017 [26]) | Fractional amplitude of low-frequency fluctuations (fALFF) | The ratio of the low-frequency power spectrum, specifically in the range of 0.01–0.08 Hz, to the entire signal frequency range. | ||
| Structure of Hemodynamic Response Function | (Jacobs et al., 2008 [28]) | HRF Structure | Using the structure of the HRF, like peak time, amplitude or other signal characteristics not mentioned above. | Could not identify an age-specific HRF. Longer peak times of the HRF 0 to 2 yrs. |
3.2. Study Characteristics
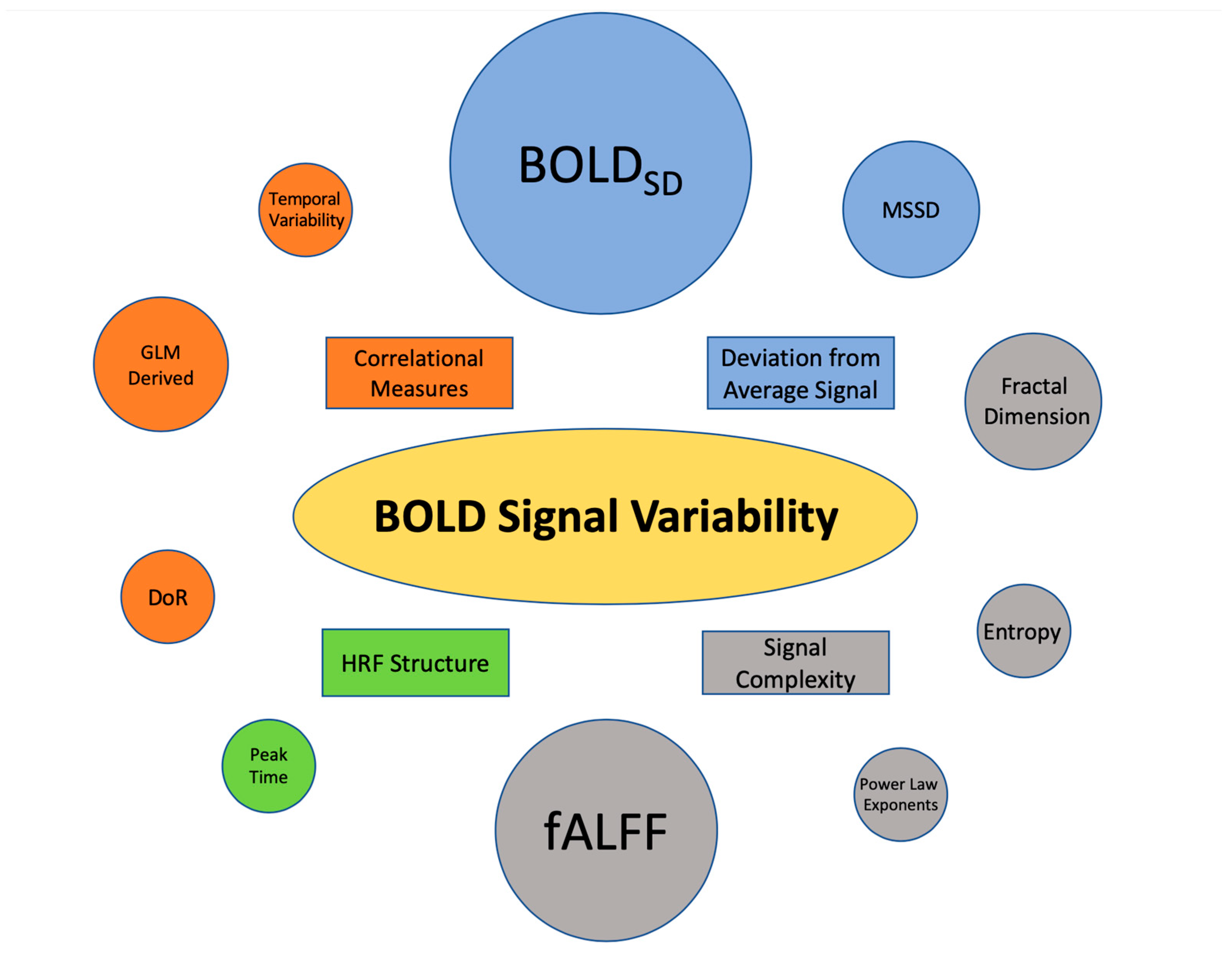
3.3. BOLD SV Metrics
3.4. Findings Associated with Deviation from the Average BOLD Signal
3.4.1. Standard Deviation of the BOLD Signal (BOLDSD)
3.4.2. Mean Successive Squared Difference (MSSD)
3.5. Findings Associated with Correlational Measures of BOLD SV
3.5.1. Temporal Variability
3.5.2. Multilinear and General Linear Model (GLM)-Derived Variance Measurement
3.5.3. Difference of Residuals
3.6. Findings Associated with Signal Complexity
3.6.1. Entropy/Sample Entropy
3.6.2. Fractal Dimensionality
3.6.3. Power-Based Metrics
3.6.4. Fractional Amplitude of Low-Frequency Fluctuation (fALFF)
3.7. Findings Associated with Characteristics of the Hemodynamic Response Function (HRF)
4. Discussion
4.1. Summary of Evidence
4.1.1. Metric Utilization
4.1.2. The Inverted U Trend and BOLD SV
4.1.3. BOLD SV Trends in Mental and Neurological Conditions
4.2. Recommendations for Clinical Applications
4.3. Future Directions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Author and Year | Title | Objectives of Included Articles | Application |
|---|---|---|---|
| Bray et al., 2017 [26] | Age-Associated Patterns in Gray Matter Volume, Cerebral Perfusion and BOLD Oscillations in Children and Adolescents | Characterize patterns in GMV, CBF, fALFF, age, and their relation to structural and functional connectivity. | Identification of structural and functional relationships in pediatric brain development |
| Easson, et al., 2019 [17] | BOLD Signal Variability and complexity in children and adolescents with and without autism spectrum disorder | Assess both BOLD variability and BOLD complexity in children and adolescents with ASD and age-matched typically developing participants. | Identification of pathophysiology of ASD |
| Thompson et al., 2021 [7] | Changes in BOLD variability are linked to the development of variable response inhibition: BOLD variability and variable response inhibition | Effect on intra-individual variability in a response inhibition task and behavioral variability across the lifespan. | Identification of response inhibition trends during development |
| Roberts et al., 2020 [18] | Creative internally directed cognition is associated with reduced BOLD variability | Identify the relationship between BOLD variability, mean BOLD Signal, and creativity tasks that required flexible thinking in various fMRI tasks. | Identify trends of BOLD SV and creativity/flexibility |
| Zöller et al., 2017 [4] | Disentangling resting-state BOLD variability and PCC functional connectivity in 22q11.2 deletion syndrome | Investigate relationships between 22q11.2 deletion syndrome associated alterations in BOLD SV and age-relationship throughout the cortex of children and adolescents. | Clinical: identify pathophysiology and symptom severity of 22q11.2 deletion and schizophrenia |
| Malins et al., 2018 [21] | Individual Differences in Reading Skill Are Related to Tiral-by-Trial Neural Activation Variability in the Reading Network | Determine whether trial-by-trial neural activation variability accounts for variance in reading skill and in what direction. | Identify trends of BOLD SV and variance in reading skill |
| Nomi et al., 2017 [16] | Moment-to-Moment BOLD Signal Variability Reflects Regional Changes in Neural Flexibility across the Lifespan | Determine regional variability trends across the lifespan using rs-fMRI data across ages 6–85. | Identify brain variability trends in healthy brain development |
| Mulligan et al., 2014 [23] | Neural correlates of inhibitory control and functional genetic variation in the dopamine D4 receptor gene | Demonstrate carriers of DRD4 (7R+) have reduced prefrontal inhibitory control than non-carriers (7R−) confirmed by locally reduced BOLD % signal change and Go/No-Go task performance. | Clinical: identify pathophysiology of genetic alterations (addition of 7 tandem repeats) of the DRD4 receptor gene |
| Zhang et al., 2016 [22] | Neural, electrophysiological, and anatomical basis of brain-network variability and its characteristic changes in mental disorders | Characterize temporal variability of the functional architecture of pediatrics with mental disorders in associated regions and networks and establish functional-structural relationships. | Clinical: identify pathophysiology and symptom severity of ADHD, ASD, and schizophrenia and identify SV trends in typically developing patients |
| Zöller et al., 2017 [29] | Psychotic symptoms influence the development of anterior cingulate BOLD variability in 22q11.2 deletion syndrome | Use the BOLD signal and BOLDSD to assess brain dynamics in patients with psychotic symptoms seen in schizophrenia. | Clinical: identify pathophysiology and symptom severity of schizophrenia |
| Dona et al., 2017 [24] | Temporal fractal analysis of the rs-BOLD signal identifies brain abnormalities in autism spectrum disorder | Utilize a model-free complexity analysis based on the fractal dimension of the rs-BOLD signal to identify BOLD SV trends in children with ASD. | Identify pathophysiology and symptom severity ASD |
| Jacobs et al., 2008 [28] | Variability of the hemodynamic response as a function of age and frequency of epileptic discharge in children with epilepsy | Identify age-related changes in the HRF after interictal epileptic discharge and identify the relationship between the number of spikes and a change in the HRF. | Clinical: Identify Pathophysiology of Epilepsy/interictal epileptic discharge in development |
| Seidel et al., 2020 [19] | Evaluation of spontaneous regional brain activity in weight-recovered anorexia nervosa | Investigate intrinsic regional brain activity differences between recAN, acAN, and healthy controls using resting-state measures of fALFF, ReHo (as reported in acAN, MSSD, DC, and VHMC (as new measures of spontaneous regional brain activity). | Clinical: Identify long-term effects and pathophysiology of recAN and patients |
| Anderson et al., 2013 [20] | Complexity of low-frequency blood oxygen level-dependent fluctuations covaries with local connectivity | Determine if BOLD fluctuations exhibit temporal complexity associated with local connectivity (ReHo), across gray matter regions utilizing power law behavior. | Identify associations between grey matter ReHo and temporal complexity |
| Dona et al., 2017 [25] | Fractal Analysis of Brain Blood Oxygenation Level Dependent (BOLD) Signals from Children with Mild Traumatic Brain Injury (mTBI) | Utilize FD to quantify local neural activity in the brain as a metric of functional changes in mTBI patients. | Clinical: Identification of pathophysiology of mTBIs. |
| Wang et al., 2021 [10] | The longitudinal relationship between BOLD signal variability changes and white matter maturation during early childhood | Investigate longitudinal relationships between BOLD SV, age, and white matter structure in early childhood. | Identify variability trends between brain region SV, structure, and age. |
| Zheng et al., 2021 [27] | Frequency-specific alterations of the resting-state BOLD signals in nocturnal enuresis: an fMRI Study | Use associations of spontaneous brain activity and scores on urinary intention-related wakefulness in 5 frequency bands to detect abnormalities of activity in local brain regions of children with nocturnal enuresis. | Identify pathophysiology of NE |
| Scoring | |||||||
|---|---|---|---|---|---|---|---|
| Reporting | External Validity | Bias | Confounding | Power | Total | % | |
| Author(s) (Year) | Items 1–10 | Items 11–13 | Items 14–20 | Items 21–26 | Item 27 * | Total/28 | Percentage |
| Bray et al., 2017 [26] | 6 | 2 | 3 | 2 | 0 | 13 | 46.4% |
| Easson et al., 2019 [17] | 7 | 1 | 3 | 3 | 0 | 14 | 50% |
| Thompson et al., 2021 [7] | 7 | 0 | 2 | 2 | 1 | 12 | 42.9% |
| Roberts et al., 2020 [18] | 7 | 0 | 3 | 2 | 0 | 12 | 42.9% |
| Zöller et al., 2017 [4] | 8 | 1 | 3 | 3 | 0 | 15 | 53.6% |
| Malins et al., 2018 [21] | 7 | 1 | 4 | 1 | 0 | 13 | 46.4% |
| Nomi et al., 2017 [16] | 7 | 1 | 2 | 2 | 0 | 12 | 42.9% |
| Mulligan et al., 2014 [23] | 7 | 1 | 3 | 3 | 0 | 14 | 50% |
| Zhang et al., 2016 [22] | 8 | 1 | 3 | 2 | 0 | 14 | 50% |
| Zöller et al., 2018 [30] | 7 | 1 | 3 | 2 | 0 | 13 | 46.4% |
| Dona et al., 2017 [24] | 7 | 1 | 3 | 2 | 1 | 14 | 50% |
| Jacobs et al., 2008 [28] | 7 | 1 | 3 | 1 | 0 | 12 | 42.9% |
| Seidel et al., 2020 [19] | 8 | 0 | 3 | 3 | 1 | 15 | 53.6% |
| Anderson et al., 2013 [20] | 6 | 2 | 3 | 1 | 0 | 12 | 42.9% |
| Dona et al., 2017 [25] | 7 | 2 | 3 | 3 | 0 | 15 | 53.6% |
| Wang et al., 2021 [10] | 7 | 2 | 3 | 3 | 0 | 15 | 53.6% |
| Zheng et al., 2021 [27] | 7 | 2 | 3 | 3 | 0 | 15 | 53.6% |
| Total | 120/187 | 19/51 | 50/119 | 38/102 | 3/27 | 230/486 | 47.3% |
| % | 64.2% | 37.3% | 42.0% | 37.3% | 11.1% | 47.3% | X |
References
- Garrett, D.D.; Kovacevic, N.; McIntosh, A.R.; Grady, C.L. Blood Oxygen Level-Dependent Signal Variability Is More than Just Noise. J. Neurosci. 2010, 30, 4914–4921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spalatro, A.V.; Amianto, F.; Huang, Z.; D’Agata, F.; Bergui, M.; Abbate Daga, G.; Fassino, S.; Northoff, G. Neuronal Variability of Resting State Activity in Eating Disorders: Increase and Decoupling in Ventral Attention Network and Relation with Clinical Symptoms. Eur. Psychiatry 2019, 55, 10–17. [Google Scholar] [CrossRef]
- Nomi, J.S.; Schettini, E.; Voorhies, W.; Bolt, T.S.; Heller, A.S.; Uddin, L.Q. Resting-State Brain Signal Variability in Prefrontal Cortex Is Associated with ADHD Symptom Severity in Children. Front. Hum. Neurosci. 2018, 12, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zöller, D.; Schaer, M.; Scariati, E.; Padula, M.C.; Eliez, S.; Van De Ville, D. Disentangling Resting-State BOLD Variability and PCC Functional Connectivity in 22q11.2 Deletion Syndrome. Neuroimage 2017, 149, 85–97. [Google Scholar] [CrossRef]
- Jones, T.B.; Bandettini, P.A.; Birn, R.M. Integration of Motion Correction and Physiological Noise Regression in FMRI. NeuroImage 2008, 42, 582–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grady, C.L.; Garrett, D.D. Understanding Variability in the BOLD Signal and Why It Matters for Aging. Brain Imaging Behav. 2014, 8, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.; Schel, M.A.; Steinbeis, N. Changes in BOLD Variability Are Linked to the Development of Variable Response Inhibition. Neuroimage 2021, 228, 117691. [Google Scholar] [CrossRef]
- Garrett, D.D.; Samanez-Larkin, G.R.; MacDonald, S.W.S.; Lindenberger, U.; McIntosh, A.R.; Grady, C.L. Moment-to-Moment Brain Signal Variability: A next Frontier in Human Brain Mapping? Neurosci. Biobehav. Rev. 2013, 37, 610–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mella, N.; de Ribaupierre, S.; Eagleson, R.; de Ribaupierre, A. Cognitive Intraindividual Variability and White Matter Integrity in Aging. Sci. World J. 2013, 2013, 350623. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ghaderi, A.; Long, X.; Reynolds, J.E.; Lebel, C.; Protzner, A.B. The Longitudinal Relationship between BOLD Signal Variability Changes and White Matter Maturation during Early Childhood. NeuroImage 2021, 242, 118448. [Google Scholar] [CrossRef]
- Pur, D.R.; Eagleson, R.A.; de Ribaupierre, A.; Mella, N.; de Ribaupierre, S. Moderating Effect of Cortical Thickness on BOLD Signal Variability Age-Related Changes. Front. Aging Neurosci. 2019, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Pur, D.; Eagleson, R.; Ribaupierre, S. de C.4 Distinct BOLD Signal Variability Changes in Temporal and Occipital Cortices in Pediatric Epilepsy. Can. J. Neurol. Sci. 2022, 49, S7. [Google Scholar] [CrossRef]
- Zou, Q.-H.; Zhu, C.-Z.; Yang, Y.; Zuo, X.-N.; Long, X.-Y.; Cao, Q.-J.; Wang, Y.-F.; Zang, Y.-F. An Improved Approach to Detection of Amplitude of Low-Frequency Fluctuation (ALFF) for Resting-State FMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The Feasibility of Creating a Checklist for the Assessment of the Methodological Quality Both of Randomised and Non-Randomised Studies of Health Care Interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomi, J.S.; Bolt, T.S.; Ezie, C.E.C.; Uddin, L.Q.; Heller, A.S. Moment-to-Moment BOLD Signal Variability Reflects Regional Changes in Neural Flexibility across the Lifespan. J. Neurosci. 2017, 37, 5539–5548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easson, A.K.; McIntosh, A.R. BOLD Signal Variability and Complexity in Children and Adolescents with and without Autism Spectrum Disorder. Dev. Cogn. Neurosci. 2019, 36, 100630. [Google Scholar] [CrossRef]
- Roberts, R.P.; Grady, C.L.; Addis, D.R. Creative, Internally-Directed Cognition Is Associated with Reduced BOLD Variability. NeuroImage 2020, 219, 116758. [Google Scholar] [CrossRef] [PubMed]
- Seidel, M.; Geisler, D.; Borchardt, V.; King, J.A.; Bernardoni, F.; Jaite, C.; Roessner, V.; Calhoun, V.; Walter, M.; Ehrlich, S. Evaluation of Spontaneous Regional Brain Activity in Weight-Recovered Anorexia Nervosa. Transl. Psychiatry 2020, 10, 395. [Google Scholar] [CrossRef]
- Anderson, J.S.; Zielinski, B.A.; Nielsen, J.A.; Ferguson, M.A. Complexity of Low-frequency Blood Oxygen Level-dependent Fluctuations Covaries with Local Connectivity. Hum. Brain Mapp. 2013, 35, 1273–1283. [Google Scholar] [CrossRef]
- Malins, J.G.; Pugh, K.R.; Buis, B.; Frost, S.J.; Hoeft, F.; Landi, N.; Mencl, W.E.; Kurian, A.; Staples, R.; Molfese, P.J.; et al. Individual Differences in Reading Skill Are Related to Trial-by-Trial Neural Activation Variability in the Reading Network. J. Neurosci. 2018, 38, 2981–2989. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cheng, W.; Liu, Z.; Zhang, K.; Lei, X.; Yao, Y.; Becker, B.; Liu, Y.; Kendrick, K.M.; Lu, G.; et al. Neural, Electrophysiological and Anatomical Basis of Brain-Network Variability and Its Characteristic Changes in Mental Disorders. Brain 2016, 139, 2307–2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulligan, R.C.; Kristjansson, S.D.; Reiersen, A.M.; Parra, A.S.; Anokhin, A.P. Neural Correlates of Inhibitory Control and Functional Genetic Variation in the Dopamine D4 Receptor Gene. Neuropsychologia 2014, 62, 306–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dona, O.; Hall, G.B.; Noseworthy, M.D. Temporal Fractal Analysis of the Rs-BOLD Signal Identifies Brain Abnormalities in Autism Spectrum Disorder. PLoS ONE 2017, 12, e0190081. [Google Scholar] [CrossRef] [Green Version]
- Dona, O.; Noseworthy, M.D.; DeMatteo, C.; Connolly, J.F. Fractal Analysis of Brain Blood Oxygenation Level Dependent (BOLD) Signals from Children with Mild Traumatic Brain Injury (MTBI). PLoS ONE 2017, 12, e0169647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, S. Age-associated Patterns in Gray Matter Volume, Cerebral Perfusion and BOLD Oscillations in Children and Adolescents. Hum. Brain Mapp. 2017, 38, 2398–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Sun, J.; Lv, Y.; Wang, M.; Du, X.; Jia, X.; Ma, J. Frequency-Specific Alterations of the Resting-State BOLD Signals in Nocturnal Enuresis: An FMRI Study. Sci. Rep. 2021, 11, 12042. [Google Scholar] [CrossRef]
- Jacobs, J.; Hawco, C.; Kobayashi, E.; Boor, R.; LeVan, P.; Stephani, U.; Siniatchkin, M.; Gotman, J. Variability of the Hemodynamic Response as a Function of Age and Frequency of Epileptic Discharge in Children with Epilepsy. Neuroimage 2008, 40, 601–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zöller, D.; Padula, M.C.; Sandini, C.; Schneider, M.; Scariati, E.; Van De Ville, D.; Schaer, M.; Eliez, S. Psychotic Symptoms Influence the Development of Anterior Cingulate BOLD Variability in 22q11.2 Deletion Syndrome. Schizophr. Res. 2018, 193, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Lake, D.E.; Moorman, J.R. Sample Entropy. In Methods in Enzymology; Numerical Computer Methods, Part E.; Academic Press: Cambridge, MA, USA, 2004; Volume 384, pp. 172–184. [Google Scholar]
- Raghavendra, B.S.; Dutt, D.N. Computing Fractal Dimension of Signals Using Multiresolution Box-Counting Method. Int. J. Inf. Math. Sci. 2010, 6, 50–65. [Google Scholar] [CrossRef]
- Castiglioni, P.; Faes, L.; Valenza, G. Assessing Complexity in Physiological Systems through Biomedical Signals Analysis. Entropy 2020, 22, 1005. [Google Scholar] [CrossRef]
- Garrett, D.D.; Kovacevic, N.; McIntosh, A.R.; Grady, C.L. The Importance of Being Variable. J. Neurosci. 2011, 31, 4496–4503. [Google Scholar] [CrossRef] [Green Version]
- Glass, L.; Mackey, M.C. From Clocks to Chaos: The Rhythms of Life; Princeton University Press: Princeton, NJ, USA, 1988; ISBN 978-0-691-08496-1. [Google Scholar]
- Amaral, L.A.N.; Díaz-Guilera, A.; Moreira, A.A.; Goldberger, A.L.; Lipsitz, L.A. Emergence of Complex Dynamics in a Simple Model of Signaling Networks. Proc. Natl. Acad. Sci. USA 2004, 101, 15551–15555. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.N.; Hausdorff, J.M.; Ivanov, P.C.; Peng, C.-K.; Stanley, H.E. Fractal Dynamics in Physiology: Alterations with Disease and Aging. Proc. Natl. Acad. Sci. USA 2002, 99, 2466–2472. [Google Scholar] [CrossRef]
- Li, S.-C.; Lindenberger, U.; Bäckman, L. Dopaminergic Modulation of Cognition across the Life Span. Neurosci. Biobehav. Rev. 2010, 34, 625–630. [Google Scholar] [CrossRef]
- Guitart-Masip, M.; Salami, A.; Garrett, D.; Rieckmann, A.; Lindenberger, U.; Bäckman, L. BOLD Variability Is Related to Dopaminergic Neurotransmission and Cognitive Aging. Cereb. Cortex 2016, 26, 2074–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, A.S.; Shah, V.D.; Baker, S.L.; Vogel, J.W.; O’Neil, J.P.; Janabi, M.; Schwimmer, H.D.; Marks, S.M.; Jagust, W.J. Aging Affects Dopaminergic Neural Mechanisms of Cognitive Flexibility. J. Neurosci. 2016, 36, 12559–12569. [Google Scholar] [CrossRef] [Green Version]
- Crespi, B.; Badcock, C. Psychosis and Autism as Diametrical Disorders of the Social Brain. Behav. Brain Sci. 2008, 31, 241–261, discussion 261-320. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Hocke, L.M.; Frederick, B.B. Low Frequency Systemic Hemodynamic “Noise” in Resting State BOLD FMRI: Characteristics, Causes, Implications, Mitigation Strategies, and Applications. Front. Neurosci. 2019, 13, 787. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Cunningham, J.P.; Glover, G.H. Influence of Heart Rate on the BOLD Signal: The Cardiac Response Function. NeuroImage 2009, 44, 857–869. [Google Scholar] [CrossRef] [Green Version]
- D’Esposito, M.; Deouell, L.Y.; Gazzaley, A. Alterations in the BOLD FMRI Signal with Ageing and Disease: A Challenge for Neuroimaging. Nat. Rev. Neurosci. 2003, 4, 863–872. [Google Scholar] [CrossRef]
- Howseman, A.; Zeki, S.; Josephs, O.; Henson, R.N.A. Event-Related Functional Magnetic Resonance Imaging: Modelling, Inference and Optimization. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1999, 354, 1215–1228. [Google Scholar] [CrossRef] [Green Version]
- Logothetis, N.K.; Pauls, J.; Augath, M.; Trinath, T.; Oeltermann, A. Neurophysiological Investigation of the Basis of the FMRI Signal. Nature 2001, 412, 150–157. [Google Scholar] [CrossRef]
- Tong, Y.; Hocke, L.M.; Fan, X.; Janes, A.C.; deB Frederick, B. Can Apparent Resting State Connectivity Arise from Systemic Fluctuations? Front. Hum. Neurosci. 2015, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Hadders-Algra, M. Early Diagnostics and Early Intervention in Neurodevelopmental Disorders—Age-Dependent Challenges and Opportunities. J. Clin. Med. 2021, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Alavash, M.; Lim, S.-J.; Thiel, C.; Sehm, B.; Deserno, L.; Obleser, J. Dopaminergic Modulation of Hemodynamic Signal Variability and the Functional Connectome during Cognitive Performance. NeuroImage 2018, 172, 341–356. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinatolo, M.F.; Pur, D.R.; Eagleson, R.; de Ribaupierre, S. The Role of Blood Oxygen Level Dependent Signal Variability in Pediatric Neuroscience: A Systematic Review. Life 2023, 13, 1587. https://doi.org/10.3390/life13071587
Dinatolo MF, Pur DR, Eagleson R, de Ribaupierre S. The Role of Blood Oxygen Level Dependent Signal Variability in Pediatric Neuroscience: A Systematic Review. Life. 2023; 13(7):1587. https://doi.org/10.3390/life13071587
Chicago/Turabian StyleDinatolo, Michael F., Daiana Roxana Pur, Roy Eagleson, and Sandrine de Ribaupierre. 2023. "The Role of Blood Oxygen Level Dependent Signal Variability in Pediatric Neuroscience: A Systematic Review" Life 13, no. 7: 1587. https://doi.org/10.3390/life13071587
APA StyleDinatolo, M. F., Pur, D. R., Eagleson, R., & de Ribaupierre, S. (2023). The Role of Blood Oxygen Level Dependent Signal Variability in Pediatric Neuroscience: A Systematic Review. Life, 13(7), 1587. https://doi.org/10.3390/life13071587






