Physiologically Aggregated LacZ Applied in Trehalose Galactosylation in a Recycled Batch Mode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
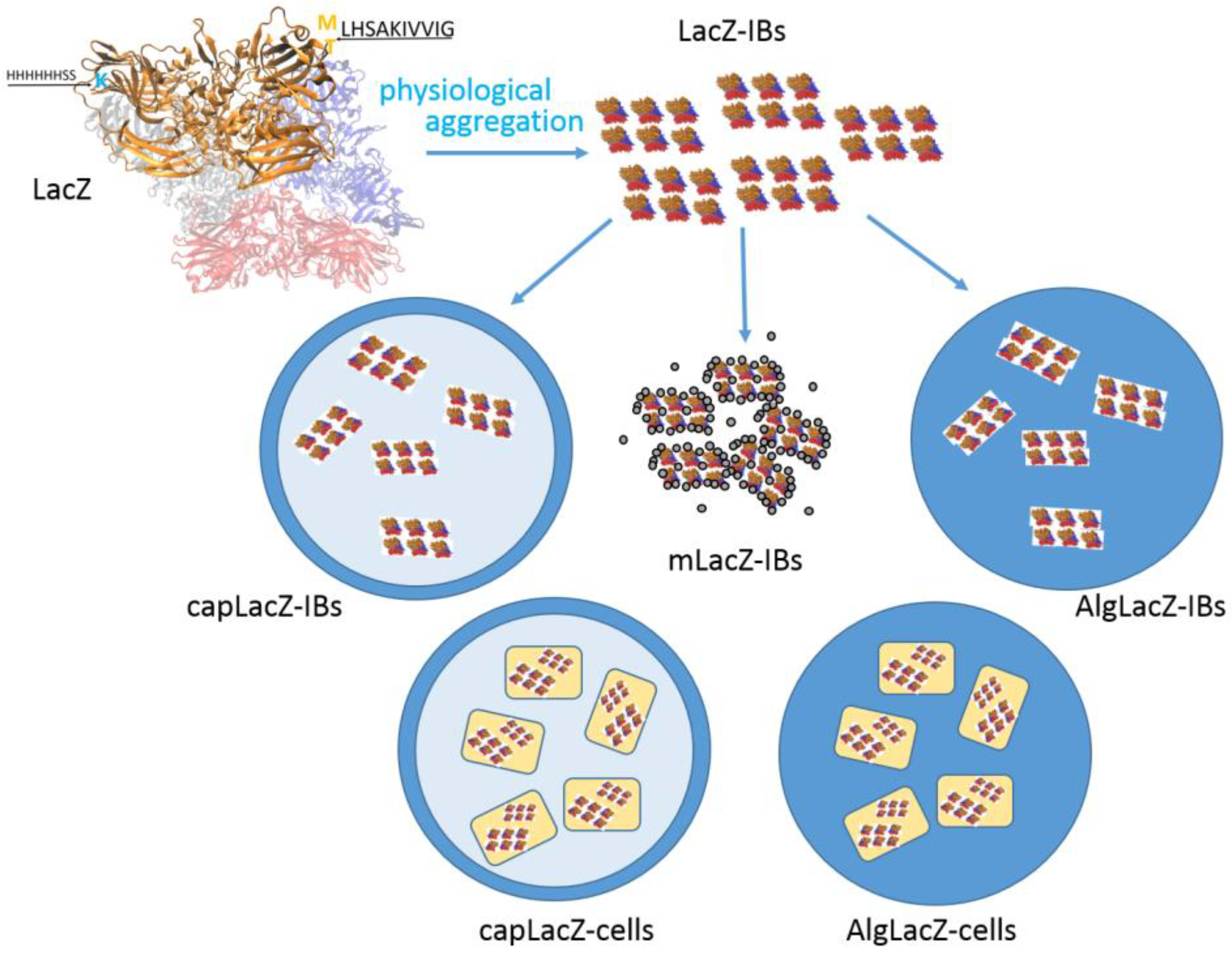
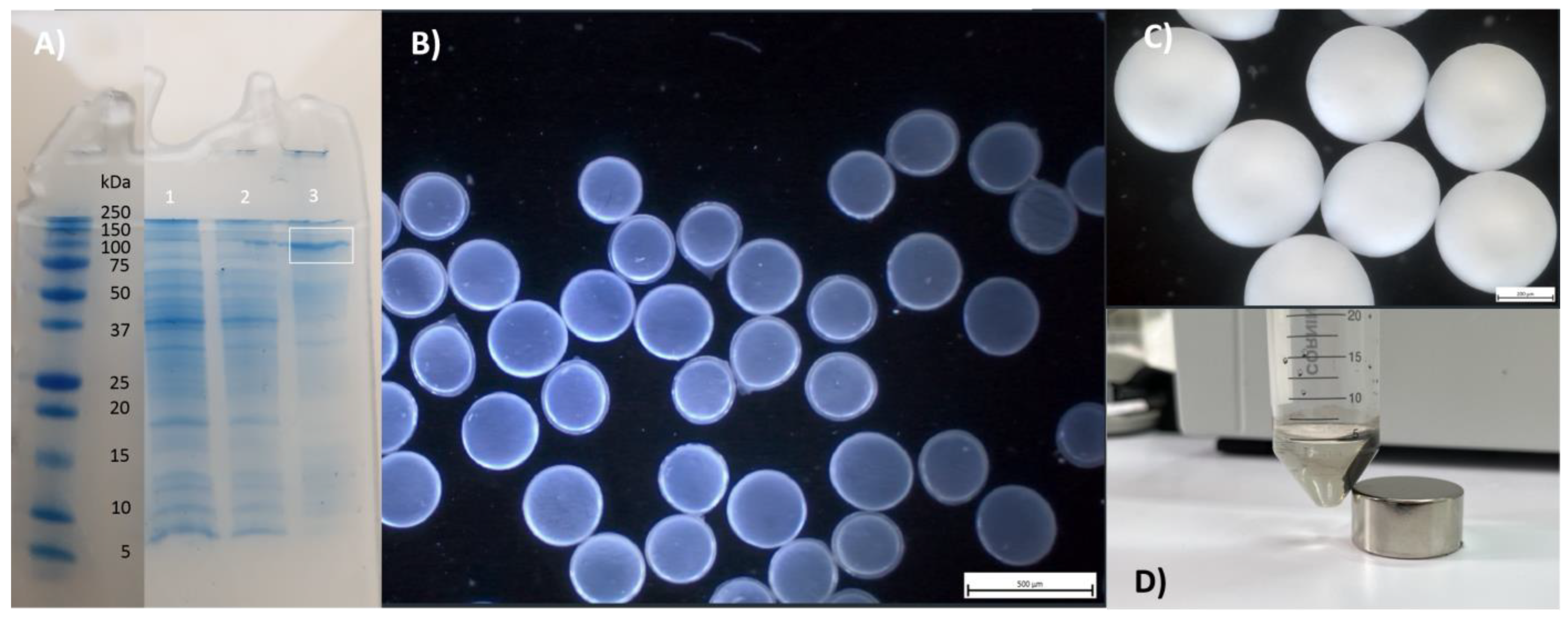
2.2. Cloning, Expression and Isolation of LacZ-IBs
2.3. Entrapment of LacZ-IBs and Whole Cells into Alg Beads
2.4. Encapsulation of LacZ-IBs and Whole Cells into Alg/CS/PMCG Capsules
2.5. Magnetic Modification of LacZ-IBs
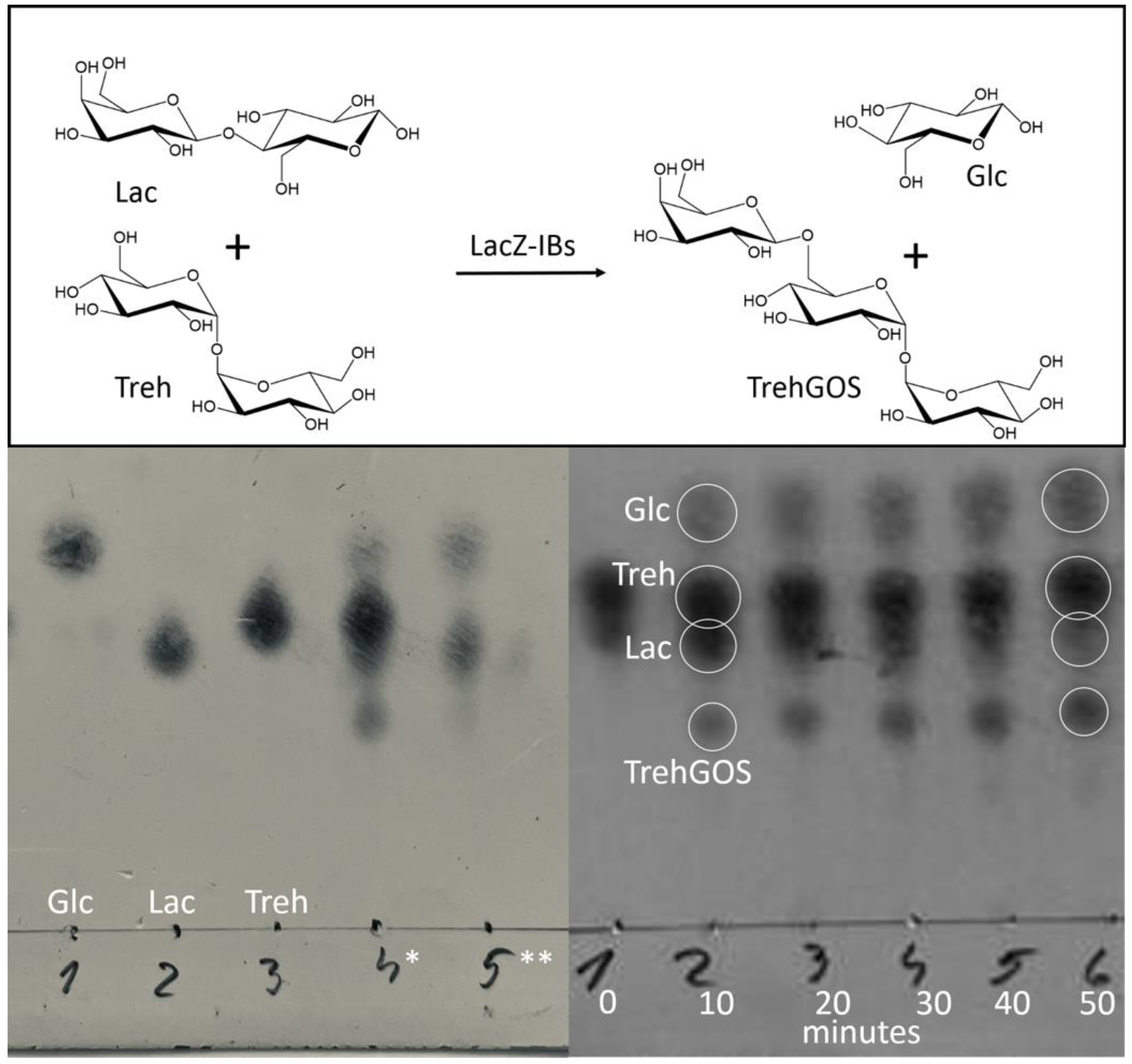
2.6. Transgalactosylation by LacZ-IBs and TLC-Monitoring
| LacZ | β-galactosidase enzyme |
| LacZ-IBs | active inclusion bodies of LacZ |
| free LacZ-IBs | LacZ-IBs recovered from the reaction mixture by centrifugation |
| AlgLacZ-IBs | LacZ-IBs entrapped into calcium alginate beads (Alg) |
| capLacZ-IBs | LacZ-IBs encapsulated into Alg/CS/PMCG capsules |
| mLacZ-IBs | magnetized LacZ-IBs |
| AlgLacZ-cells | whole cells expressing LacZ-IBs entrapped into Alg-beads |
| capLacZ-cells | whole cells expressing LacZ-IBs encapsulated into Alg/CS/PMCG-capsules |
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juers, D.H.; Matthews, B.W.; Huber, R.E. LacZ β-galactosidase: Structure and function of an enzyme of historical and molecular biological importance. Protein Sci. 2012, 21, 1792–1807. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.V.; Sambyal, K. β-Galactosidase as an industrial enzyme: Production and potential. Chem. Pap. 2023, 77, 11–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhu, L.; He, P.; Wang, Q. High sensitivity detection of Escherichia coli based on the measurement of β-galactosidase activity by microchip capillary electrophoresis combined with field-amplified sample injection. Anal. Methods 2019, 11, 1558–1565. [Google Scholar] [CrossRef]
- Forsgård, R.A. Lactose digestion in humans: Intestinal lactase appears to be constitutive whereas the colonic microbiome is adaptable. Am. J. Clin. Nutr. 2019, 110, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Hu, W.; Liu, W.; Zhao, L.; Huang, D.; Liu, X.; Chan, H.; Zhang, Y.; Zeng, J.; Coker, O.O.; et al. Streptococcus thermophilus inhibits colorectal tumorigenesis through secreting β-galactosidase. Gastroenterology 2021, 160, 1179–1193.e14. [Google Scholar] [CrossRef]
- Ambrogi, V.; Bottacini, F.; Cao, L.; Kuipers, B.; Schoterman, M.; van Sinderen, D. Galacto-oligosaccharides as infant prebiotics: Production, application, bioactive activities and future perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Maráz, A.; Kovács, Z.; Benjamins, E.; Pázmándi, M. Recent developments in microbial production of high-purity galacto-oligosaccharides. World J. Microbiol. Biotechnol. 2022, 38, 95. [Google Scholar] [CrossRef]
- Vera, C.; Córdova, A.; Aburto, C.; Guerrero, C.; Suárez, S.; Illanes, A. Synthesis and purification of galacto-oligosaccharides: State of the art. World J. Microbiol. Biotechnol. 2016, 32, 197. [Google Scholar] [CrossRef]
- Huerta, L.M.; Vera, C.; Guerrero, C.; Wilson, L.; Illanes, A. Synthesis of galacto-oligosaccharides at very high lactose concentrations with immobilized β-galactosidases from Aspergillus oryzae. Process Biochem. 2011, 46, 245–252. [Google Scholar] [CrossRef]
- Benjamins, E.; Boxem, L.; KleinJan-Noeverman, J.; Broekhuis, T.A. Assessment of repetitive batch-wise synthesis of galacto-oligosaccharides from lactose slurry using immobilised β-galactosidase from Bacillus circulans. Int. Dairy J. 2014, 38, 160–168. [Google Scholar] [CrossRef]
- Albayrak, N.; Yang, S.T. Production of galacto-oligosaccharides from lactose by aspergillus oryzae beta-galactosidase immobilized on cotton cloth. Biotechnol. Bioeng. 2002, 77, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Damin, B.I.S.; Kovalski, F.C.; Fischer, J.; Piccin, J.S.; Dettmer, A. Challenges and perspectives of the β-galactosidase enzyme. Appl. Microbiol. Biotechnol. 2021, 105, 5281–5298. [Google Scholar] [CrossRef]
- Katrolia, P.; Liu, X.; Li, G.; Kopparapu, N.K. Enhanced properties and lactose hydrolysis efficiencies of food-grade β-galactosidases immobilized on various supports: A comparative approach. Appl. Biochem. Biotechnol. 2019, 188, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Richmond, M.L.; Gray, J.I.; Stine, C.M. Beta-galactosidase: Review of recent research related to technological application, nutritional concerns, and immobilization. J. Dairy Sci. 1981, 64, 1759–1771. [Google Scholar] [CrossRef]
- Movahedpour, A.; Ahmadi, N.; Ghalamfarsa, F.; Ghesmati, Z.; Khalifeh, M.; Maleksabet, A.; Shabaninejad, Z.; Taheri-Anganeh, M.; Savardashtaki, A. β-Galactosidase: From its source and applications to its recombinant form. Biotechnol. Appl. Biochem. 2022, 69, 612–628. [Google Scholar] [CrossRef]
- Ölçücü, G.; Klaus, O.; Jaeger, K.; Drepper, T.; Krauss, U. Emerging solutions for in vivo biocatalyst immobilization: Tailor-made catalysts for industrial biocatalysis. ACS Sustain. Chem. Eng. 2021, 9, 8919–8945. [Google Scholar] [CrossRef]
- Marco, A.; Ferrer-Miralles, N.; Garcia-Fruitos, E.; Mitraki, A.; Peternel, S.; Rinas, U.; Trujillo-Roldan, A.; Valdez-Cruz, N.A.; Vazquez, E.; Villaverde, A. Bacterial inclusion bodies are industrially exploitable amyloids. FEMS Microbiol. Rev. 2019, 43, 63–72. [Google Scholar] [CrossRef]
- Belková, M.; Köszagová, R.; Nahálka, J. Active Inclusion Bodies: The Unexpected Journey. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e5951. [Google Scholar] [CrossRef]
- Köszagová, R.; Nahálka, J. Inclusion bodies in biotechnology. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 1191–1196. [Google Scholar] [CrossRef]
- Köszagová, R.; Hrabárová, E.; Achbergerová, L.; Nahálka, J. Insoluble protein applications: The use of bacterial inclusion bodies as biocatalysts. Methods Mol. Biol. 2022, 2406, 501–515. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Boland, B.; Yu, W.H.; Corti, O.; Mollereau, B.; Henriques, A.; Bezard, E.; Pastores, G.M.; Rubinsztein, D.C.; Nixon, R.A.; Duchen, M.R.; et al. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2018, 17, 660–688. [Google Scholar] [CrossRef]
- Nahalka, J. 1-L transcription in Alzheimer’s disease. Curr. Issues Mol. Biol. 2022, 44, 3533–3551. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoon, Y.; Lee, S. Mechanism of neuroprotection by trehalose: Controversy surrounding autophagy induction. Cell Death Dis. 2018, 9, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifeh, M.; Barreto, G.; Sahebkar, A. Therapeutic potential of trehalose in neurodegenerative diseases: The knowns and unknowns. Neural Regen. Res. 2021, 16, 2026–2027. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Tapia, H.; Goddard, J.M.; Gibney, P.A. Trehalose and its applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5004–5037. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Gibney, P.A. Dietary trehalose as a bioactive nutrient. Nutrients 2023, 15, 1393. [Google Scholar] [CrossRef]
- Walmagh, M.; Zhao, R.; Desmet, T. Trehalose analogues: Latest insights in properties and biocatalytic production. Int. J. Mol. Sci. 2015, 16, 13729–13745. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Lee, K.; Han, N.; Park, K.; Lee, S. Enzymatic synthesis and characterization of galactosyl trehalose trisaccharides. Food Sci. Biotechnol. 2007, 16, 127–132. [Google Scholar]
- Gallego-Lobillo, P.; Doyagüez, E.G.; Jimeno, M.L.; Villamiel, M.; Hernandez-Hernandez, O. Enzymatic synthesis and structural characterization of novel trehalose-based oligosaccharides. J. Agric. Food Chem. 2021, 69, 12541–12553. [Google Scholar] [CrossRef]
- Hrabarova, E.; Belkova, M.; Koszagova, R.; Nahalka, J. Pull-down into active inclusion bodies and their application in the detection of (poly)-phosphates and metal-ions. Front. Bioeng. Biotechnol. 2022, 10, 833192. [Google Scholar] [CrossRef] [PubMed]
- Nahálka, J.; Vikartovská, A.; Hrabárová, E. A crosslinked inclusion body process for sialic acid synthesis. J. Biotechnol. 2008, 134, 146–153. [Google Scholar] [CrossRef]
- Flores, S.S.; Clop, P.D.; Barra, J.L.; Argaraña, C.E.; Perillo, M.A.; Nolan, V.; Sánchez, J.M. His-tag β-galactosidase supramolecular performance. Biophys. Chem. 2022, 281, 106739. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.S.; Nolan, V.; Perillo, M.A.; Sánchez, J.M. Superactive β-galactosidase inclusion bodies. Colloids Surf. B Biointerfaces 2019, 173, 769–775. [Google Scholar] [CrossRef]
- Sanchez, J.M.; López-Laguna, H.; Serna, N.; Unzueta, U.; Clop, P.D.; Villaverde, A.; Vazquez, E. Engineering the performance of artificial inclusion bodies built of catalytic β-galactosidase. ACS Sustain. Chem. Eng. 2021, 9, 2552–2558. [Google Scholar] [CrossRef]
- Mobayed, F.H.; Nunes, J.C.; Gennari, A.; de Andrade, B.C.; Ferreira, M.L.V.; Pauli, P.; Renard, G.; Chies, J.M.; Volpato, G.; Volken de Souza, C.F. Effect of by-products from the dairy industry as alternative inducers of recombinant β-galactosidase expression. Biotechnol. Lett. 2021, 43, 589–599. [Google Scholar] [CrossRef]
- Smidsrød, O.; Skjåk-Bræk, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021, 118, 106782. [Google Scholar] [CrossRef]
- Prüße, U.; Dalluhn, J.; Breford, J.; Vorlop, K.D. Production of spherical beads by Jet Cutting. Chem. Eng. Technol. 2000, 23, 1105–1110. [Google Scholar] [CrossRef]
- Lacík, I.; Briššová, M.; Anilkumar, A.V.; Powers, A.C.; Wang, T. New capsule with tailored properties for the encapsulation of living cells. J. Biomed. Mater. Res. 1998, 39, 52–60. [Google Scholar] [CrossRef]
- Nahalka, J.; Dib, I.; Nidetzky, B. Encapsulation of trigonopsis variabilis D-amino acid oxidase and fast comparison of the operational stabilities of free and immobilized preparations of the enzyme. Biotechnol. Bioeng. 2008, 99, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, A.V.; Lacik, I.; Wang, T.G. A novel reactor for making uniform capsules. Biotechnol. Bioeng. 2001, 75, 581–589. [Google Scholar] [CrossRef]
- Koszagova, R.; Krajcovic, T.; Palencarova-Talafova, K.; Patoprsty, V.; Vikartovska, A.; Pospiskova, K.; Safarik, I.; Nahalka, J. Magnetization of active inclusion bodies: Comparison with centrifugation in repetitive biotransformations. Microb. Cell Fact. 2018, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Estel, L.; Poux, M.; Benamara, N.; Polaert, I. Continuous flow-microwave reactor: Where are we? Chem. Eng Process. Process Intensif. 2017, 113, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Bučko, M.; Vikartovská, A.; Gemeiner, P.; Lacík, I.; Kolláriková, G.; Marison, I.W. Nocardia tartaricans cells immobilized in sodium alginate–cellulose sulfate–poly(methylene-co-guanidine)capsules: Mechanical resistance and operational stability. J. Chem. Technol. Biotechnol. 2006, 81, 500–504. [Google Scholar] [CrossRef]
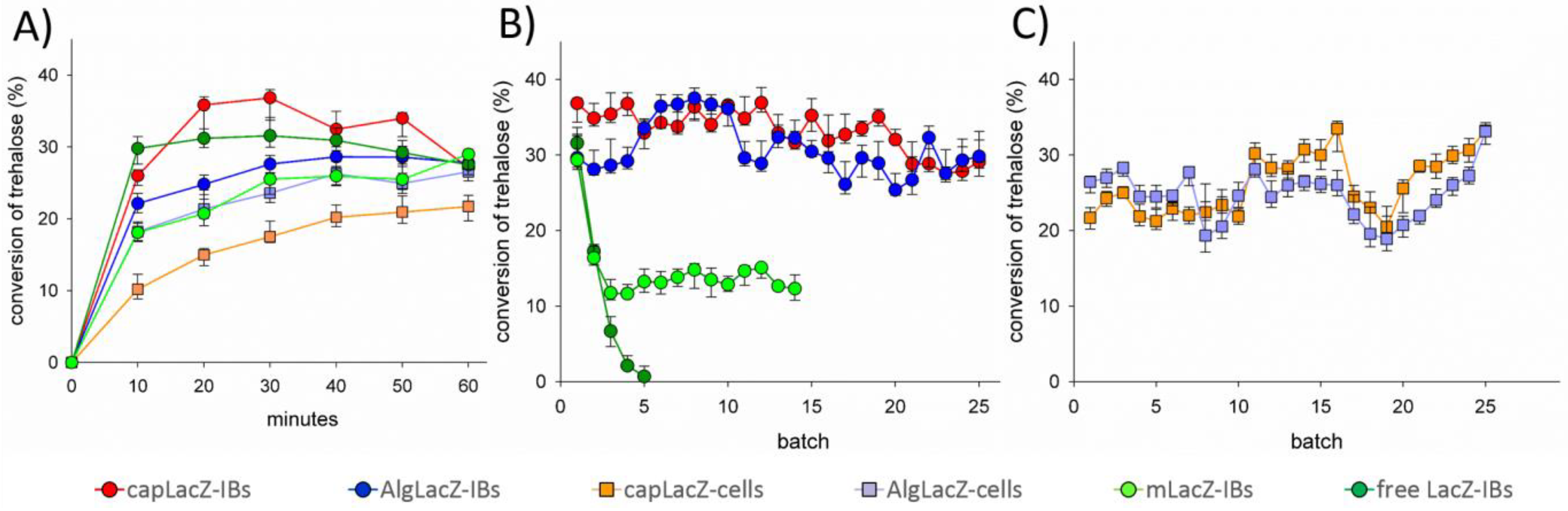
| Biocatalyst | Cells 1 (mg) | LacZ-IBs 1 (mg) | Max Treh Conversion (%) |
|---|---|---|---|
| free LacZ-IBs | 1.00 | 0.255 | 32 |
| AlgLacZ-IBs | 2.50 | 0.638 | 29 |
| capLacZ-IBs | 1.25 | 0.319 | 37 |
| mLacZ-IBs | 2.50 | 0.638 | 26 |
| AlgLacZ-cells | 5.00 | 1.275 | 25 |
| capLacZ-cells | 5.00 | 1.275 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkova, M.; Janegova, T.; Hrabarova, E.; Nahalka, J. Physiologically Aggregated LacZ Applied in Trehalose Galactosylation in a Recycled Batch Mode. Life 2023, 13, 1619. https://doi.org/10.3390/life13081619
Belkova M, Janegova T, Hrabarova E, Nahalka J. Physiologically Aggregated LacZ Applied in Trehalose Galactosylation in a Recycled Batch Mode. Life. 2023; 13(8):1619. https://doi.org/10.3390/life13081619
Chicago/Turabian StyleBelkova, Martina, Tatiana Janegova, Eva Hrabarova, and Jozef Nahalka. 2023. "Physiologically Aggregated LacZ Applied in Trehalose Galactosylation in a Recycled Batch Mode" Life 13, no. 8: 1619. https://doi.org/10.3390/life13081619
APA StyleBelkova, M., Janegova, T., Hrabarova, E., & Nahalka, J. (2023). Physiologically Aggregated LacZ Applied in Trehalose Galactosylation in a Recycled Batch Mode. Life, 13(8), 1619. https://doi.org/10.3390/life13081619







