Regulatory T Cell-Targeted Immunomodulatory Therapy for Long-Term Clinical Improvement of Atopic Dermatitis: Hypotheses and Perspectives
Abstract
:1. Introduction
2. Unmet Needs of Patients with AD
3. Hypothesis on the Pathogenesis of AD
4. Previous Reports on the Long-Term Clinical Improvement of AD
5. Five Questions and Three Hypotheses on the Regulatory T Cell-Targeted Immunomodulatory Strategies to Achieve a Long-Term Clinical Improvement of AD
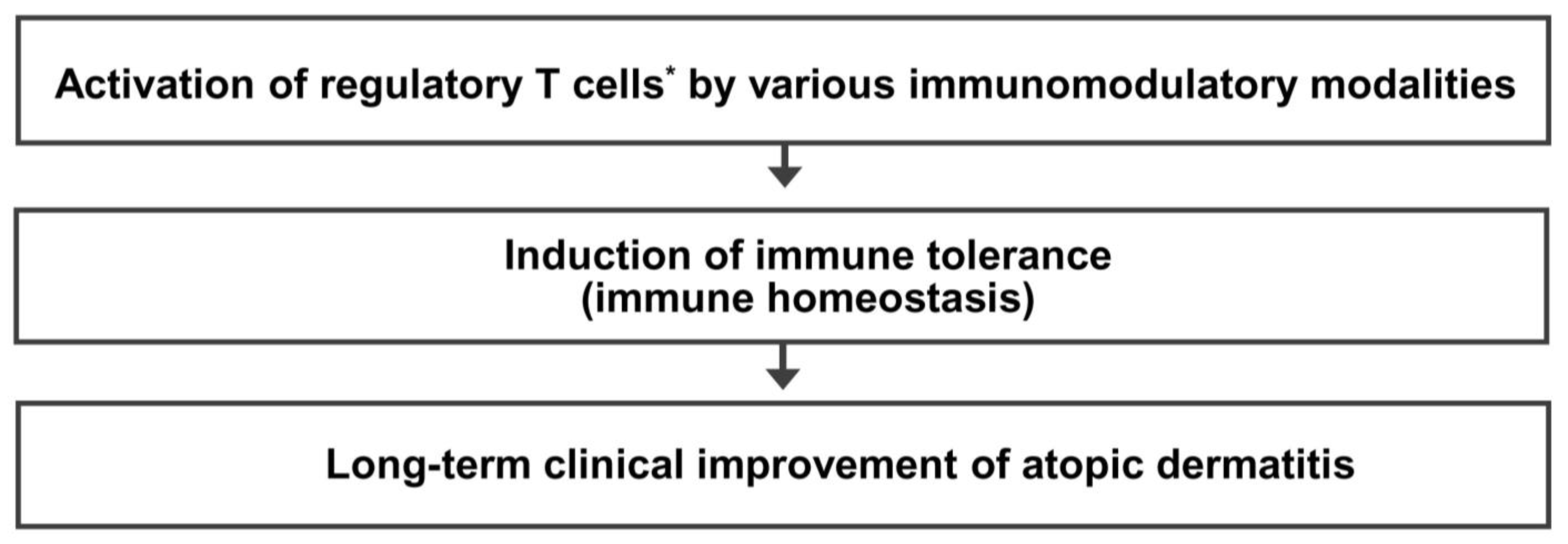
6. Mechanism of Immune Tolerance and Rationale of Regulatory T Cell-Targeted Immunomodulatory Therapy for AD
7. Immunomodulatory Strategies Activating Regulatory T Cells for AD: In Vivo Activation
7.1. Allergen Immunotherapy (Activation of Allergen-Specific Tr1 Cells)
7.2. Microbial Therapy
7.3. Vitamin D
7.4. Polyvalent Human IgG from Multiple Healthy Blood Donors
7.5. Intramuscular Administration of Autologous Polyvalent IgG
7.6. Monoclonal Antibodies to Antigens on the Surface of T Cells or Antigen-Presenting Cells
7.7. Other Chemical Candidates for Regulatory T Cell-Targeted Therapy of AD
7.7.1. Sirolimus
7.7.2. Metformin
7.7.3. Butyrate
7.8. Adoptive Cell Therapy with Ex Vivo Expanded Regulatory T Cells
7.8.1. Autologous Polyclonal Regulatory T Cells
7.8.2. Autologous Antigen-Stimulated Regulatory T Cells
7.8.3. Genetically Engineered Regulatory T Cells
7.8.4. Limitations of Adoptive Cell Therapy with Regulatory T Cells
8. Combinations of Different Modalities Activating Regulatory T Cells
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wollenberg, A.; Christen-Zach, S.; Taieb, A.; Paul, C.; Thyssen, J.P.; de Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.J.; et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2717–2744. [Google Scholar] [CrossRef]
- Ratchataswan, T.; Banzon, T.M.; Thyssen, J.P.; Weidinger, S.; Guttman-Yassky, E.; Phipatanakul, W. Biologics for Treatment of Atopic Dermatitis: Current Status and Future Prospect. J. Allergy Clin. Immunol. Pract. 2021, 9, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Nahm, D.H. Personalized Immunomodulatory Therapy for Atopic Dermatitis: An Allergist’s View. Ann. Dermatol. 2015, 27, 355–363. [Google Scholar] [CrossRef]
- Wang, F.P.; Tang, X.J.; Wei, C.Q.; Xu, L.R.; Mao, H.; Luo, F.M. Dupilumab treatment in moderate-to-severe atopic dermatitis: A systematic review and meta-analysis. J. Dermatol. Sci. 2018, 90, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, X.; Zhao, K.; Meng, F.; Li, L.; Mu, Z.; Han, X. Efficacy and Safety of Janus Kinase Inhibitors for the Treatment of Atopic Dermatitis: A Systematic Review and Meta-Analysis. Dermatology 2022, 238, 725–735. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Teixeira, H.D.; Simpson, E.L.; Papp, K.A.; Pangan, A.L.; Blauvelt, A.; Thaci, D.; Chu, C.Y.; Hong, H.C.; Katoh, N.; et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): Results from two replicate double-blind, randomised controlled phase 3 trials. Lancet 2021, 397, 2151–2168. [Google Scholar] [CrossRef]
- Verhagen, J.; Akdis, M.; Traidl-Hoffmann, C.; Schmid-Grendelmeier, P.; Hijnen, D.; Knol, E.F.; Behrendt, H.; Blaser, K.; Akdis, C.A. Absence of T-regulatory cell expression and function in atopic dermatitis skin. J. Allergy Clin. Immunol. 2006, 117, 176–183. [Google Scholar] [CrossRef]
- Fyhrquist, N.; Lehtimaki, S.; Lahl, K.; Savinko, T.; Lappetelainen, A.M.; Sparwasser, T.; Wolff, H.; Lauerma, A.; Alenius, H. Foxp3+ cells control Th2 responses in a murine model of atopic dermatitis. J. Investig. Dermatol. 2012, 132, 1672–1680. [Google Scholar] [CrossRef] [Green Version]
- Vieira, B.L.; Lim, N.R.; Lohman, M.E.; Lio, P.A. Complementary and Alternative Medicine for Atopic Dermatitis: An Evidence-Based Review. Am. J. Clin. Dermatol. 2016, 17, 557–581. [Google Scholar] [CrossRef]
- Genuis, S.J. Sensitivity-related illness: The escalating pandemic of allergy, food intolerance and chemical sensitivity. Sci. Total Env. 2010, 408, 6047–6061. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.S.; Park, M.R.; Lee, S.W.; Kim, E.H.; Cho, J.B.; Kim, J.; Han, Y.; Jung, K.; Cheong, H.K. Relationship between indoor air pollutant levels and residential environment in children with atopic dermatitis. Allergy Asthma Immunol. Res. 2014, 6, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 993–999; discussion 1000. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Jeon, B.H.; Kim, J.; Kim, Y.M.; Han, Y.; Ahn, K.; Cheong, H.K. Exposure to phthalates and bisphenol A are associated with atopic dermatitis symptoms in children: A time-series analysis. Environ. Health 2017, 16, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byremo, G.; Rod, G.; Carlsen, K.H. Effect of climatic change in children with atopic eczema. Allergy 2006, 61, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Vahavihu, K.; Ylianttila, L.; Salmelin, R.; Lamberg-Allardt, C.; Viljakainen, H.; Tuohimaa, P.; Reunala, T.; Snellman, E. Heliotherapy improves vitamin D balance and atopic dermatitis. Br. J. Dermatol. 2008, 158, 1323–1328. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Noh, S.; Bae, B.G.; Shin, J.U.; Park, C.O.; Lee, K.H. Retrospective Analysis on the Effects of House Dust Mite Specific Immunotherapy for More Than 3 Years in Atopic Dermatitis. Yonsei Med. J. 2016, 57, 393–398. [Google Scholar] [CrossRef]
- Chait, I.; Allkins, V. Remission of life-long atopic dermatitis after hyposensitisation to house dust mite. Practitioner 1985, 229, 609–612. [Google Scholar] [PubMed]
- Leroy, B.P.; Lachapelle, J.-M.; Somville, M.; Jacquemin, M.; Saint-Remy, J. Injection of allergen-antibody complexes is an effective treatment of atopic dermatitis. Dermatology 1991, 182, 98–106. [Google Scholar] [CrossRef]
- Tuft, L. Studies in atopic dermatitis. V. Problems in inhalant hyposensitization and results of treatment. J. Allergy 1960, 31, 1–11. [Google Scholar] [CrossRef]
- Hua, T.C.; Hwang, C.Y.; Chen, Y.J.; Chu, S.Y.; Chen, C.C.; Lee, D.D.; Chang, Y.T.; Wang, W.J.; Liu, H.N. The natural course of early-onset atopic dermatitis in Taiwan: A population-based cohort study. Br. J. Dermatol. 2014, 170, 130–135. [Google Scholar] [CrossRef]
- Sykes, M. Immune tolerance: Mechanisms and application in clinical transplantation. J. Intern. Med. 2007, 262, 288–310. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, P.; Thomas, R. Immune tolerance therapies for autoimmune diseases: Shifting the goalpost to cure. Curr. Opin. Pharmacol. 2022, 65, 102242. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Akdis, C.A. Therapeutic manipulation of immune tolerance in allergic disease. Nat. Rev. Drug Discov. 2009, 8, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Putterman, C.; Diamond, B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: A paradigm for autoimmune disease. Proc. Natl. Acad. Sci. USA 1996, 93, 2019–2024. [Google Scholar] [CrossRef]
- Mohamed Khosroshahi, L.; Rezaei, N. Dysregulation of the immune response in coronavirus disease 2019. Cell Biol. Int. 2021, 45, 702–707. [Google Scholar] [CrossRef]
- O’Garra, A.; Vieira, P. Regulatory T cells and mechanisms of immune system control. Nat. Med. 2004, 10, 801–805. [Google Scholar] [CrossRef]
- Miyara, M.; Sakaguchi, S. Natural regulatory T cells: Mechanisms of suppression. Trends Mol. Med. 2007, 13, 108–116. [Google Scholar] [CrossRef]
- Miyara, M.; Wing, K.; Sakaguchi, S. Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory T-cell activation and expansion. J. Allergy Clin. Immunol. 2009, 123, 749–755; quiz 756–757. [Google Scholar] [CrossRef]
- Schmitt, E.G.; Williams, C.B. Generation and function of induced regulatory T cells. Front. Immunol. 2013, 4, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L.M.R.; Muller, Y.D.; Bluestone, J.A.; Tang, Q. Next-generation regulatory T cell therapy. Nat. Rev. Drug Discov. 2019, 18, 749–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Riemann, M.; Andreas, N.; Fedoseeva, M.; Meier, E.; Weih, D.; Freytag, H.; Schmidt-Ullrich, R.; Klein, U.; Wang, Z.-Q.; Weih, F. Central immune tolerance depends on crosstalk between the classical and alternative NF-κB pathways in medullary thymic epithelial cells. J. Autoimmun. 2017, 81, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Hogquist, K.A. T-cell tolerance: Central and peripheral. Cold Spring Harb. Perspect. Biol. 2012, 4, a006957. [Google Scholar] [CrossRef] [Green Version]
- Akdis, C.A.; Akdis, M.; Blesken, T.; Wymann, D.; Alkan, S.S.; Muller, U.; Blaser, K. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J. Clin. Investig. 1996, 98, 1676–1683. [Google Scholar] [CrossRef] [Green Version]
- Akdis, C.A.; Blesken, T.; Akdis, M.; Wuthrich, B.; Blaser, K. Role of interleukin 10 in specific immunotherapy. J. Clin. Investig. 1998, 102, 98–106. [Google Scholar] [CrossRef]
- Francis, J.N.; Till, S.J.; Durham, S.R. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J. Allergy Clin. Immunol. 2003, 111, 1255–1261. [Google Scholar] [CrossRef]
- Jutel, M.; Akdis, M.; Budak, F.; Aebischer-Casaulta, C.; Wrzyszcz, M.; Blaser, K.; Akdis, C.A. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur. J. Immunol. 2003, 33, 1205–1214. [Google Scholar] [CrossRef]
- Akdis, M. Healthy immune response to allergens: T regulatory cells and more. Curr. Opin. Immunol. 2006, 18, 738–744. [Google Scholar] [CrossRef]
- Akdis, M.; Verhagen, J.; Taylor, A.; Karamloo, F.; Karagiannidis, C.; Crameri, R.; Thunberg, S.; Deniz, G.; Valenta, R.; Fiebig, H. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 2004, 199, 1567–1575. [Google Scholar] [CrossRef]
- Vaseghi-Shanjani, M.; Smith, K.L.; Sara, R.J.; Modi, B.P.; Branch, A.; Sharma, M.; Lu, H.Y.; James, E.L.; Hildebrand, K.J.; Biggs, C.M.; et al. Inborn errors of immunity manifesting as atopic disorders. J. Allergy Clin. Immunol. 2021, 148, 1130–1139. [Google Scholar] [CrossRef]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef]
- Halabi-Tawil, M.; Ruemmele, F.M.; Fraitag, S.; Rieux-Laucat, F.; Neven, B.; Brousse, N.; De Prost, Y.; Fischer, A.; Goulet, O.; Bodemer, C. Cutaneous manifestations of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Br. J. Dermatol. 2009, 160, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Adriani, M.; Jones, K.; Kirby, M.; Anderson, S.; Silvin, C.; Wincowitch, S.; Candotti, F. Defects of Regulatory T Cell function In The Wiskott-Aldrich Syndrome. (49.25). J. Immunol. 2010, 184, 49.25. [Google Scholar] [CrossRef]
- Saurat, J.H. Eczema in primary immune-deficiencies. Clues to the pathogenesis of atopic dermatitis with special reference to the Wiskott-Aldrich syndrome. Acta Derm. -Venereol. Suppl. 1985, 114, 125–128. [Google Scholar]
- Bousquet, J.; Lockey, R.; Malling, H.J. Allergen immunotherapy: Therapeutic vaccines for allergic diseases. A WHO position paper. J. Allergy Clin. Immunol. 1998, 102, 558–562. [Google Scholar] [CrossRef]
- Bae, J.M.; Choi, Y.Y.; Park, C.O.; Chung, K.Y.; Lee, K.H. Efficacy of allergen-specific immunotherapy for atopic dermatitis: A systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2013, 132, 110–117. [Google Scholar] [CrossRef]
- Yepes-Nunez, J.J.; Guyatt, G.H.; Gomez-Escobar, L.G.; Perez-Herrera, L.C.; Chu, A.W.L.; Ceccaci, R.; Acosta-Madiedo, A.S.; Wen, A.; Moreno-Lopez, S.; MacDonald, M.; et al. Allergen immunotherapy for atopic dermatitis: Systematic review and meta-analysis of benefits and harms. J. Allergy Clin. Immunol. 2023, 151, 147–158. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ. J. 2015, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucuksezer, U.C.; Ozdemir, C.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol. Int. 2020, 69, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.G.; Gregori, S.; Bacchetta, R.; Battaglia, M.; Gagliani, N. The Biology of T Regulatory Type 1 Cells and Their Therapeutic Application in Immune-Mediated Diseases. Immunity 2018, 49, 1004–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iinuma, T.; Kiuchi, M.; Hirahara, K.; Kurita, J.; Kokubo, K.; Yagyu, H.; Yoneda, R.; Arai, T.; Sonobe, Y.; Fukuyo, M.; et al. Single-cell immunoprofiling after immunotherapy for allergic rhinitis reveals functional suppression of pathogenic T(H)2 cells and clonal conversion. J. Allergy Clin. Immunol. 2022, 150, 850–860.e5. [Google Scholar] [CrossRef]
- Mercenier, A.; Pavan, S.; Pot, B. Probiotics as biotherapeutic agents: Present knowledge and future prospects. Curr. Pharm. Des. 2003, 9, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Rather, I.A.; Bajpai, V.K.; Kumar, S.; Lim, J.; Paek, W.K.; Park, Y.H. Probiotics and Atopic Dermatitis: An Overview. Front. Microbiol. 2016, 7, 507. [Google Scholar] [CrossRef] [Green Version]
- Reid, G.; Sanders, M.E.; Gaskins, H.R.; Gibson, G.R.; Mercenier, A.; Rastall, R.; Roberfroid, M.; Rowland, I.; Cherbut, C.; Klaenhammer, T.R. New scientific paradigms for probiotics and prebiotics. J. Clin. Gastroenterol. 2003, 37, 105–118. [Google Scholar] [CrossRef]
- Isolauri, E.; Arvola, T.; Sutas, Y.; Moilanen, E.; Salminen, S. Probiotics in the management of atopic eczema. Clin. Exp. Allergy 2000, 30, 1604–1610. [Google Scholar] [CrossRef] [Green Version]
- Isolauri, E. Probiotics in human disease. Am. J. Clin. Nutr. 2001, 73, 1142S–1146S. [Google Scholar] [CrossRef] [Green Version]
- Cuello-Garcia, C.A.; Brozek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nunez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schunemann, H.J. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.J.; Lee, S.H.; Yu, J.; Jeong, S.K.; Hong, S.J. Effects of Lactobacillus rhamnosus on allergic march model by suppressing Th2, Th17, and TSLP responses via CD4+CD25+Foxp3+ Tregs. Clin. Immunol. 2014, 153, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.T. Bacterial components plus vitamin D: The ultimate solution to the asthma (autoimmune disease) epidemic? J. Allergy Clin. Immunol. 2011, 127, 1128–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R.; Mantaring, J.B.V., 3rd; Chan Shih Yen, E.; Recto, M.S.T.; Sison, O.T.; Alejandria, M.M. Comparative effectiveness of probiotic strains for the treatment of pediatric atopic dermatitis: A systematic review and network meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Guo, J.; Cao, Z.; Shen, M. The efficacy of probiotics supplementation for the treatment of atopic dermatitis in adults: A systematic review and meta-analysis. J. Dermatol. Treat. 2022, 33, 2800–2809. [Google Scholar] [CrossRef]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R.; Recto, M.S.T.; Castor, M.A.R.; Casis-Hao, R.J.; Nano, A.L.M. Comparative effectiveness of probiotic strains on the prevention of pediatric atopic dermatitis: A systematic review and network meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 1255–1270. [Google Scholar] [CrossRef]
- Boyle, R.J.; Bath-Hextall, F.J.; Leonardi-Bee, J.; Murrell, D.F.; Tang, M.L. Probiotics for the treatment of eczema: A systematic review. Clin. Exp. Allergy 2009, 39, 1117–1127. [Google Scholar] [CrossRef]
- Makrgeorgou, A.; Leonardi-Bee, J.; Bath-Hextall, F.J.; Murrell, D.F.; Tang, M.L.; Roberts, A.; Boyle, R.J. Probiotics for treating eczema. Cochrane Database Syst. Rev. 2018, 11, CD006135. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Pujari, R.; Banerjee, G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol. Cell Biol. 2021, 99, 255–273. [Google Scholar] [CrossRef]
- Shibata, R.; Kimura, M.; Takahashi, H.; Mikami, K.; Aiba, Y.; Takeda, H.; Koga, Y. Clinical effects of kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clin. Exp. Allergy 2009, 39, 1397–1403. [Google Scholar] [CrossRef]
- Ahn, K. The Effect of Prebiotics on Atopic Dermatitis. Allergy Asthma Immunol. Res. 2023, 15, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Burrello, C.; Garavaglia, F.; Cribiù, F.M.; Ercoli, G.; Lopez, G.; Troisi, J.; Colucci, A.; Guglietta, S.; Carloni, S.; Guglielmetti, S. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat. Commun. 2018, 9, 5184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-H.; Kim, K.; Kim, W. Gut microbiota restoration through fecal microbiota transplantation: A new atopic dermatitis therapy. Exp. Mol. Med. 2021, 53, 907–916. [Google Scholar] [CrossRef]
- Mashiah, J.; Karady, T.; Fliss-Isakov, N.; Sprecher, E.; Slodownik, D.; Artzi, O.; Samuelov, L.; Ellenbogen, E.; Godneva, A.; Segal, E.; et al. Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe atopic dermatitis. Immun. Inflamm. Dis. 2022, 10, e570. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.A.; Rahimzadeh, M.; Brierley, C.; Gration, B.; Doree, C.; Kimber, C.E.; Plaza Cajide, A.; Lamikanra, A.A.; Roberts, D.J. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS ONE 2019, 14, e0222313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidbury, R.; Sullivan, A.F.; Thadhani, R.I.; Camargo, C.A., Jr. Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: A pilot study. Br. J. Dermatol. 2008, 159, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Hong, S.; Kim, H.J.; Lee, S.H.; Yum, H.Y. Correlation between serum vitamin D level and the severity of atopic dermatitis associated with food sensitization. Allergy Asthma Immunol. Res. 2013, 5, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Unger, W.W.; Laban, S.; Kleijwegt, F.S.; van der Slik, A.R.; Roep, B.O. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD-L1. Eur. J. Immunol. 2009, 39, 3147–3159. [Google Scholar] [CrossRef]
- Barrat, F.J.; Cua, D.J.; Boonstra, A.; Richards, D.F.; Crain, C.; Savelkoul, H.F.; de Waal-Malefyt, R.; Coffman, R.L.; Hawrylowicz, C.M.; O’Garra, A. In vitro generation of interleukin 10–producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)–and Th2-inducing cytokines. J. Exp. Med. 2002, 195, 603–616. [Google Scholar] [CrossRef]
- Urry, Z.; Xystrakis, E.; Richards, D.F.; McDonald, J.; Sattar, Z.; Cousins, D.J.; Corrigan, C.J.; Hickman, E.; Brown, Z.; Hawrylowicz, C.M. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J. Clin. Investig. 2009, 119, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Dimeloe, S.; Nanzer, A.; Ryanna, K.; Hawrylowicz, C. Regulatory T cells, inflammation and the allergic response-The role of glucocorticoids and Vitamin D. J. Steroid Biochem. Mol. Biol. 2010, 120, 86–95. [Google Scholar] [CrossRef]
- Gorman, S.; Geldenhuys, S.; Judge, M.; Weeden, C.E.; Waithman, J.; Hart, P.H. Dietary Vitamin D Increases Percentages and Function of Regulatory T Cells in the Skin-Draining Lymph Nodes and Suppresses Dermal Inflammation. J. Immunol. Res. 2016, 2016, 1426503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhou, Q.; Zhang, G.; Tian, X.; Li, Y.; Wang, Z.; Zhao, Y.; Chen, Y.; Luo, Z. Vitamin D Supplementation and Allergic Diseases during Childhood: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3947. [Google Scholar] [CrossRef]
- Buckley, R.H.; Schiff, R.I. The use of intravenous immune globulin in immunodeficiency diseases. N. Engl. J. Med. 1991, 325, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kazatchkine, M.D.; Kaveri, S.V. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N. Engl. J. Med. 2001, 345, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, E.W. Intravenous immune globulin in autoimmune and inflammatory diseases. N. Engl. J. Med. 2012, 367, 2015–2025. [Google Scholar] [CrossRef]
- Kessel, A.; Ammuri, H.; Peri, R.; Pavlotzky, E.R.; Blank, M.; Shoenfeld, Y.; Toubi, E. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J. Immunol. 2007, 179, 5571–5575. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.C.; Yang, K.D.; Lin, C.Y.; Huang, A.Y.; Hsiao, C.C.; Lin, M.T.; Tsai, Y.G. Intravenous immunoglobulin therapy enhances suppressive regulatory T cells and decreases innate lymphoid cells in children with immune thrombocytopenia. Pediatr. Blood Cancer 2020, 67, e28075. [Google Scholar] [CrossRef]
- Tsurikisawa, N.; Saito, H.; Oshikata, C.; Tsuburai, T.; Akiyama, K. High-dose intravenous immunoglobulin treatment increases regulatory T cells in patients with eosinophilic granulomatosis with polyangiitis. J. Rheumatol. 2012, 39, 1019–1025. [Google Scholar] [CrossRef] [Green Version]
- Maddur, M.S.; Kaveri, S.V.; Bayry, J. Circulating normal IgG as stimulator of regulatory T cells: Lessons from intravenous immunoglobulin. Trends Immunol. 2017, 38, 789–792. [Google Scholar] [CrossRef]
- Ephrem, A.; Chamat, S.; Miquel, C.; Fisson, S.; Mouthon, L.; Caligiuri, G.; Delignat, S.; Elluru, S.; Bayry, J.; Lacroix-Desmazes, S.; et al. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: A critical factor in controlling experimental autoimmune encephalomyelitis. Blood 2008, 111, 715–722. [Google Scholar] [CrossRef]
- Perez, E.E.; Orange, J.S.; Bonilla, F.; Chinen, J.; Chinn, I.K.; Dorsey, M.; El-Gamal, Y.; Harville, T.O.; Hossny, E.; Mazer, B.; et al. Update on the use of immunoglobulin in human disease: A review of evidence. J. Allergy Clin. Immunol. 2017, 139, S1–S46. [Google Scholar] [CrossRef] [Green Version]
- Clinical Research Group for Histaglobin. Double blind controlled study on the efficacy of histaglobin on chronic urticaria and eczematous dermatitis (in Japanese). Nishinipponifuka 1980, 42, 470–477. [Google Scholar]
- Pons-Guiraud, A. Value of Allerglobulin in the treatment of atopic dermatitis in children and young adults. A double-blind randomized study. Rev. Med. Interne 1986, 7, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Jerne, N.K. Towards a network theory of the immune system. Ann. Immunol. 1974, 125C, 373–389. [Google Scholar]
- Lopez-Requena, A.; Mateo De Acosta, C.; Vazquez, A.M.; Perez, R. Immunogenicity of autologous immunoglobulins: Principles and practices. Mol. Immunol. 2007, 44, 3076–3082. [Google Scholar] [CrossRef]
- Shoenfeld, Y. The idiotypic network in autoimmunity: Antibodies that bind antibodies that bind antibodies. Nat. Med. 2004, 10, 17–18. [Google Scholar] [CrossRef]
- Schulz, R.; Werner, B.; Behn, U. Self-tolerance in a minimal model of the idiotypic network. Front. Immunol. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Wallmann, J.; Pali-Scholl, I.; Jensen-Jarolim, E. Anti-ids in allergy: Timeliness of a classic concept. World Allergy Organ. J. 2010, 3, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahm, D.H.; Cho, S.M.; Kim, M.E.; Kim, Y.J.; Jeon, S.Y. Autologous immunoglobulin therapy in patients with severe recalcitrant atopic dermatitis: A preliminary report. Allergy Asthma Immunol. Res. 2014, 6, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Nahm, D.H.; Kim, M.E.; Cho, S.M. Effects of Intramuscular Injection of Autologous Immunoglobulin on Clinical Severity and Serum IgE Concentration in Patients with Atopic Dermatitis. Dermatology 2015, 231, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Nahm, D.H.; Ahn, A.; Kim, M.E.; Cho, S.M.; Park, M.J. Autologous Immunoglobulin Therapy in Patients With Severe Recalcitrant Atopic Dermatitis: Long-Term Changes of Clinical Severity and Laboratory Parameters. Allergy Asthma Immunol. Res. 2016, 8, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Kim, M.E.; Kwon, B.; Nahm, D.H. Immunomodulatory effects induced by intramuscular administration of autologous total immunoglobulin G in patients with atopic dermatitis. Int. Immunopharmacol. 2017, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nahm, D.H.; Ye, Y.M.; Shin, Y.S.; Park, H.S.; Kim, M.E.; Kwon, B.; Cho, S.M.; Han, J. Efficacy, Safety, and Immunomodulatory Effect of the Intramuscular Administration of Autologous Total Immunoglobulin G for Atopic Dermatitis: A Randomized Clinical Trial. Allergy Asthma Immunol. Res. 2020, 12, 949–963. [Google Scholar] [CrossRef]
- Kwon, B.; Yang, S.J.; Cho, S.M.; Kim, M.E.; Nahm, D.H. Intramuscular administration of autologous total immunoglobulin G induces immunomodulatory effects on T cells in healthy human subjects: An open-labeled prospective single-arm trial. Medicine 2022, 101, e29486. [Google Scholar] [CrossRef] [PubMed]
- Sgnotto, F.D.R.; de Oliveira, M.G.; Lira, A.A.L.; Inoue, A.H.S.; Titz, T.O.; Orfali, R.L.; Bento-de-Souza, L.; Sato, M.N.; Aoki, V.; Duarte, A.J.S.; et al. IgG from atopic dermatitis patients induces IL-17 and IL-10 production in infant intrathymic TCD4 and TCD8 cells. Int. J. Dermatol. 2018, 57, 434–440. [Google Scholar] [CrossRef]
- Fagundes, B.O.; de Sousa, T.R.; Nascimento, A.; Fernandes, L.A.; Sgnotto, F.D.R.; Orfali, R.L.; Aoki, V.; Duarte, A.; Sanabani, S.S.; Victor, J.R. IgG from Adult Atopic Dermatitis (AD) Patients Induces Nonatopic Neonatal Thymic Gamma-Delta T Cells (gammadeltaT) to Acquire IL-22/IL-17 Secretion Profile with Skin-Homing Properties and Epigenetic Implications Mediated by miRNA. Int. J. Mol. Sci. 2022, 23, 6872. [Google Scholar] [CrossRef]
- Victor, J.R. Do different IgG repertoires play a role in B- and T-cell functional modulation during ontogeny? The “hooks without bait” theory. Immunol. Cell Biol. 2020, 98, 540–548. [Google Scholar] [CrossRef]
- Croft, M.; So, T.; Duan, W.; Soroosh, P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol. Rev. 2009, 229, 173–191. [Google Scholar] [CrossRef] [Green Version]
- Redmond, W.L.; Ruby, C.E.; Weinberg, A.D. The role of OX40-mediated co-stimulation in T-cell activation and survival. Crit. Rev. Immunol. 2009, 29, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Ishii, N.; Takahashi, T.; Soroosh, P.; Sugamura, K. OX40-OX40 ligand interaction in T-cell-mediated immunity and immunopathology. Adv. Immunol. 2010, 105, 63–98. [Google Scholar] [CrossRef]
- Kumar, P.; Marinelarena, A.; Raghunathan, D.; Ragothaman, V.K.; Saini, S.; Bhattacharya, P.; Fan, J.; Epstein, A.L.; Maker, A.V.; Prabhakar, B.S. Critical role of OX40 signaling in the TCR-independent phase of human and murine thymic Treg generation. Cell Mol. Immunol. 2019, 16, 138–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruby, C.E.; Yates, M.A.; Hirschhorn-Cymerman, D.; Chlebeck, P.; Wolchok, J.D.; Houghton, A.N.; Offner, H.; Weinberg, A.D. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J. Immunol. 2009, 183, 4853–4857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkachev, V.; Furlan, S.N.; Watkins, B.; Hunt, D.J.; Zheng, H.B.; Panoskaltsis-Mortari, A.; Betz, K.; Brown, M.; Schell, J.B.; Zeleski, K.; et al. Combined OX40L and mTOR blockade controls effector T cell activation while preserving T(reg) reconstitution after transplant. Sci. Transl. Med. 2017, 9, eaan3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttman-Yassky, E.; Simpson, E.L.; Reich, K.; Kabashima, K.; Igawa, K.; Suzuki, T.; Mano, H.; Matsui, T.; Esfandiari, E.; Furue, M. An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: A multicentre, double-blind, placebo-controlled phase 2b study. Lancet 2023, 401, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Kahan, B.D. Sirolimus: A comprehensive review. Expert. Opin. Pharmacother. 2001, 2, 1903–1917. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Eisen, H.J. Mechanistic Target of Rapamycin (mTOR) Inhibitors. Handb. Exp. Pharmacol. 2022, 272, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.; Czystowska, M.; Szajnik, M.; Mandapathil, M.; Whiteside, T.L. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS ONE 2009, 4, e5994. [Google Scholar] [CrossRef] [Green Version]
- Lai, Z.W.; Kelly, R.; Winans, T.; Marchena, I.; Shadakshari, A.; Yu, J.; Dawood, M.; Garcia, R.; Tily, H.; Francis, L.; et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: A single-arm, open-label, phase 1/2 trial. Lancet 2018, 391, 1186–1196. [Google Scholar] [CrossRef]
- Yang, F.; Tanaka, M.; Wataya-Kaneda, M.; Yang, L.; Nakamura, A.; Matsumoto, S.; Attia, M.; Murota, H.; Katayama, I. Topical application of rapamycin ointment ameliorates Dermatophagoides farina body extract-induced atopic dermatitis in NC/Nga mice. Exp. Dermatol. 2014, 23, 568–572. [Google Scholar] [CrossRef]
- Kim, J.W.; Choe, J.Y.; Park, S.H. Metformin and its therapeutic applications in autoimmune inflammatory rheumatic disease. Korean J. Intern. Med. 2022, 37, 13–26. [Google Scholar] [CrossRef]
- Son, H.J.; Lee, J.; Lee, S.Y.; Kim, E.K.; Park, M.J.; Kim, K.W.; Park, S.H.; Cho, M.L. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediat. Inflamm. 2014, 2014, 973986. [Google Scholar] [CrossRef] [Green Version]
- Nyambuya, T.M.; Dludla, P.V.; Mxinwa, V.; Mokgalaboni, K.; Ngcobo, S.R.; Tiano, L.; Nkambule, B.B. The impact of metformin and aspirin on T-cell mediated inflammation: A systematic review of in vitro and in vivo findings. Life Sci. 2020, 255, 117854. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.S.; Alarfaj, S.J.; Saif, D.S.; El-Naggar, M.E.; Elsokary, M.A.; Elsawah, H.K.; Abdelsattar Zaki, S.; Wahsh, E.A.; Abo Mansour, H.E.; Mosalam, E.M. The AMPK modulator metformin as adjunct to methotrexate in patients with rheumatoid arthritis: A proof-of-concept, randomized, double-blind, placebo-controlled trial. Int. Immunopharmacol. 2021, 95, 107575. [Google Scholar] [CrossRef]
- Pei, X.; Ling, Y.; Zhu, K.; Chen, W.; Gu, M. Metformin Attenuates Atopic Dermatitis by Inhibiting CD40 Expression in CD11c+DC via the mTOR Pathway. Nanosci. Nanotechnol. Lett. 2017, 9, 934–940. [Google Scholar] [CrossRef]
- Jiang, L.; Fu, H.; Yang, H.K.; Xu, W.; Wang, J.; Yang, S.T. Butyric acid: Applications and recent advances in its bioproduction. Biotechnol. Adv. 2018, 36, 2101–2117. [Google Scholar] [CrossRef] [PubMed]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Lupton, J.R. Microbial degradation products influence colon cancer risk: The butyrate controversy. J. Nutr. 2004, 134, 479–482. [Google Scholar] [CrossRef] [Green Version]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef] [Green Version]
- Hoeppli, R.E.; Wu, D.; Cook, L.; Levings, M.K. The environment of regulatory T cell biology: Cytokines, metabolites, and the microbiome. Front. Immunol. 2015, 6, 61. [Google Scholar] [CrossRef] [Green Version]
- Scheppach, W.; Sommer, H.; Kirchner, T.; Paganelli, G.M.; Bartram, P.; Christl, S.; Richter, F.; Dusel, G.; Kasper, H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992, 103, 51–56. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, S.H.; Kim, I.S.; Yu, D.Y.; Kim, S.C.; Lee, S.H.; Lee, S.S.; Yun, C.H.; Choi, I.S.; Cho, K.K. Anti-Inflammatory Effects of a Mixture of Lactic Acid Bacteria and Sodium Butyrate in Atopic Dermatitis Murine Model. J. Med. Food 2018, 21, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F.; Adcock, I.M.; Benito-Villalvilla, C.; Bianchini, R.; Bjermer, L.; Boyman, O.; Caramori, G.; Cari, L.; Fan Chung, K.; Diamant, Z. Immune modulation via T regulatory cell enhancement: Disease-modifying therapies for autoimmunity and their potential for chronic allergic and inflammatory diseases—An EAACI position paper of the Task Force on Immunopharmacology (TIPCO). Allergy 2021, 76, 90–113. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Terada, T.; Kitatani, K.; Kawata, R.; Nabe, T. Roles of type 1 regulatory T (Tr1) cells in allergen-specific immunotherapy. Front. Allergy 2022, 3, 981126. [Google Scholar] [CrossRef] [PubMed]
- Bellinghausen, I.; Khatri, R.; Saloga, J. Current Strategies to Modulate Regulatory T Cell Activity in Allergic Inflammation. Front. Immunol. 2022, 13, 912529. [Google Scholar] [CrossRef]
- Marek-Trzonkowska, N.; Mysliwiec, M.; Dobyszuk, A.; Grabowska, M.; Derkowska, I.; Juscinska, J.; Owczuk, R.; Szadkowska, A.; Witkowski, P.; Mlynarski, W.; et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets—Results of one year follow-up. Clin. Immunol. 2014, 153, 23–30. [Google Scholar] [CrossRef]
- Sawitzki, B.; Harden, P.N.; Reinke, P.; Moreau, A.; Hutchinson, J.A.; Game, D.S.; Tang, Q.; Guinan, E.C.; Battaglia, M.; Burlingham, W.J.; et al. Regulatory cell therapy in kidney transplantation (The ONE Study): A harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 2020, 395, 1627–1639. [Google Scholar] [CrossRef]
- Roemhild, A.; Otto, N.M.; Moll, G.; Abou-El-Enein, M.; Kaiser, D.; Bold, G.; Schachtner, T.; Choi, M.; Oellinger, R.; Landwehr-Kenzel, S.; et al. Regulatory T cells for minimising immune suppression in kidney transplantation: Phase I/IIa clinical trial. BMJ 2020, 371, m3734. [Google Scholar] [CrossRef]
- Hu, M.; Rogers, N.M.; Li, J.; Zhang, G.Y.; Wang, Y.M.; Shaw, K.; O’Connell, P.J.; Alexander, S.I. Antigen Specific Regulatory T Cells in Kidney Transplantation and Other Tolerance Settings. Front. Immunol. 2021, 12, 717594. [Google Scholar] [CrossRef]
- Leventhal, J.; LeFever, A.; Skaro, A.; Gallon, L.; Mathew, J.; Stare, D.; Konieczna, I.; He, J.; Johnson, G. Interim Results of a Phase 1 Trial of Treg Adoptive Cell Transfer (TRACT) in Living Donor Kidney Transplant Recipients. Am. J. Transpl. 2015, 15, 3031. [Google Scholar]
- Mfarrej, B.; Tresoldi, E.; Stabilini, A.; Paganelli, A.; Caldara, R.; Secchi, A.; Battaglia, M. Generation of donor-specific Tr1 cells to be used after kidney transplantation and definition of the timing of their in vivo infusion in the presence of immunosuppression. J. Transl. Med. 2017, 15, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, I.; Bashuda, H.; Uchida, K.; Seino, K.I.; Habu, S.; Nakajima, I.; Fuchinoue, S.; Okumura, K.; Teraoka, S. A Clinical Trial With Adoptive Transfer of Ex Vivo-induced, Donor-specific Immune-regulatory Cells in Kidney Transplantation-A Second Report. Transplantation 2020, 104, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.N.; Salim, K.; Levings, M.K. Manufacturing next-generation regulatory T-cell therapies. Curr. Opin. Biotechnol. 2022, 78, 102822. [Google Scholar] [CrossRef]
- Foster, M.C.; Savoldo, B.; Lau, W.; Rubinos, C.; Grover, N.; Armistead, P.; Coghill, J.; Hagan, R.S.; Morrison, K.; Buchanan, F.B.; et al. Utility of a safety switch to abrogate CD19.CAR T-cell-associated neurotoxicity. Blood 2021, 137, 3306–3309. [Google Scholar] [CrossRef]
- Filley, A.C.; Henriquez, M.; Dey, M. CART Immunotherapy: Development, Success, and Translation to Malignant Gliomas and Other Solid Tumors. Front. Oncol. 2018, 8, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, J.; Yakoub-Agha, I. Chimeric antigen-receptor T-cell therapy for hematological malignancies and solid tumors: Clinical data to date, current limitations and perspectives. Curr. Res. Transl. Med. 2017, 65, 93–102. [Google Scholar] [CrossRef]
- Prasad, V. Tisagenlecleucel—The first approved CAR-T-cell therapy: Implications for payers and policy makers. Nat. Rev. Clin. Oncol. 2018, 15, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O. CD19 as an attractive target for antibody-based therapy. MAbs 2012, 4, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.; Wang, Y.; Lu, X.; Han, W. Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy. J. Natl. Cancer Inst. 2016, 108, djv439. [Google Scholar] [CrossRef] [Green Version]
- Vairy, S.; Garcia, J.L.; Teira, P.; Bittencourt, H. CTL019 (tisagenlecleucel): CAR-T therapy for relapsed and refractory B-cell acute lymphoblastic leukemia. Drug Des. Devel. Ther. 2018, 12, 3885–3898. [Google Scholar] [CrossRef] [Green Version]
- Madduri, D.; Dhodapkar, M.V.; Lonial, S.; Jagannath, S.; Cho, H.J. SOHO State of the Art Updates and Next Questions: T-Cell-Directed Immune Therapies for Multiple Myeloma: Chimeric Antigen Receptor-Modified T Cells and Bispecific T-Cell-Engaging Agents. Clin. Lymphoma Myeloma Leuk. 2019, 19, 537–544. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, O.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Abdeladhim, M.; Zhang, A.H.; Kropp, L.E.; Lindrose, A.R.; Venkatesha, S.H.; Mitre, E.; Scott, D.W. Engineered ovalbumin-expressing regulatory T cells protect against anaphylaxis in ovalbumin-sensitized mice. Clin. Immunol. 2019, 207, 49–54. [Google Scholar] [CrossRef]
- Schmetterer, K.G.; Haiderer, D.; Leb-Reichl, V.M.; Neunkirchner, A.; Jahn-Schmid, B.; Kung, H.J.; Schuch, K.; Steinberger, P.; Bohle, B.; Pickl, W.F. Bet v 1-specific T-cell receptor/forkhead box protein 3 transgenic T cells suppress Bet v 1-specific T-cell effector function in an activation-dependent manner. J. Allergy Clin. Immunol. 2011, 127, 238–245, 245.e1–e3. [Google Scholar] [CrossRef]
- Voo, K.S.; Wang, Y.H.; Santori, F.R.; Boggiano, C.; Wang, Y.H.; Arima, K.; Bover, L.; Hanabuchi, S.; Khalili, J.; Marinova, E.; et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 4793–4798. [Google Scholar] [CrossRef]
- McClymont, S.A.; Putnam, A.L.; Lee, M.R.; Esensten, J.H.; Liu, W.; Hulme, M.A.; Hoffmuller, U.; Baron, U.; Olek, S.; Bluestone, J.A.; et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J. Immunol. 2011, 186, 3918–3926. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.M.; Sharma, G.; Verma, R.; Byun, S.; Rudra, D.; Im, S.H. Inflammation-induced Id2 promotes plasticity in regulatory T cells. Nat. Commun. 2018, 9, 4736. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Boeld, T.J.; Eder, R.; Huehn, J.; Floess, S.; Wieczorek, G.; Olek, S.; Dietmaier, W.; Andreesen, R.; Edinger, M. Loss of FOXP3 expression in natural human CD4+ CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur. J. Immunol. 2009, 39, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Inomata, T.; Chen, Y.; Foulsham, W.; Stevenson, W.; Shiang, T.; Bluestone, J.A.; Dana, R. Pathological conversion of regulatory T cells is associated with loss of allotolerance. Sci. Rep. 2018, 8, 7059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safari, F.; Farajnia, S.; Arya, M.; Zarredar, H.; Nasrolahi, A. CRISPR and personalized Treg therapy: New insights into the treatment of rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2018, 40, 201–211. [Google Scholar] [CrossRef]
- Tang, Q.; Bluestone, J.A. Regulatory T-cell therapy in transplantation: Moving to the clinic. Cold Spring Harb. Perspect. Med. 2013, 3, a015552. [Google Scholar] [CrossRef]
- Hesse, L.; van Ieperen, N.; Petersen, A.H.; Elberink, J.; van Oosterhout, A.J.M.; Nawijn, M.C. High dose vitamin D(3) empowers effects of subcutaneous immunotherapy in a grass pollen-driven mouse model of asthma. Sci. Rep. 2020, 10, 20876. [Google Scholar] [CrossRef]
- Jerzynska, J.; Stelmach, W.; Rychlik, B.; Lechanska, J.; Podlecka, D.; Stelmach, I. The clinical effect of vitamin D supplementation combined with grass-specific sublingual immunotherapy in children with allergic rhinitis. Allergy Asthma Proc. 2016, 37, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Chiewchalermsri, C.; Sangkanjanavanich, S.; Pradubpongsa, P.; Mitthamsiri, W.; Jaisupa, N.; Jindarat, S.; Buranapraditkun, S.; Jacquet, A.; Sangasapaviliya, A.; Boonpiyathad, T. Randomized, Double-Blind, Placebo-Controlled Trial of Vitamin D Supplementation in the Build-up Phase of House Dust Mite-Specific Immunotherapy. Allergy Asthma Immunol. Res. 2023, 15, 336–347. [Google Scholar] [CrossRef]
- Leroy, B.P.; Boden, G.; Lachapelle, J.M.; Jacquemin, M.G.; Saint-Remy, J.M. A novel therapy for atopic dermatitis with allergen-antibody complexes: A double-blind, placebo-controlled study. J. Am. Acad. Dermatol. 1993, 28, 232–239. [Google Scholar] [CrossRef]


|
|
| Strategies with proven clinical efficacy by at least one randomized clinical trial |
|
| Strategies without proven clinical efficacy in patients with atopic dermatitis by a clinical trial |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahm, D.-H. Regulatory T Cell-Targeted Immunomodulatory Therapy for Long-Term Clinical Improvement of Atopic Dermatitis: Hypotheses and Perspectives. Life 2023, 13, 1674. https://doi.org/10.3390/life13081674
Nahm D-H. Regulatory T Cell-Targeted Immunomodulatory Therapy for Long-Term Clinical Improvement of Atopic Dermatitis: Hypotheses and Perspectives. Life. 2023; 13(8):1674. https://doi.org/10.3390/life13081674
Chicago/Turabian StyleNahm, Dong-Ho. 2023. "Regulatory T Cell-Targeted Immunomodulatory Therapy for Long-Term Clinical Improvement of Atopic Dermatitis: Hypotheses and Perspectives" Life 13, no. 8: 1674. https://doi.org/10.3390/life13081674
APA StyleNahm, D.-H. (2023). Regulatory T Cell-Targeted Immunomodulatory Therapy for Long-Term Clinical Improvement of Atopic Dermatitis: Hypotheses and Perspectives. Life, 13(8), 1674. https://doi.org/10.3390/life13081674





